Simple Summary
The developmental origins of health and disease (DOHaD) hypothesis posits that stressful exposures during early development alter biological processes and increase the risk for chronic disease later in life. A widely accepted example of the DOHaD hypothesis is the strong link between exposure to maternal obesity and the development of obesity in offspring. Mechanisms explaining this relationship are not fully understood. Therefore, this study investigates both the hypothalamus and inflammation as important variables in the relationship between maternal and offspring obesity. The hypothalamus is the master regulator of energy homeostasis in the body. Inflammation in the hypothalamus is associated with the development of obesity. We used an established mouse model of maternal obesity to study body composition, energy homeostasis, and inflammation in male and female offspring. Like in our previous work, both male and female offspring exposed to maternal obesity had a phenotype consistent with energy excess. We found decreased markers of inflammation in the body and hypothalamus of offspring exposed to maternal obesity compared with offspring exposed to a control pregnancy. Inflammation was different between male and female offspring. Future studies focused on understanding how inflammation affects hypothalamic development in males and females may provide neuroprotective strategies to improve hypothalamic function thereby decreasing obesity risk.
Abstract
Maternal obesity is a well-established risk factor for offspring obesity development. The relationship between maternal and offspring obesity is mediated in part by developmental programming of offspring metabolic circuitry, including hypothalamic signaling. Dysregulated hypothalamic inflammation has also been linked to development of obesity. We utilized an established C57Bl/6J mouse model of high-fat, high-sugar diet induced maternal obesity to evaluate the effect of maternal obesity on systemic and hypothalamic TNF-α, IL-6, and IL-1β levels in neonatal and adult offspring. The offspring of dams with obesity demonstrated increased adiposity and decreased activity compared to control offspring. Maternal obesity was associated with decreased plasma TNF-α, IL-6 and IL-1β in adult female offspring and decreased plasma IL-6 in neonatal male offspring. Neonatal female offspring of obese dams had decreased TNF-α gene expression in the hypothalamus compared to control females, while neonatal and adult male offspring of obese dams had decreased IL-6 gene expression in the hypothalamus compared to control males. In summary, our results highlight important sex differences in the inflammatory phenotype of offspring exposed to maternal obesity. Sex-specific immunomodulatory mechanisms should be considered in future efforts to develop therapeutic interventions for obesity prevention and treatment.
Keywords:
inflammation; obesity; fetal development; hypothalamus; energy metabolism; sex differences 1. Introduction
The obesity epidemic is a major public health crisis affecting the reproductive health of millions of women [1]. In the United States, nearly 40% of women of reproductive age are now classified as obese [2]. In addition to higher rates of pregnancy complications, offspring born to mothers with obesity have increased risk of obesity and metabolic syndrome later in life, perpetuating a transgenerational cycle [1,2,3,4]. Specifically, children born to obese women have increased body weight and adiposity at birth and higher BMI and blood pressure later in childhood compared to offspring of non-obese women [1,3,4,5]. These findings persist even when correcting for postnatal diet and lifestyle factors [4,5].
Animal models have repeatedly shown that maternal obesity causes increased adiposity and alters adipose tissue function in offspring [3]. Within the hypothalamus, maternal obesity impacts the circuitry responsible for the regulation of hunger, satiety, and energy expenditure [3,6]. While it is clear that maternal obesity affects the developmental programming of metabolic circuitry in offspring, successful therapeutic interventions to disrupt the transmission of obesity from a mother to her offspring are yet to be identified [1,3,6,7,8,9,10].
The neonatal–perinatal medicine field has applied neuroprotective strategies to infants born premature and term infants who have sustained developmental injury such as hypoxic–ischemic injury [11,12]. While these interventions have not yet been studied in offspring born to mothers exposed to maternal obesity, our group is interested in the potential of this application. The hypothalamus, which plays a key role in energy homeostasis through the regulation of energy intake and expenditure, is highly sensitive to injury during key periods of development [13,14,15]. Likewise, models of hypothalamic injury cause obesity. We have previously shown that a maternal high-fat, high-sugar diet increases adiposity, decreases energy expenditure, and decreases neuronal activity and oxidative energy production in the hypothalamus of adult mouse offspring, suggesting developmental injury [6]. Specific mechanisms that mediate the relationship between exposure to maternal obesity and abnormal hypothalamic function in offspring remain unclear.
We hypothesize that a potential mediating variable between maternal and offspring obesity is inflammatory-mediated hypothalamic injury. Obesity is an inflammatory state, and dysregulated inflammation within the hypothalamus has been implicated in the development of obesity in adults [16,17,18,19,20]. The role of hypothalamic inflammation in the transmission of obesity from a mother to her offspring has received limited study [21,22,23,24,25]. However, previous work by our group established that early inflammatory stress contributes to long-term transcriptomic and behavior changes in rodent hippocampus [26,27,28], highlighting that early inflammatory-mediated injury can cause long-term changes within the developing brain.
The aim of this study was to investigate the relationships between maternal obesity, offspring metabolism, and hypothalamic inflammation. We utilized an established mouse model of diet-induced maternal obesity [29] to characterize offspring metabolic phenotypes, as well as systemic and hypothalamic inflammation. TNF-α, IL-1β, and IL-6 were chosen as inflammatory targets as they are most commonly associated with and reported in the obesity literature [16,25,30,31,32,33,34,35,36,37].
2. Materials and Methods
2.1. Animal Model
C57Bl/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were group-housed under standard conditions in an animal care facility with 12 h light: dark cycles. At approximately 6 weeks of age, females were randomized to a control diet (CON; D12489B, Research Diets, New Brunswick, NJ, USA, 10.6 kcal% fat, 16.8 kcal% protein, 72.6 kcal% carbohydrate, 240 g/kg sucrose) or high-fat-high-sucrose obesogenic (OB) diet. The OB diet included a high-fat pellet (Western Diet D12079B, Research Diets, New Brunswick, NJ, USA, 40.0 kcal% fat, 17.0 kcal% protein, 43.0 kcal% carbohydrate, 340 g/kg sucrose) and 20% sucrose solution supplemented with vitamins (AIN Vitamin Mixture; MP Biomedicals, Solon, OH, USA) and minerals (AIN-93M Mineral Mix; MP Biomedicals, Solon, OH, USA) [6,9,29]. All animals were fed ad libitum and had free access to water. When OB females gained 25% of their body weight (approximately 10–12 weeks of age), they were age-matched with CON females and mated with males maintained on a standard chow (Teklad Global 18% Protein Rodent Diet 2918, ENVIGO, Madison, WI, USA; 18 kcal% fat, 24 kcal% protein, 58 kcal% carbohydrate). Dams were maintained on CON or OB diet throughout pregnancy and lactation because hypothalamic development in mice occurs during both the embryonic and early postnatal periods [38]. Dam macronutrient intake from pregnancy through lactation was estimated by weighing food pellet and sucrose solutions every 48 h.
