Effect of Metaldehyde on Survival, Enzyme Activities, and Histopathology of the Apple Snail Pomacea canaliculata (Lamarck 1822)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Snail Collection
2.2. Experimental Design
2.3. Biochemical Analysis
2.4. Histological Preparation
2.5. Data Analysis
3. Results
3.1. Snail Activity
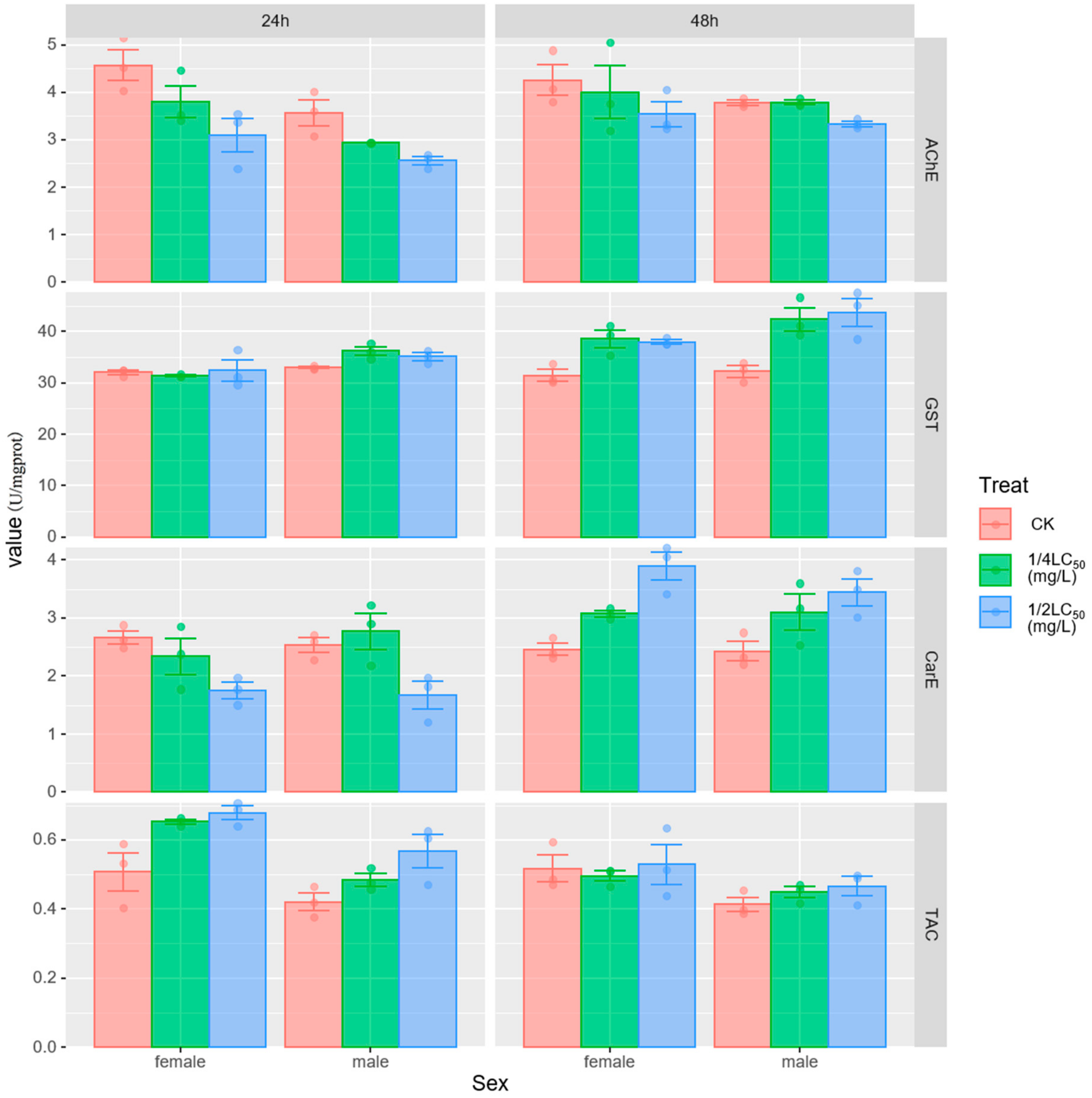
3.2. Enzyme Activities
3.3. Histopathology
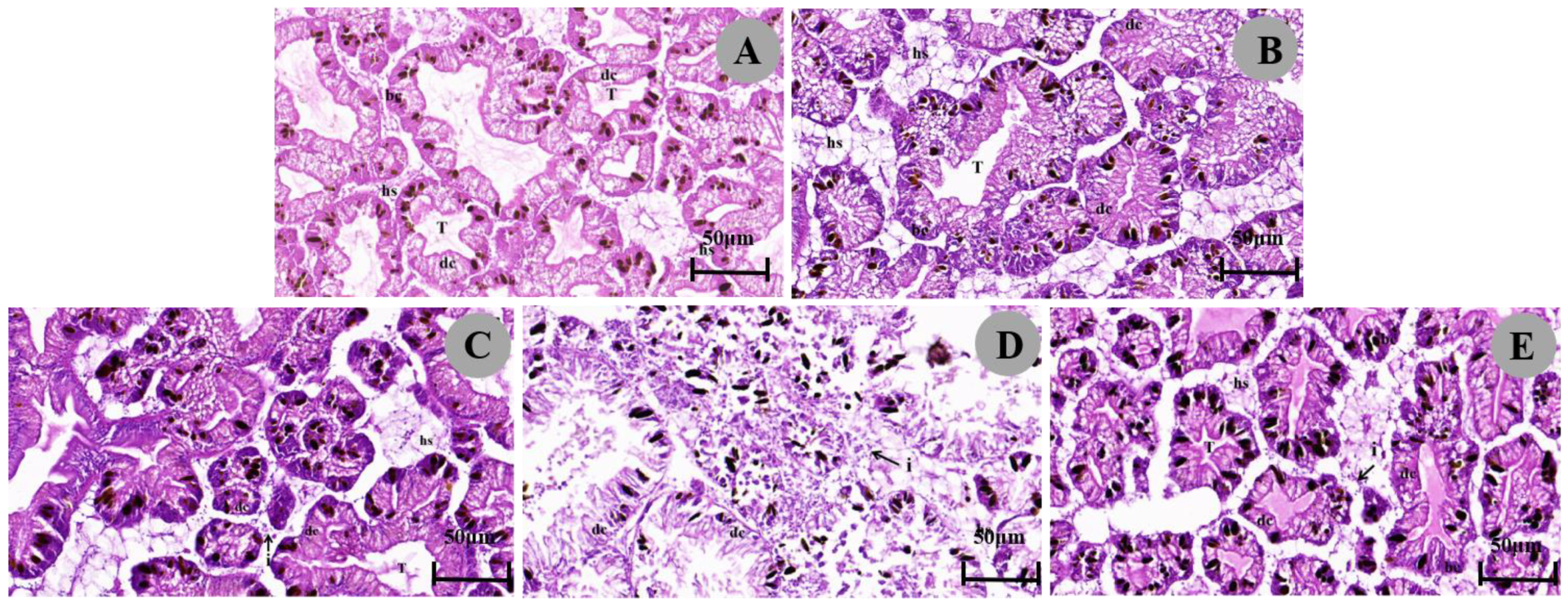
3.3.1. Histopathological Response of the Digestive Glands
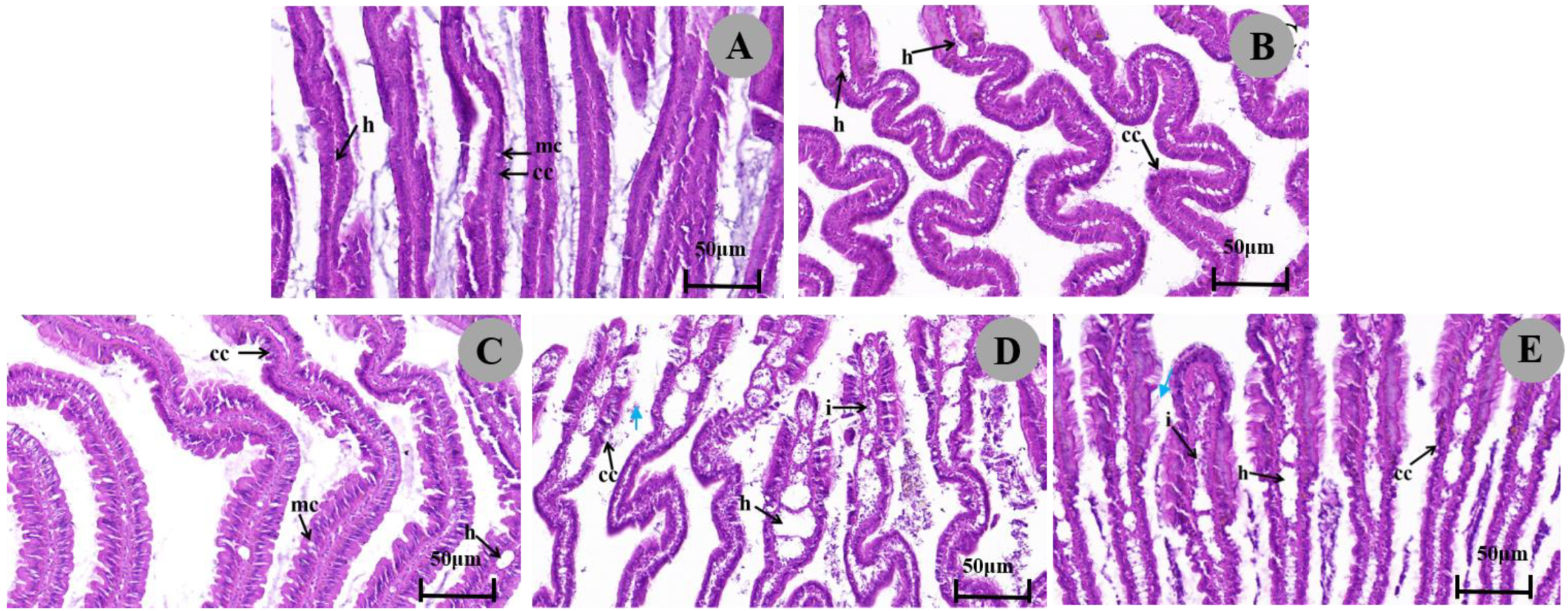
3.3.2. Histopathological Reaction of the Gills
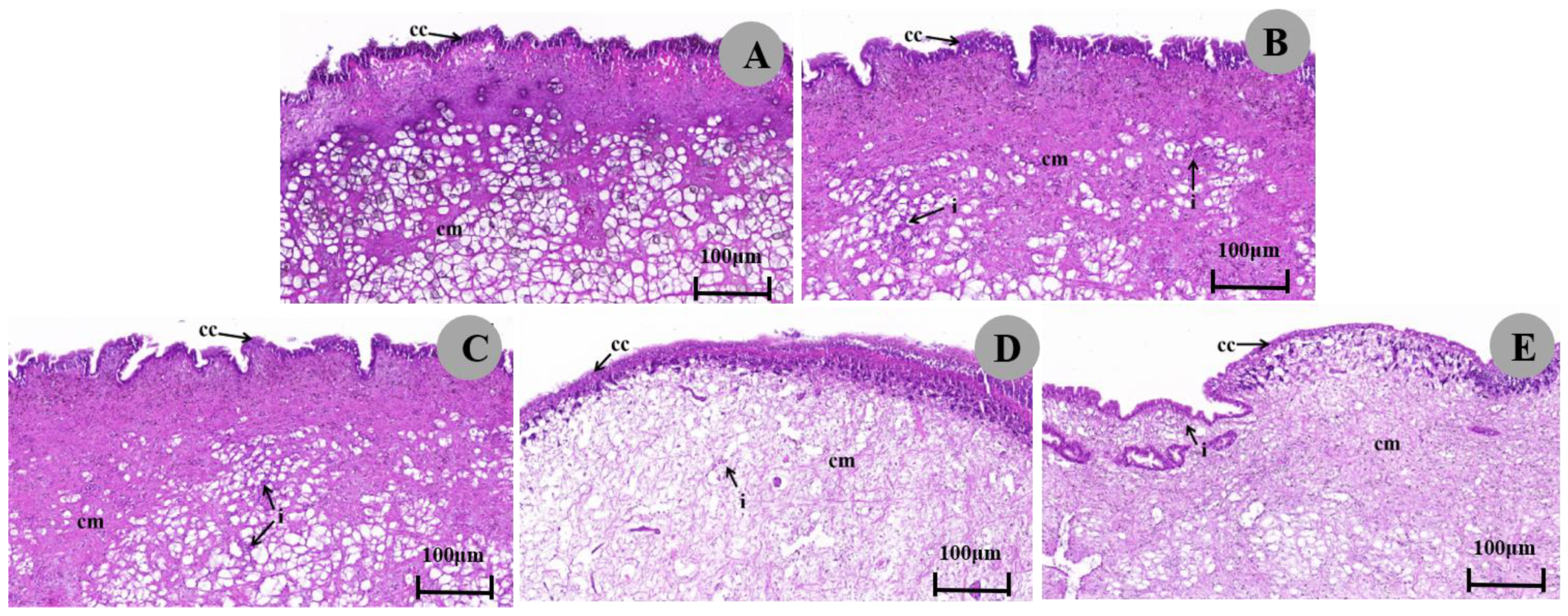
3.3.3. Histopathological Response of the Ventral Foot
4. Discussion
4.1. Effect of Metaldehyde on Enzyme Activity
4.2. Effect of Metaldehyde on the Histopathology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, K.A.; Joshi, R.C.; Thiengo, S.C.; Cowie, R.H. Out of South America: Multiple Origins of Non-Native Apple Snails in Asia: Invasive Ampullariids in Asia. Divers. Distrib. 2008, 14, 701–712. [Google Scholar] [CrossRef]
- Wada, T. Strategies for Controlling the Apple Snail Pomacea canaliculata (Lamarck) (Gastropoda: Ampullariidae) in Japanese Direct-Sown Paddy Fields. Jpn. Agric. Res. Q. JARQ 2004, 38, 75–80. [Google Scholar] [CrossRef]
- Boland, B.B.; Meerhoff, M.; Fosalba, C.; Mazzeo, N.; Barnes, M.A.; Burks, R.L. Juvenile Snails, Adult Appetites: Contrasting Resource Consumption between Two Species of Applesnails (Pomacea). J. Molluscan Stud. 2007, 74, 47–54. [Google Scholar] [CrossRef]
- Wang, W.; Huang, S.; Liu, F.; Sun, Y.; Wang, X.; Yao, J.; Li, S.; Liu, Y.; Luo, B.; Zhang, X.; et al. Control of the Invasive Agricultural Pest Pomacea canaliculata with a Novel Molluscicide: Efficacy and Safety to Nontarget Species. J. Agric. Food Chem. 2022, 70, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, N.O.L.; Bronmark, C. Size-Dependent Effects of an Invasive Herbivorous Snail (Pomacea canaliculata) on Macrophytes and Periphyton in Asian Wetlands. Freshw. Biol. 2006, 51, 695–704. [Google Scholar] [CrossRef]
- Cowie, R.H.; Hayes, K.A.; Thiengo, S.C. What Are Apple Snails? Confused Taxonomy and Some Preliminary Resolution; PhilRice: Nueva Ecija, Philippines, 2006.