Offspring were delivered spontaneously and studied during the neonatal period on postnatal day 9 (PN9) and in adulthood at approximately 4 months of age. Litters were randomly culled to equal size at PN9 (4 pups/L). The offspring utilized in this study were a subset from a larger study, and thus all offspring underwent intraperitoneal injection of normal saline (20 μL) on PN7. Offspring were weaned on PN21 and maintained on standard chow.
2.2. Blood and Tissue Collection
Blood and tissue collection from fasted animals were performed as previously described [6,9]. Dams and offspring were euthanized by rapid decapitation. Whole blood was collected by truncal blood sampling (offspring) and cardiac puncture (dams). Whole blood glucose was measured via glucometer (Contour Next, Ascensia Diabetes Care, Parsippany, NJ, USA). Plasma was isolated by spinning whole blood at 5000 RPM for 10 min; supernatant was decanted and stored at −80 °C. Offspring whole brains were removed, and the hypothalamus was rapidly dissected on ice, flash-frozen in liquid nitrogen, and stored at −80 °C until the time of analysis.
2.3. Plasma Analyses
Plasma cytokines (TNF-α, IL-1β, and IL-6) were quantified by the Cytokine Reference Laboratory at the University of Minnesota using automated multiplex ELISA per manufacturer’s instructions (Ella, Simple Plex, ProteinSimple, San Jose, CA, USA). Plasma cholesterol, triglycerides, phospholipids, non-esterified fatty acids (NEFA), insulin, and leptin were measured at the Mouse Metabolic Phenotyping Center at the University of Cincinnati by standard protocol as previously described [9].
2.4. Hypothalamic Gene Expression Analyses
RNA was isolated from whole hypothalamus (Rneasy Mini Kit, Qiagen, Redwood City, CA, USA) and converted to cDNA (High-Capacity RNA-to-cDNA Kit, Applied Biosystems, Waltham, MA, USA). Relative gene expression was measured using Real-Time qPCR with TaqMan primers (Supplemental Table S1, ThermoFisher Scientific, Carlsbad, CA, USA). Gene expression was normalized to 18S rRNA using the cycle threshold (ΔΔCT) method. We have previously confirmed 18S rRNA (M-value < 1.5) as a suitable reference gene between groups [8,39].
2.5. In Vivo Physiology Analyses
Fat mass, fat free mass, and adiposity (fat mass/body weight, %) were measured using Echo-MRI (EchoMRI, Echo Medical Systems, Houston, TX, USA) as previously described [6,9]. Energy intake was calculated based on the amount of chow consumed, as measured by BioDAQ episodic Food Intake Monitor for mice (Research Diet, New Brunswick, NJ, USA). Basal metabolic rate (BMR) was measured using indirect calorimetry in singly housed freely moving mice after a period of acclimation (Oxymax, Columbus Instruments, Columbus, OH, USA) and calculated as previously described by our group and others [6,9,40,41]. Activity levels were measured using automated singly housed cages after a period of acclimation (Oxymax, Columbus Instruments, OH, USA).
2.6. Statistical Analysis and Data Presentation
The exposure variable for this study was maternal diet (CON, OB), and experimental numbers (N/group) were determined using our group’s previous work [6,8,9]. Group differences were measured by unpaired t-test. As there are recognized differences in metabolic outcomes and response to inflammation in males and females, we analyzed the sexes separately. p-values < 0.05 were considered statistically significant. Data are represented as mean ± SEM, with N representing the number of animals per group. Statistical analysis and graphics were performed using GraphPad Prism version 9 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Dam Characteristics
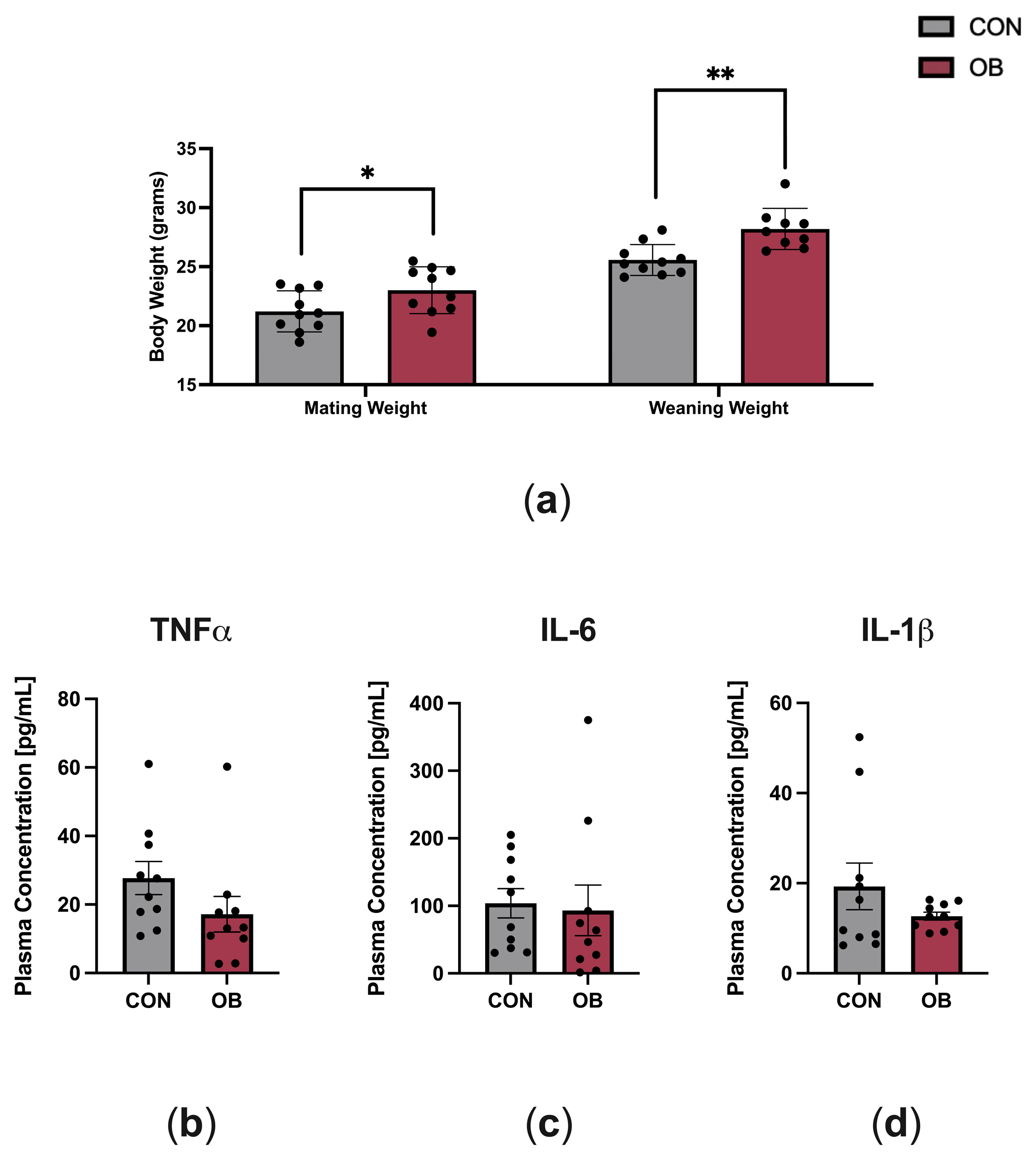
The characteristics of dams fed CON and OB diets are shown in Figure 1. OB dams weighed 8% more than CON dams at mating (p = 0.046) and weighed 10% more than CON dams at weaning (Figure 1a, p = 0.002). To determine the effect of maternal diet on maternal systemic inflammation, key plasma cytokines implicated in obesity (TNF-α, IL-6, IL-1β) were measured in dams at weaning (PN21). There were no differences in plasma cytokine levels between CON and OB dams (Figure 1b–d).
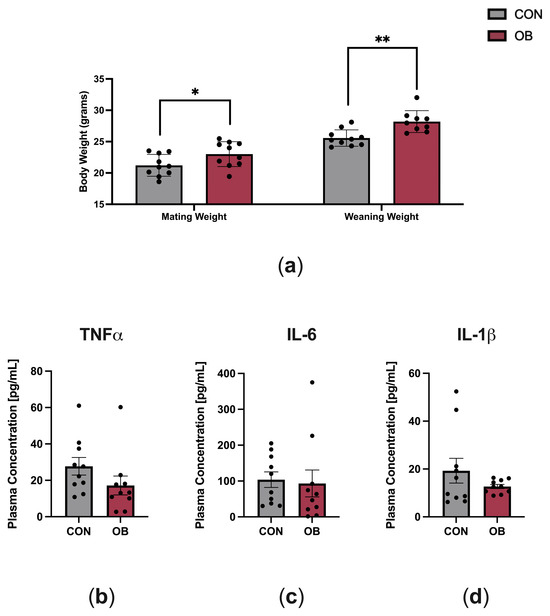
Figure 1.
Characteristics of dams fed CON or OB diets. (a) Body weights at mating and weaning were significantly higher in OB versus CON dams. (b–d) Plasma cytokines measured on postnatal day 21 (TNF-α, IL-6, IL-1β) did not significantly differ between CON and OB dams. Results expressed as mean ± SEM; significance determined by unpaired t-test, * p < 0.05, ** p < 0.01. Gray bars: CON. Red bars: OB. N = 9–10/group.
3.2. Offspring Phenotype
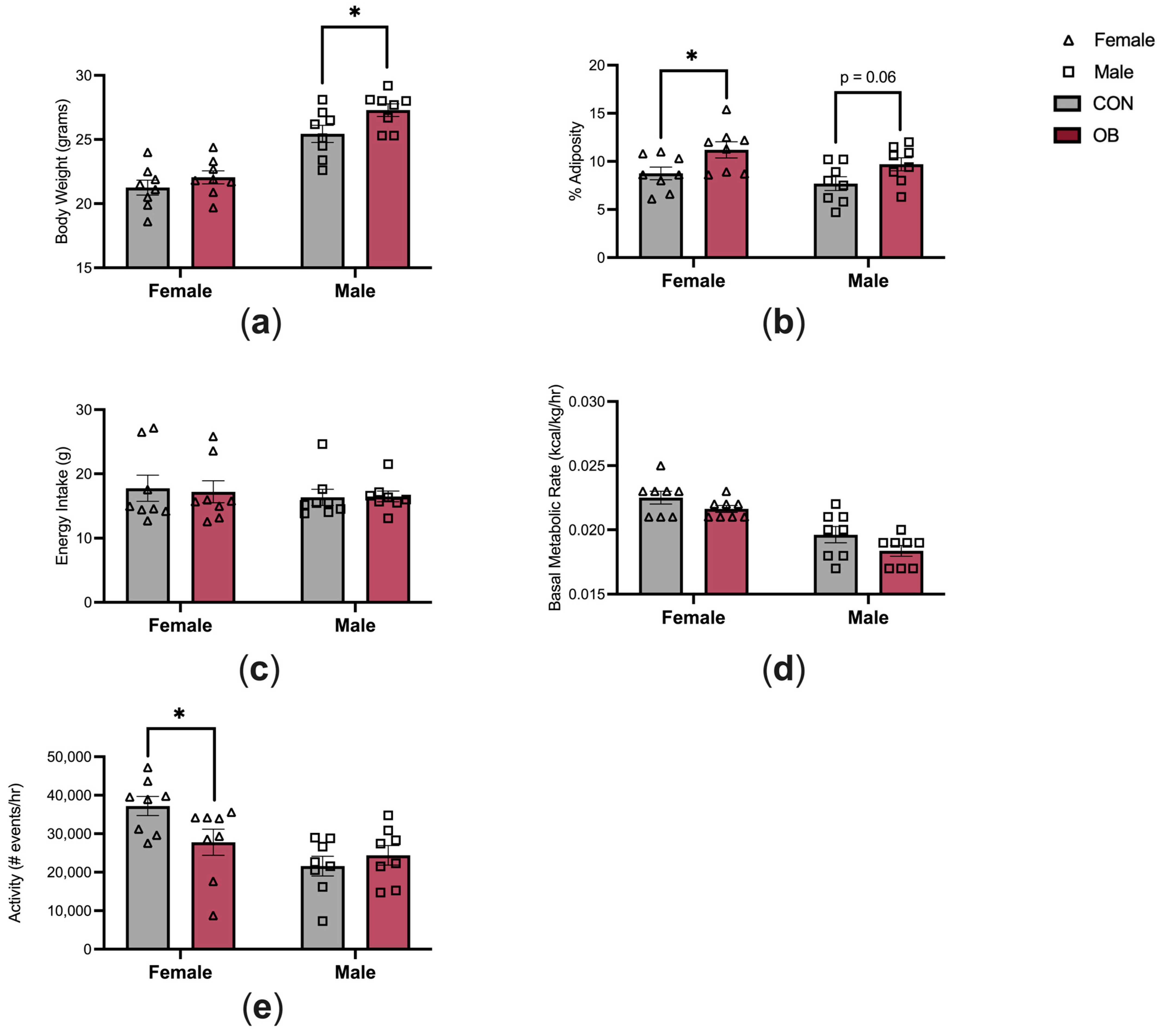
The characteristics of the offspring energy balance are shown in Figure 2. Adult male offspring in the OB group were 7% heavier than in CON males (Figure 2a, p = 0.04). Body weights did not differ between OB and CON females. Adiposity was 28% higher in OB females versus CON females (Figure 2b, p = 0.04), and there was a non-significant trend towards increased adiposity in OB males compared to CON males (Figure 2b, p = 0.06). Energy intake and BMR were not affected by maternal diet or offspring sex (Figure 2c,d). Offspring activity level was 25% lower in OB females compared to CON females (Figure 2e, p = 0.04). Activity level did not differ between OB and CON males.