- Liu, J.; Li, J.; Wang, Z.; Yang, H. Transcriptome Sequencing and Bioinformatics Analysis of Ovarian Tissues from Pomacea canaliculata in Guangdong and Hunan. Mediat. Inflamm. 2022, 2022, 3917036. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.K.; Kwong, K.L.; Qiu, J.-W. Complex Interactions among Fish, Snails and Macrophytes: Implications for Biological Control of an Invasive Snail. Biol. Invasions 2009, 11, 2223–2232. [Google Scholar] [CrossRef]
- Kannan, A.; Rama Rao, S.; Ratnayeke, S.; Yow, Y.-Y. The Efficiency of Universal Mitochondrial DNA Barcodes for Species Discrimination of Pomacea canaliculata and Pomacea maculata. PeerJ 2020, 8, e8755. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Liu, S.-W.; He, C.; Yu, X.-P. Distribution and the Origin of Invasive Apple Snails, Pomacea canaliculata and P. Maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 2018, 8, 1185. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Liu, H.-X.; Hu, L.; Liu, Q.; Wei, F.-R.; Guo, Y.-H.; Steinmann, P.; Hu, W.; Zhou, X.-N.; et al. Phylogenetic Evidence for Multiple and Secondary Introductions of Invasive Snails: Pomacea Species in the People’s Republic of China. Divers. Distrib. 2013, 19, 147–156. [Google Scholar] [CrossRef]
- Cao, M.-Y.; Fan, Y.-Y.; Yin, C.-L.; Yu, X.-P. Identification of HSF and HSP Gene Family in Pomacea canaliculata and Their Expression Pattern under Temperature Stress. J. Agric. Biotechnol. 2023, 30, 370–382. [Google Scholar]
- Wang, J.; Xing, Y.; Dai, Y.; Li, Y.; Xiang, W.; Dai, J.; Xu, F. A Novel Gelatin-Based Sustained-Release Molluscicide for Control of the Invasive Agricultural Pest and Disease Vector Pomacea canaliculata. Molecules 2022, 27, 4268. [Google Scholar] [CrossRef] [PubMed]
- Castle, G.D.; Mills, G.A.; Gravell, A.; Jones, L.; Townsend, I.; Cameron, D.G.; Fones, G.R. Review of the Molluscicide Metaldehyde in the Environment. Environ. Sci. Water Res. Technol. 2017, 3, 415–428. [Google Scholar] [CrossRef]