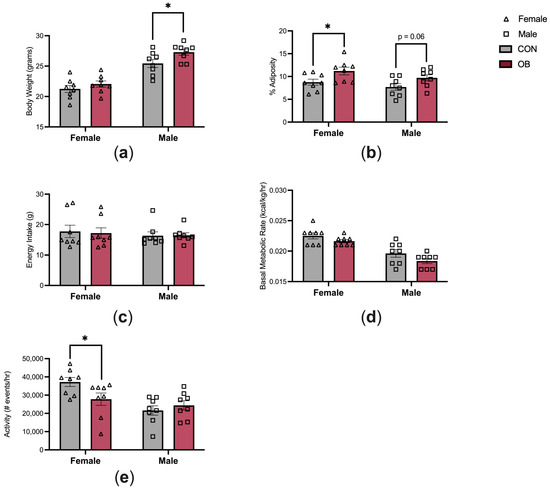
Figure 2.
Maternal obesity alters offspring energy balance. (a) OB males are heavier than CON males, and (b) OB females have increased adiposity compared to CON females. (c,d) Energy intake and BMR are similar between groups. (e) OB female offspring have decreased activity compared to CON females. Results expressed as mean ± SEM; significance determined by unpaired t-test with males and females analyzed separately. * p < 0.05. Open triangles: female offspring. Open squares: male offspring. Gray bars: CON offspring. Red bars: OB offspring. N = 8/group/sex.
The results of offspring metabolic profile are shown in Table 1. Insulin, leptin, NEFA, cholesterol, triglyceride, and phospholipid levels were measured in plasma at 4 months of age. The plasma metabolic profile did not differ between CON and OB offspring.

Table 1.
Adult offspring metabolic profile.
3.3. Offspring Inflammation
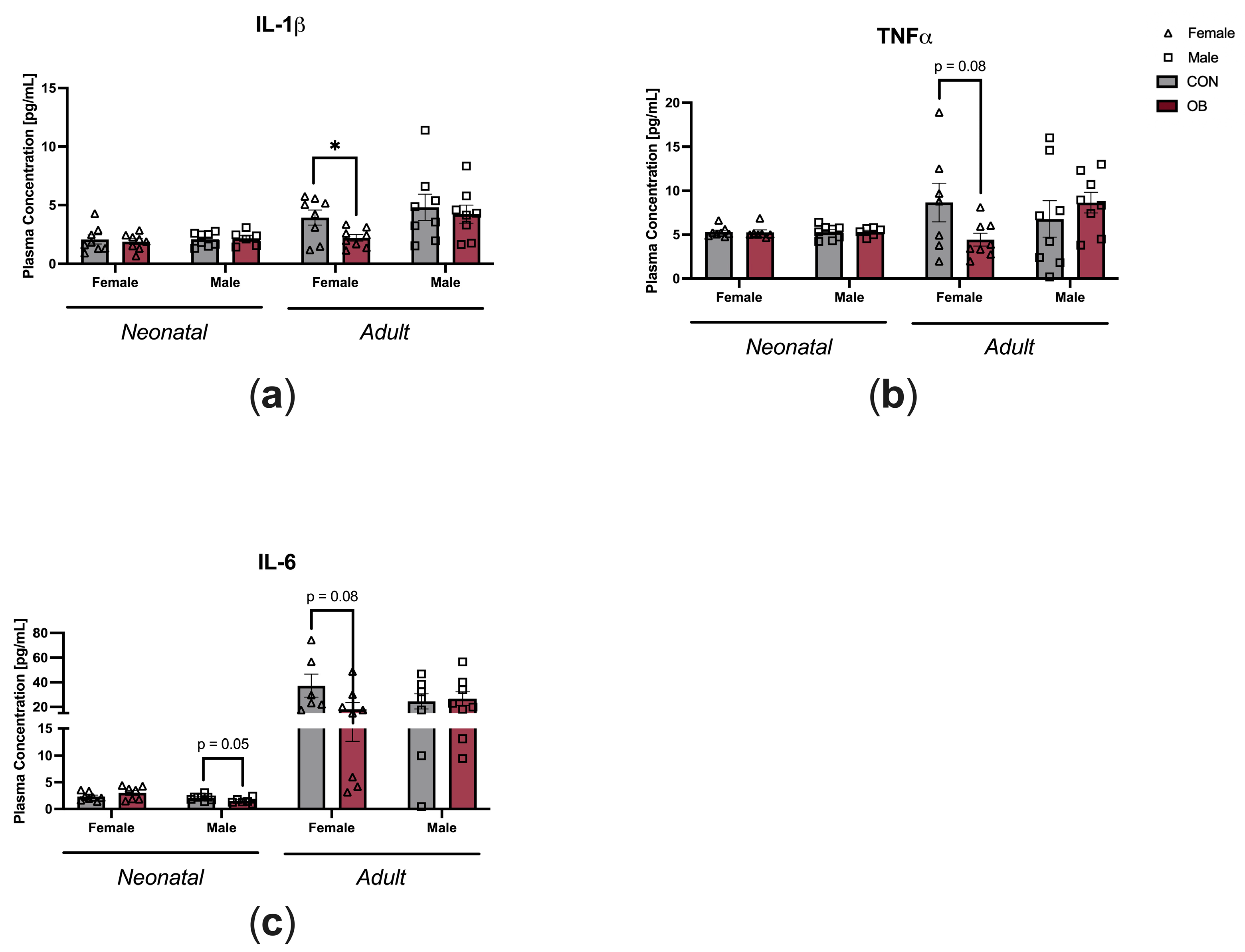
To determine the impact of maternal diet on offspring systemic inflammation, plasma cytokine levels were measured in neonatal and adult offspring (Figure 3). Plasma IL-1β was decreased in OB adult female offspring compared to CON adult female offspring (Figure 3a, p = 0.03). There was a nonsignificant trend towards decreased plasma TNF-α in OB adult female offspring compared to CON adult female offspring (Figure 3b, p = 0.08). There were nonsignificant trends toward decreased IL-6 in OB neonatal male offspring and OB adult female offspring compared to their respective controls (Figure 3c, males p = 0.05; females p = 0.08). Plasma cytokines in female neonatal offspring and adult male offspring were not affected by maternal diet (Figure 3a–c).
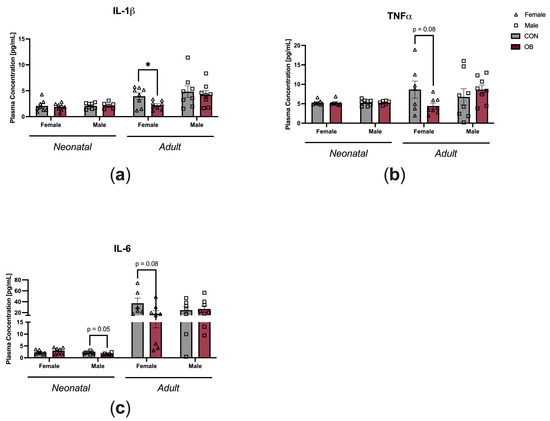
Figure 3.
Maternal obesity is associated with decreased plasma cytokine levels in adult female offspring and decreased plasma IL-6 in neonatal male offspring. Plasma concentrations of (a) IL-1β, (b) TNF-α, and (c) IL-6 measured in neonatal and adult offspring of CON and OB dams. Results expressed as mean ± SEM; significance determined by unpaired t-test, * p < 0.05. Open triangles: female offspring. Open squares: male offspring. Gray bars: CON offspring. Red bars: OB offspring. N = 6–8/group.
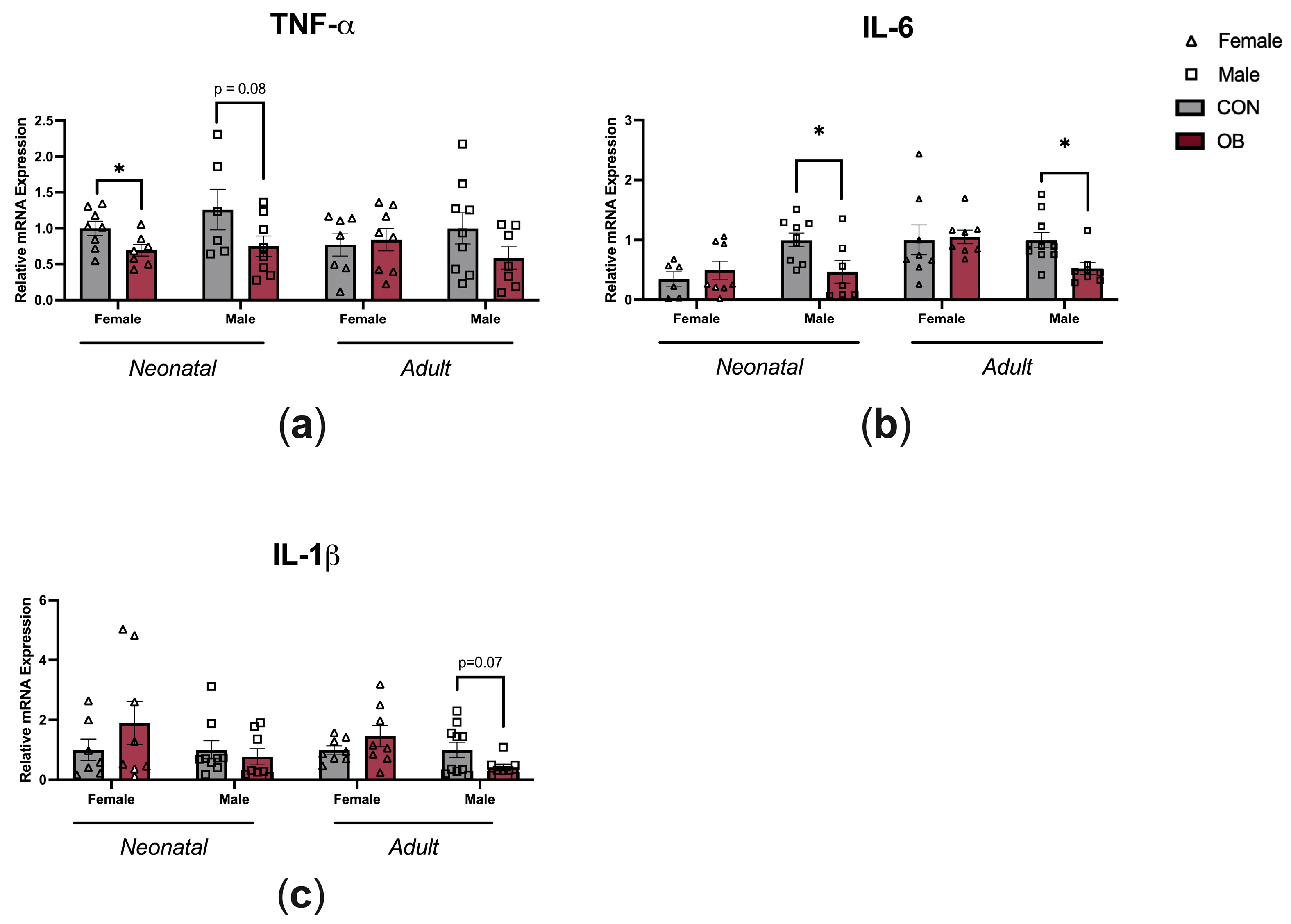
To determine whether maternal OB diet causes inflammatory changes within the hypothalamus, gene expression of TNF-α, IL-6, and IL-1β were measured in the hypothalamic tissue of neonatal and adult offspring (Figure 4). TNF-α expression was lower in the female OB offspring compared to the CON offspring hypothalamus (Figure 4a, p = 0.04), and there was a non-significant trend towards lower TNF-α in neonatal male offspring hypothalamus (a, p = 0.08). IL-6 expression was lower in neonatal and adult OB male offspring hypothalamus compared to CON offspring hypothalamus (b, p = 0.02; p = 0.01). There was a nonsignificant trend towards lower IL-1β expression in the adult OB offspring hypothalamus compared to the CON offspring hypothalamus (c, p = 0.07).