- Castro-Gutierrez, V.; Pickering, L.; Cambronero-Heinrichs, J.; Holden, B.; Haley, J.; Jarvis, P.; Jefferson, B.; Helgason, T.; Moir, J.; Hassard, F. Bioaugmentation of Pilot-Scale Slow Sand Filters Can Achieve Compliant Levels for the Micropollutant Metaldehyde in a Real Water Matrix. Water Res. 2022, 211, 118071. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Shao, X.; Lin, H.; Hu, J. Dissipation, Residues and Risk Assessment of Metaldehyde and Niclosamide Ethanolamine in Pakchoi after Field Application. Food Chem. 2017, 229, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in Aquatic Environments and Their Removal by Adsorption Methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, C.; Xu, P.J.; Ma, Y.Q. Analysis of Molluscicide Metaldehyde in Vegetables by Dispersive Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2011, 28, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Machado, A.M.D.; da Silva, R.H.S.; Faria, A.C.; Machado, L.S.; Santos, H.; Braga, S.d.M.; Torres, B.B.J.; Miguel, M.P.; Chaves, A.R.; et al. Fatal Metaldehyde Poisoning in a Dog Confirmed by Gas Chromatography. BMC Vet. Res. 2020, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M. Impact of Methomyl Lannate on Physiological Parameters of the Land Snail Eobania Vermiculata. J. Basic. Appl. Zool. 2016, 74, 1–7. [Google Scholar] [CrossRef][Green Version]
- Abo Bakr, Y. Histopathological Changes Induced by Metaldehyde in Eobania Vermiculata (Müller 1774). Alex. Sci. Exch. J. Int. Q. J. Sci. Agric. Environ. 2011, 32, 300–310. [Google Scholar] [CrossRef][Green Version]
- Abobakr, Y.; Gad, A.F.; Abou-Elnasr, H.S.; Abdelgalil, G.M.; Hussein, H.I.; Selim, S. Contact Toxicity and Biochemical Impact of Metaldehyde against the White Garden Snail Theba pisana (Müller, 1774). Pest Manag. Sci. 2021, 77, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. BioMed Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mu, X.; Wei, H.; Yang, Y.; Xu, M.; Luo, D.; Hu, Y. Effects of Sublethal Dose of Metaldehyde on the Activities of AchE, GSTs and MFO in Pomacea canaliculata. Plant Prot. 2016, 42, 58–62. [Google Scholar]
- Arrighetti, F.; Ambrosio, E.; Astiz, M.; Capítulo, A.R.; Lavarías, S. Differential Response between Histological and Biochemical Biomarkers in the Apple Snail Pomacea canaliculata (Gasteropoda: Amullariidae) Exposed to Cypermethrin. Aquat. Toxicol. 2018, 194, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Longshaw, M.; Lyons, B.P.; Jones, G.; Green, M.; Feist, S.W. Histopathological Biomarkers in Estuarine Fish Species for the Assessment of Biological Effects of Contaminants. Mar. Environ. Res. 2003, 55, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Gaber, O.A.; Asran, A.E.A.; Elfayoumi, H.M.K.; El-Shahawy, G.; Khider, F.K.; Abdel-Tawab, H.; Mahmoud, K.A. Influence of Methomyl (Copter 90%) on Certain Biochemical Activities and Histological Structures of Land Snails Monacha Cartusiana. Saudi J. Biol. Sci. 2022, 29, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, S.B.; Otludil, B. Accumulation and Histopathological Effects of Cadmium on the Great Pond Snail Lymnaea Stagnalis Linnaeus, 1758 (Gastropoda: Pulmonata). Environ. Toxicol. Pharmacol. 2020, 78, 103403. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Lu, Y.; Zheng, X.; He, X.; Guo, J.; Fu, Q.; Xu, H.; Lu, Z. Sublethal Effects of Chlorantraniliprole on Growth, Biochemical and Molecular Parameters in Two Chironomids, Chironomus Kiiensis and Chironomus Javanus. Ecotoxicol. Environ. Saf. 2023, 253, 114658. [Google Scholar] [CrossRef] [PubMed]
- Buikema, A.L.; Niederlehner, B.R.; Cairns, J. Introduction to Toxicity Testing. In Biological Monitoring in Water Pollution; Elsevier: Amsterdam, The Netherlands, 1982; pp. 239–262. ISBN 978-0-08-028730-0. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- van Asperen, K. A Study of Housefly Esterases by Means of a Sensitive Colorimetric Method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Zayed, K.M.; Guo, Y.-H.; Lv, S.; Zhang, Y.; Zhou, X.-N. Molluscicidal and Antioxidant Activities of Silver Nanoparticles on the Multi-Species of Snail Intermediate Hosts of Schistosomiasis. PLoS Negl. Trop. Dis. 2022, 16, e0010667. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, Y.; Lu, Z.; Ma, Y.; Ran, X.; Yan, X.; Zhang, M.; Qiu, X.; Luo, L.; Yue, G.; et al. Sublethal Effects of Niclosamide on the Aquatic Snail Pomacea canaliculata. Ecotoxicol. Environ. Saf. 2023, 259, 115064. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Bride, J.M.; Hoffmann, F.; Karch, F. Acetylcholinesterase. Two Types of Modifications Confer Resistance to Insecticide. J. Biol. Chem. 1992, 267, 14270–14274. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Pereira, H.M.V.S.; Lemos, M.F.L.; Soares, A.M.V.M. Effects of Imidacloprid Exposure on Chironomus Riparius Meigen Larvae: Linking Acetylcholinesterase Activity to Behaviour. Ecotoxicol. Environ. Saf. 2011, 74, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, C.; Li, Y.; Li, X. Biochemical Responses to the Toxicity of the Biocide Abamectin on the Freshwater Snail Physa Acuta. Ecotoxicol. Environ. Saf. 2014, 101, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Singh, D.K. Inhibition Kinetics of Certain Enzymes in the Nervous Tissue of Vector Snail Lymnaea Acuminata by Active Molluscicidal Components of Sapindus Mukorossi and Terminalia Chebula. Chemosphere 2011, 85, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Ezemonye, L.; Tongo, I. Sublethal Effects of Endosulfan and Diazinon Pesticides on Glutathione-S-Transferase (GST) in Various Tissues of Adult Amphibians (Bufo regularis). Chemosphere 2010, 81, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Sellami, B.; Khazri, A.; Mezni, A.; Louati, H.; Dellali, M.; Aissa, P.; Mahmoudi, E.; Beyrem, H.; Sheehan, D. Effect of Permethrin, Anthracene and Mixture Exposure on Shell Components, Enzymatic Activities and Proteins Status in the Mediterranean Clam Venerupis Decussata. Aquat. Toxicol. 2015, 158, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, H.; Xu, K.; Wu, J.; Zhang, J. Collecting Differently Sized Particles on Water Surface by Maneuvering Pedal Waves on the Foot of the Water Snail Pomacea canaliculata. Soft Matter 2022, 18, 7850–7858. [Google Scholar] [CrossRef] [PubMed]
- Sturba, L.; Fattorini, N.; Liberatori, G.; Vannuccini, M.L.; Nannoni, F.; Protano, G.; Tursi, A.; Corsi, I. Multi-Model Inference Analysis of Toxicological Responses and Levels of Heavy Metals in Soft Tissue of Land Snail Cornu Aspersum Caged in Proximity to an Industrial Setting. Ecol. Indic. 2020, 117, 106688. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Phillips, B.M.; Anderson, B.S.; Miller, J.L.; Miller, M.J.; Hammock, B.D. Applications of Carboxylesterase Activity in Environmental Monitoring and Toxicity Identification Evaluations (TIEs). In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2008; Volume 195, pp. 117–178. ISBN 978-0-387-77029-1. [Google Scholar]