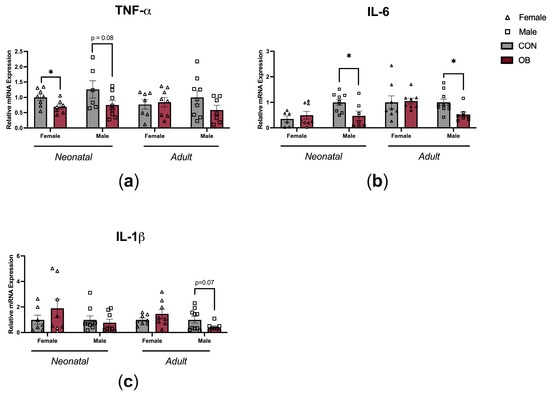
Figure 4.
Maternal OB diet downregulates hypothalamic cytokine expression in neonatal and adult offspring in a sex-specific manner. Hypothalamic gene expression of (a) TNF-α, (b) IL-6, and (c) IL-1β measured in neonatal and adult offspring of CON and OB dams. Results expressed as mean ± SEM; significance determined by unpaired t-test, * p < 0.05. Open triangles: female offspring. Open squares: male offspring. Gray bars: CON offspring. Red bars: OB offspring. N = 6–10/group.
4. Discussion
The objective of this study was to better understand the role of inflammation in the developmental origins of obesity. The main finding of the study is that male and female offspring exposed to maternal obesity have different inflammatory phenotypes despite a common metabolic phenotype consistent with energy excess. We found that offspring exposed to maternal diet-induced obesity have decreased plasma levels and downregulated hypothalamic gene expression of TNF-α, IL-6, and IL-1β. In the following paragraphs, we examine our results in the context of the existing literature, review the study’s limitations, and discuss key insights relevant to the development of targeted obesity prevention strategies.
Contrary to our hypothesis, we did not observe elevated plasma cytokines in male or female offspring of obese dams. We suspect that systemic inflammation was not increased in our study due to the specific metabolic phenotype observed in offspring exposed to maternal obesity. The excess secretion of TNF-α, IL-6, and IL-1β from adipocytes may cause chronic and low-grade inflammation involved in obesity-associated systemic and hepatic insulin resistance [33,42,43,44]. However, our mouse model does not cause significant systemic insulin resistance in OB offspring [6,9]. Offspring exposed to maternal obesity in our study had normal leptin levels in contrast to previous work demonstrating elevated leptin and insulin levels in offspring exposed to maternal obesity [21,22]. These findings suggest a comparatively milder metabolic phenotype produced by our mouse model. In addition, our cohort was fed a standard diet (vs. a obesogenic diet associated with inflammation) and was studied in the fasting state, which may have contributed to the non-elevation of inflammatory markers observed in offspring exposed to maternal obesity despite having higher fat mass. Importantly, we observed phenotypic and hypothalamic inflammatory changes in adult offspring despite an absence of systemic inflammation or altered insulin and leptin levels. These findings indicate that lasting hypothalamic injury occurs even in the absence of ongoing exposure to dietary excess or overt metabolic derangements.
Consistent with previous work by our group, we found lower activity levels in female offspring exposed to maternal obesity [6,9]. We postulate that decreased activity contributes to decreased plasma TNF-α, IL-6, and IL-1β levels observed in these animals. Exercise is a known inducer of TNF-α, IL-6, and IL-1β [45,46]. Therefore, it is possible that female offspring exposed to maternal obesity have lower systemic TNF-α, IL-6, and IL-1β related to lower activity levels compared to control offspring or male offspring exposed to maternal obesity.
We report longitudinal decreased IL-6 gene expression in the hypothalamus of male offspring exposed to maternal obesity compared to controls. Although increased hypothalamic IL-6 has been associated with an obesogenic phenotype, there is also a growing body of literature suggesting that hypothalamic IL-6 may be involved in mechanisms that are protective against obesity [32,47]. For example, hypothalamic IL-6 expression is lower in mice that are genetically prone to obesity, and IL-6 knock-out mice have increased body weight and decreased appetite stimulating neuropeptide (Pomc) expression in the hypothalamus [32]. Cytokines exert a neurotrophic effect in the developing brain, and specifically exogenous IL-6 has been shown to induce hypothalamic neurogenesis in mice [32]. Cytokine-induced apoptosis has also been described as a mechanism to eliminate overabundant neurons [14,32]. Therefore, it is plausible that the downregulation of hypothalamic IL-6 gene expression observed in offspring exposed to maternal obesity alters patterns of hypothalamic neurogenesis and apoptosis.
TNF-α, IL-6, and IL-1β induce anorexia through hypothalamic appetite signaling [43]. These cytokines induce appetite and weight loss when administered peripherally or directly into the brain [48,49,50]. Mechanistically, previous work has shown that decreased appetite is caused by decreases in corticotropin-releasing factor (CRF) and the stimulation of orexigenic neuropeptide Y (NPY) [50]. We report decreased hypothalamic TNF-α and IL-6 gene expression in neonatal offspring exposed to maternal obesity. Lower levels, which would theoretically increase appetite, may be an adaptation to the exposure to excess macronutrients from maternal obesity. Future studies to test this hypothesis would be important to investigate the relationship between early hypothalamic cytokine expression and energy homeostasis later in life.
We found lower TNF-α gene expression in the neonatal but not adult hypothalamus of offspring exposed to maternal obesity. We have previously shown, through pathway analysis, that the TNF gene was dysregulated in the hypothalamus of adult offspring exposed to maternal obesity and that TNF expression was moderated by sex [6]. As TNF is involved in the differentiation of neuroglia and the growth of neurites, one hypothesis is that decreased TNF levels may impair hypothalamic growth and differentiation, causing aberrant energy homeostasis leading to development of energy excess in adulthood [37,50].
Previous work demonstrates hypothalamic inflammatory changes in adults fed an obesogenic diet [14,17,18,19,20], as well as in offspring following exposure to maternal diet-induced obesity during hypothalamic development [6,21,22,23,38,51]. Furthermore, hypothalamic inflammation is decreased in diet-induced obesity-resistant WSB/EiJ mice compared to obesity-prone C57Bl/6J mice, underscoring the association between hypothalamic inflammation and the development of diet-induced obesity [52]. In contrast, the increased hypothalamic gene expression of TNF-α, IL-6, and IL-1β was not observed in our adult OB offspring despite increased body weight and adiposity. Since leptin itself has been shown to impact inflammation in mouse glial cells [22,53], we speculate that normal leptin levels in our study prohibited findings of hypothalamic inflammation.