- Cacciatore, L.C.; Verrengia Guerrero, N.; Cochón, A.C. Cholinesterase and Carboxylesterase Inhibition in Planorbarius Corneus Exposed to Binary Mixtures of Azinphos-Methyl and Chlorpyrifos. Aquat. Toxicol. 2013, 128–129, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Somuncu, S.; Atmaca, H.; Ilhan, S. Effects of Acute Exposure to Environmentally Realistic Tebuconazole Concentrations on Stress Responses of Kidney and Digestive Gland of Lymnaea Stagnalis. Environ. Toxicol. Pharmacol. 2024, 105, 104352. [Google Scholar] [CrossRef] [PubMed]
- Cossi, P.F.; Herbert, L.T.; Yusseppone, M.S.; Pérez, A.F.; Kristoff, G. Environmental Concentrations of Azinphos-Methyl Cause Different Toxic Effects without Affecting the Main Target (Cholinesterases) in the Freshwater Gastropod Biomphalaria Straminea. Ecotoxicol. Environ. Saf. 2018, 162, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Meng, Z.; Liu, B.; Yang, D.; Yu, D. Stearoyl-CoA Desaturase and Carotenoids Concertedly Enhance the Resistance of Pearl Oyster Pinctada Fucata to High Temperature Stress. Aquac. Res. 2020, 51, 4991–5004. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Sayed, S.S.M. Assessment of the Molluscicidal Activity of the Methanolic Seed Extracts of Ziziphus Spina-christi and Carica papaya on Immunological and Molecular Aspects of Biomphalaria alexandrina Snails. Aquac. Res. 2021, 52, 2014–2024. [Google Scholar] [CrossRef]
- Zaldibar, B.; Cancio, I.; Soto, M.; Marigómez, I. Changes in Cell-Type Composition in Digestive Gland of Slugs and Its Influence in Biomarkers Following Transplantation between a Relatively Unpolluted and a Chronically Metal-Polluted Site. Environ. Pollut. 2008, 156, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kruatrachue, M.; Sumritdee, C.; Pokethitiyook, P.; Singhakaew, S. Histopathological Effects of Contaminated Sediments on Golden Apple Snail (Pomacea canaliculata, Lamarck 1822). Bull. Environ. Contam. Toxicol. 2011, 86, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Dummee, V.; Tanhan, P.; Kruatrachue, M.; Damrongphol, P.; Pokethitiyook, P. Histopathological Changes in Snail, Pomacea canaliculata, Exposed to Sub-Lethal Copper Sulfate Concentrations. Ecotoxicol. Environ. Saf. 2015, 122, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, B.; Köhler, H.-R.; Triebskorn, R. Histopathological Effects of Copper and Lithium in the Ramshorn Snail, Marisa cornuarietis (Gastropoda, Prosobranchia). Chemosphere 2011, 85, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Osterauer, R.; Köhler, H.-R.; Triebskorn, R. Histopathological Alterations and Induction of Hsp70 in Ramshorn Snail (Marisa cornuarietis) and Zebrafish (Danio rerio) Embryos after Exposure to PtCl2. Aquat. Toxicol. 2010, 99, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dummee, V.; Kruatrachue, M.; Trinachartvanit, W.; Tanhan, P.; Pokethitiyook, P.; Damrongphol, P. Bioaccumulation of Heavy Metals in Water, Sediments, Aquatic Plant and Histopathological Effects on the Golden Apple Snail in Beung Boraphet Reservoir, Thailand. Ecotoxicol. Environ. Saf. 2012, 86, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.M.; El-Mesalamy, A.F.; Baghdadi, S.A.E.-W.S.; Elhanbaly, R. Histopathological Effects of Methomyl and Crude Extracts of Jatropha Curcas against the Terrestrial Snail, Monacha obstructa (Gastropoda:Hygromiidae). Chem. Biol. Technol. Agric. 2022, 9, 65. [Google Scholar] [CrossRef]
| Time (h) | LC50 (mg/L) | χ2 | r | 95% Confidence Limits |
|---|---|---|---|---|
| 24 | 3.792 | 11.957 | 0.968 | 3.516–4.103 |
| 48 | 2.195 | 12.588 | 0.943 | 2.012–2.378 |
| 72 | 1.833 | 12.923 | 0.909 | 1.661–1.997 |
| 96 | 1.706 | 15.929 | 0.867 | 1.560–1.842 |
| Factors | df | SS | MS | F | p | |
|---|---|---|---|---|---|---|
| AChE | Treat | 2 | 4.97 | 2.48 | 10.94 | <0.01 |
| Time | 1 | 1.18 | 1.18 | 5.18 | 0.03 | |
| Sex | 1 | 2.76 | 2.76 | 12.14 | <0.01 | |
| Treat × Time | 2 | 0.76 | 0.38 | 1.66 | 0.21 | |
| Treat × Sex | 2 | 0.21 | 0.11 | 0.47 | 0.63 | |
| Time × Sex | 1 | 0.58 | 0.58 | 2.56 | 0.12 | |
| Treat × Time × Sex | 2 | 0.04 | 0.02 | 0.09 | 0.92 | |
| GST | Treat | 2 | 202.92 | 101.46 | 17.09 | <0.01 |
| Time | 1 | 171.76 | 171.76 | 28.94 | <0.01 | |
| Sex | 1 | 87.24 | 87.24 | 14.70 | <0.01 | |
| Treat × Time | 2 | 114.41 | 57.21 | 9.64 | <0.01 | |
| Treat × Sex | 2 | 23.54 | 11.77 | 1.98 | 0.16 | |
| Time × Sex | 1 | 0.88 | 0.88 | 0.15 | 0.70 | |
| Treat × Time × Sex | 2 | 7.24 | 3.62 | 0.61 | 0.55 | |
| CarE | Treat | 2 | 0.54 | 0.27 | 1.95 | 0.16 |
| Time | 1 | 5.44 | 5.44 | 39.60 | <0.01 | |
| Sex | 1 | 0.01 | 0.01 | 0.10 | 0.75 | |
| Treat × Time | 2 | 6.97 | 3.48 | 25.36 | <0.01 | |