Consistent with known sex differences in immune and neuroimmune activity, we report significant differences in peripheral cytokine levels in only female offspring exposed to maternal obesity and significant differences in hypothalamic cytokine expression in only male offspring exposed to maternal obesity. Prior studies have indicated that females have a greater peripheral immune response compared to males, while males have a greater neuroimmune response compared to females [54,55]. Sex chromosome genes and sex hormones including estrogen, progesterone, and androgens contribute to the differential regulation of immune response between sexes [54,55]. Of note, a study by Morselli et al. demonstrated increased hypothalamic inflammation in male wild-type mice compared to females in the setting of a high-fat diet, as well as increased inflammatory cytokines in estrogen receptor knock-out female mice [56]. Ongoing investigation of the intersection between biological sex and how environmental factors such as exposure to maternal obesity alter the development and function of the immune system is critical to developing therapies to prevent obesity.
There are several important limitations to our study that warrant further investigation. First, a limited number of cytokines were studied. While these target cytokines were chosen based on their strong, established relationships with obesity, a large breadth of study may offer novel insights or a more complete analysis of the relationship between developmental obesity and immune function. Second, this study is limited by solely evaluating hypothalamic mRNA expression. To better understand cytokine expression, investigating protein and cytokine secretion in hypothalamus is necessary. Third, the significance of our results may have been limited by a relatively small sample size. Additionally, further studies investigating immune cell differentiation and cell tracking would improve our understanding of how developmental stress, such as maternal obesity, affects immune function in the periphery and hypothalamus. Last, immune response is a complex interaction between tissue type, the dose of the stressor, the type of immune challenge, the timing of the stressor, the chronicity of the stressor, the hormonal state, the environment, and the biological sex. While this study considers many of these variables, further investigations that include varying iterations of these complex interactions are necessary prior to development of therapies that may prevent obesity with a developmental origin.
5. Conclusions
In conclusion, results from this study provide further support to research aimed at understanding how developmental inflammatory stress alters long-term health. A better understanding of how developmental stress intersects with biological sex, neurodevelopment, endocrine hormone activity, and immune function may lead to novel prevention and treatment strategies to mitigate the obesity epidemic.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology13060399/s1: Table S1: RT-qPCR Gene Targets.
Author Contributions
Conceptualization, experimental design, and methodology, L.A.B., T.G. and M.E.P.; investigation, L.A.B., D.R.K. and A.B.; analysis, L.A.B.; writing—original draft preparation, L.A.B. and M.E.P.; writing—review and editing L.A.B., T.G. and M.E.P.; supervision, T.G. and M.E.P.; funding acquisition, L.A.B., T.G. and M.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Health Grants HD055887 (MP; K12 BIRCWH Scholar) and HD099246 (T.G.), University of Minnesota Benjamin Walker Hanson Neonatology Fund (L.B.), and the University of Minnesota Medical School, Department of Pediatrics (M.P.).
Institutional Review Board Statement
The animal study protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee (protocol no. 1708-35022 A).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions and ongoing projects.
Acknowledgments
The plasma colorimetric assays were performed at the Mouse Metabolic Phenotyping Center, University of Cincinnati (Grant DK59630). We thank Michael Ehrhardt, senior scientist in the Cytokine Reference Laboratory at the University of Minnesota, for assistance with multiplex ELISA. The in vivo metabolic studies (indirect calorimetry, activity, meal pattern analysis, and body composition) were conducted at the Integrative Biology and Physiology Phenotyping Core, the University of Minnesota Medical School. We thank Raghu Rao and Emilyn Alejandro for their contributions to LB’s scholarly oversight committee, which was instrumental in successful conduction of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet. Gynecol. 2021, 138, 489. [Google Scholar] [CrossRef] [PubMed]
- Schoonejans, J.M.; Ozanne, S.E. Developmental programming by maternal obesity: Lessons from animal models. Diabet. Med. 2021, 38, e14694. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Haines, J.; Liu, G.; Zhang, C.; Wang, X.; Field, A.E.; Chavarro, J.E.; Sun, Q. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: Results from two prospective cohort studies of mother-child pairs in the United States. BMJ 2018, 362, k2486. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, D.; Abrahante Llorens, J.E.; Buckley, L.; Tkac, I.; Rao, R.; Paulsen, M.E. Female and male C57BL/6J offspring exposed to maternal obesogenic diet develop altered hypothalamic energy metabolism in adulthood. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E448–E466. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Reynolds, C.M. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J. Dev. Orig. Health Dis. 2017, 8, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, D.; Rao, R.B.; Paulsen, M.E. Excess sucrose intake during pregnancy programs fetal brain glucocorticoid receptor expression in female but not male C57Bl/6J mice. Obes. Sci. Pract. 2021, 7, 462–472. [Google Scholar] [CrossRef]
- Kulhanek, D.; Weigel, R.; Paulsen, M.E. Maternal High-Fat-High-Carbohydrate Diet-Induced Obesity Is Associated with Increased Appetite in Peripubertal Male but Not Female C57Bl/6J Mice. Nutrients 2020, 12, 2919. [Google Scholar] [CrossRef]
- Menting, M.D.; Mintjens, S.; van de Beek, C.; Frick, C.J.; Ozanne, S.E.; Limpens, J.; Roseboom, T.J.; Hooijmans, C.R.; van Deutekom, A.W.; Painter, R.C. Maternal obesity in pregnancy impacts offspring cardiometabolic health: Systematic review and meta-analysis of animal studies. Obes. Rev. 2019, 20, 675–685. [Google Scholar] [CrossRef]