| Treat × Sex | 2 | 0.38 | 0.19 | 1.38 | 0.27 | |
| Time × Sex | 1 | 0.12 | 0.12 | 0.84 | 0.37 | |
| Treat × Time × Sex | 2 | 0.12 | 0.06 | 0.45 | 0.65 | |
| TAC | Treat | 2 | 0.06 | 0.03 | 8.25 | <0.01 |
| Time | 1 | 0.05 | 0.05 | 14.52 | <0.01 | |
| Sex | 1 | 0.08 | 0.08 | 25.129 | <0.01 | |
| Treat × Time | 2 | 0.03 | 0.01 | 3.881 | 0.03 | |
| Treat × Sex | 2 | 0.0006 | 0.0003 | 0.089 | 0.91 | |
| Time × Sex | 1 | 0.006 | 0.006 | 1.737 | 0.20 | |
| Treat × Time × Sex | 2 | 0.007 | 0.004 | 1.058 | 0.36 |
| Tissues | Histopathological Effects | Control | 1/2 LC50 (24 h) | 1/4 LC50 (24 h) | 1/2 LC50 (48 h) | 1/4 LC50 (48 h) |
|---|---|---|---|---|---|---|
| Digestive gland | Number of basophils | - | ++ | + | + | + |
| Digestive cell atrophy | - | ++ | ++ | ++ | ++ | |
| Expansion of the hemolymphatic space | - | + | + | + | + | |
| Gill | Ciliate shedding | - | ++ | - | + | - |
| Columnar cell changes | - | +++ | - | ++ | + | |
| Ventral foot | Epithelial cell atrophy | - | + | - | ++ | - |
| Columnar muscle cell dispersion | - | ++ | - | + | - | |
| Decrease in muscle fibers | - | +++ | - | ++ | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, X.; Zhang, J.; Yao, F.; Shi, Z.; Chen, Y.; Chen, Q.; Qin, Z. Effect of Metaldehyde on Survival, Enzyme Activities, and Histopathology of the Apple Snail Pomacea canaliculata (Lamarck 1822). Biology 2024, 13, 428. https://doi.org/10.3390/biology13060428
Liu J, Chen X, Zhang J, Yao F, Shi Z, Chen Y, Chen Q, Qin Z. Effect of Metaldehyde on Survival, Enzyme Activities, and Histopathology of the Apple Snail Pomacea canaliculata (Lamarck 1822). Biology. 2024; 13(6):428. https://doi.org/10.3390/biology13060428
Chicago/Turabian StyleLiu, Jimin, Xuan Chen, Jiaen Zhang, Fucheng Yao, Zhaoji Shi, Yingtong Chen, Qi Chen, and Zhong Qin. 2024. "Effect of Metaldehyde on Survival, Enzyme Activities, and Histopathology of the Apple Snail Pomacea canaliculata (Lamarck 1822)" Biology 13, no. 6: 428. https://doi.org/10.3390/biology13060428
APA StyleLiu, J., Chen, X., Zhang, J., Yao, F., Shi, Z., Chen, Y., Chen, Q., & Qin, Z. (2024). Effect of Metaldehyde on Survival, Enzyme Activities, and Histopathology of the Apple Snail Pomacea canaliculata (Lamarck 1822). Biology, 13(6), 428. https://doi.org/10.3390/biology13060428