- Juul, S.E.; Comstock, B.A.; Heagerty, P.J.; Mayock, D.E.; Goodman, A.M.; Hauge, S.; Gonzalez, F.; Wu, Y.W. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial—Background, Aims, and Study Protocol. Neonatology 2018, 113, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gali Ramamoorthy, T.; Begum, G.; Harno, E.; White, A. Developmental programming of hypothalamic neuronal circuits: Impact on energy balance control. Front. Neurosci. 2015, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Ziko, I.; De Luca, S.; Dinan, T.; Barwood, J.M.; Sominsky, L.; Cai, G.; Kenny, R.; Stokes, L.; Jenkins, T.A.; Spencer, S.J. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav. Immun. 2014, 41, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, A.A.; Leibel, R.L.; LeDuc, C.A. Neurodevelopmental Programming of Adiposity: Contributions to Obesity Risk. Endocr. Rev. 2024, 45, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- De Bona Schraiber, R.; De Mello, A.H.; Garcez, M.L.; De Bem Silveira, G.; Zacaron, R.P.; De Souza Goldim, M.P.; Budni, J.; Silveira, P.C.L.; Petronilho, F.; Ferreira, G.K.; et al. Diet-induced obesity causes hypothalamic neurochemistry alterations in Swiss mice. Metab. Brain Dis. 2019, 34, 565–573. [Google Scholar] [CrossRef]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharmacal Res. 2019, 42, 383–392. [Google Scholar] [CrossRef]
- Bae-Gartz, I.; Janoschek, R.; Kloppe, C.S.; Vohlen, C.; Roels, F.; Oberthur, A.; Alejandre Alcazar, M.A.; Lippach, G.; Muether, P.S.; Dinger, K.; et al. Running Exercise in Obese Pregnancies Prevents IL-6 Trans-signaling in Male Offspring. Med. Sci. Sports Exerc. 2016, 48, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Rother, E.; Kuschewski, R.; Alcazar, M.A.; Oberthuer, A.; Bae-Gartz, I.; Vohlen, C.; Roth, B.; Dotsch, J. Hypothalamic JNK1 and IKKbeta activation and impaired early postnatal glucose metabolism after maternal perinatal high-fat feeding. Endocrinology 2012, 153, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Bae-Gartz, I.; Janoschek, R.; Breuer, S.; Schmitz, L.; Hoffmann, T.; Ferrari, N.; Branik, L.; Oberthuer, A.; Kloppe, C.S.; Appel, S.; et al. Maternal Obesity Alters Neurotrophin-Associated MAPK Signaling in the Hypothalamus of Male Mouse Offspring. Front. Neurosci. 2019, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Luo, X.; Zhang, C. Emerging role of hypothalamus in the metabolic regulation in the offspring of maternal obesity. Front. Nutr. 2023, 10, 1094616. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Glendining, K.A.; Grattan, D.R.; Jasoni, C.L. Maternal obesity leads to increased proliferation and numbers of astrocytes in the developing fetal and neonatal mouse hypothalamus. Int. J. Dev. Neurosci. 2016, 53, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, T.; Ennis, K.; Bhandari, V.; Rao, R. Recurrent hypoinsulinemic hyperglycemia in neonatal rats increases PARP-1 and NF-kappaB expression and leads to microglial activation in the cerebral cortex. Pediatr. Res. 2015, 78, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, T.; Singh, G.; Georgieff, M.K. Fetal inflammation is associated with persistent systemic and hippocampal inflammation and dysregulation of hippocampal glutamatergic homeostasis. Pediatr. Res. 2019, 85, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Segura, B.J.; Georgieff, M.K.; Gisslen, T. Fetal inflammation induces acute immune tolerance in the neonatal rat hippocampus. J. Neuroinflammation 2021, 18, 69. [Google Scholar] [CrossRef]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity 2015, 23, 1663–1670. [Google Scholar] [CrossRef]
- Hahn, W.S.; Kuzmicic, J.; Burrill, J.S.; Donoghue, M.A.; Foncea, R.; Jensen, M.D.; Lavandero, S.; Arriaga, E.A.; Bernlohr, D.A. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1033–E1045. [Google Scholar] [CrossRef]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, V.C.; Engel, D.F.; Jara, C.P.; Mendes, N.F.; Haddad-Tovolli, R.; Prado, T.P.; Sidarta-Oliveira, D.; Morari, J.; Velloso, L.A.; Araujo, E.P. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J. Neuroinflammation 2021, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A. Role of interleukins in obesity: Implications for metabolic disease. Trends Endocrinol. Metab. 2014, 25, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, H.L.; Kraakman, M.J.; Febbraio, M.A. Adipose tissue inflammation in glucose metabolism. Rev. Endocr. Metab. Disord. 2014, 15, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014, 90, 130. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Nutritional programming of hypothalamic development: Critical periods and windows of opportunity. Int. J. Obes. Suppl. 2012, 2, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Cero, C.; Razzoli, M.; Han, R.; Sahu, B.S.; Patricelli, J.; Guo, Z.; Zaidman, N.A.; Miles, J.M.; O’Grady, S.M.; Bartolomucci, A. The neuropeptide TLQP-21 opposes obesity via C3aR1-mediated enhancement of adrenergic-induced lipolysis. Mol. Metab. 2017, 6, 148–158. [Google Scholar] [CrossRef]
- Nie, Y.; Gavin, T.P.; Kuang, S. Measurement of Resting Energy Metabolism in Mice Using Oxymax Open Circuit Indirect Calorimeter. Bio Protoc. 2015, 5, e1602. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Rahmat, A.; Patimah, I.; Abed, Y. Obesity can predict and promote systemic inflammation in healthy adults. Int. J. Cardiol. 2016, 215, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515 Pt 1, 287–291. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef] [PubMed]
- McNay, D.E.G.; Briançon, N.; Kokoeva, M.V.; Maratos-Flier, E.; Flier, J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012, 122, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Nielsen, R.R.; Vendelbo, M.H.; Moller, A.B.; Jessen, N.; Buhl, M.; Hafstrøm, T.K.; Holm, L.; Pedersen, S.B.; Pilegaard, H.; et al. Direct effects of TNF-alpha on local fuel metabolism and cytokine levels in the placebo-controlled, bilaterally infused human leg: Increased insulin sensitivity, increased net protein breakdown, and increased IL-6 release. Diabetes 2013, 62, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Leppanen, J.; Ibrahim, M.A.A.; Himmerich, H. Impact of TNF-alpha Inhibitors on Body Weight and BMI: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 481. [Google Scholar] [CrossRef]
- Choi, J.S. Effects of Maternal and Post-Weaning High-Fat Diet on Leptin Resistance and Hypothalamic Appetite Genes in Sprague Dawley Rat Offspring. Clin. Nutr. Res. 2018, 7, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Terrien, J.; Seugnet, I.; Seffou, B.; Herrero, M.J.; Bowers, J.; Chamas, L.; Decherf, S.; Duvernois-Berthet, E.; Djediat, C.; Ducos, B.; et al. Reduced central and peripheral inflammatory responses and increased mitochondrial activity contribute to diet-induced obesity resistance in WSB/EiJ mice. Sci. Rep. 2019, 9, 19696. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Okuma, Y.; Nomura, Y. Expression of leptin receptors and induction of IL-1beta transcript in glial cells. Biochem. Biophys. Res. Commun. 2000, 273, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Posillico, C.K.; Garcia-Hernandez, R.E.; Tronson, N.C. Sex differences and similarities in the neuroimmune response to central administration of poly I:C. J. Neuroinflam. 2021, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Morselli, E.; Frank, A.P.; Palmer, B.F.; Rodriguez-Navas, C.; Criollo, A.; Clegg, D.J. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int. J. Obes. 2016, 40, 206–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).