Effect of Metformin on Sertoli Cell Fatty Acid Metabolism and Blood–Testis Barrier Formation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Assays
2.2.1. Sertoli Cell Isolation and Culture
2.2.2. Culture Conditions
2.2.3. Fatty Acid Oxidation Assay
2.2.4. Determination of 3-Hydroxybutyrate Production
2.2.5. BTB Integrity Assay: Transepithelial Electrical Resistance (TER) Measurement
2.2.6. Real-Time PCR (RT-qPCR)
2.2.7. Western Blot Analysis
2.2.8. Cell Viability Test
2.2.9. Immunofluorescent (IF) Detection of Claudin 11 and ZO-1 Protein
2.3. In Vivo Assays
2.3.1. Animals
2.3.2. Experimental Design
2.3.3. Histological Analysis and Meiosis Progression
2.3.4. Blood–Testis Barrier Integrity Assay
2.3.5. Intratesticular Testosterone Determination
2.3.6. Apoptosis Determination by TUNEL
2.3.7. Daily Sperm Production (DSP)
2.4. Statistical Analysis
3. Results and Discussion
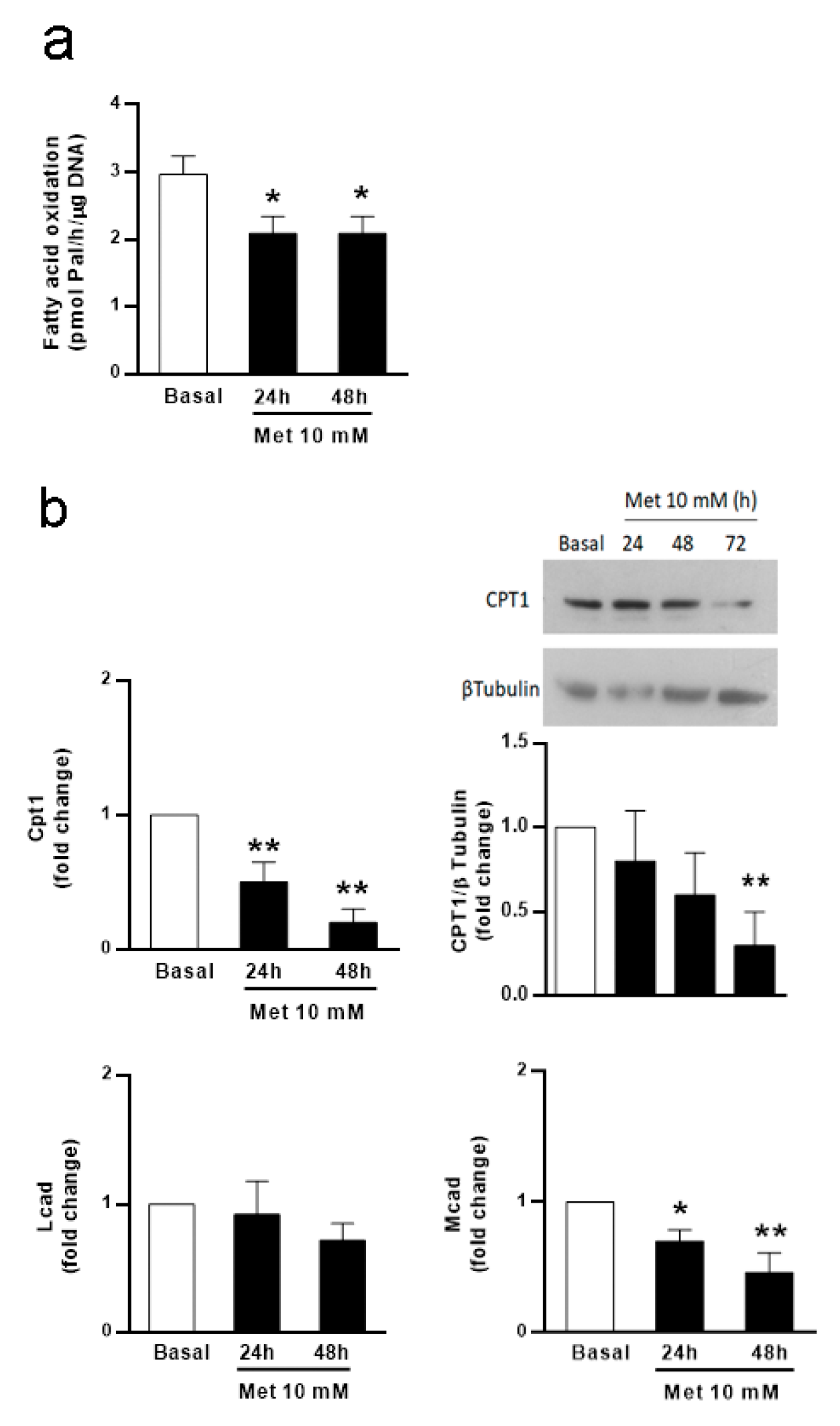
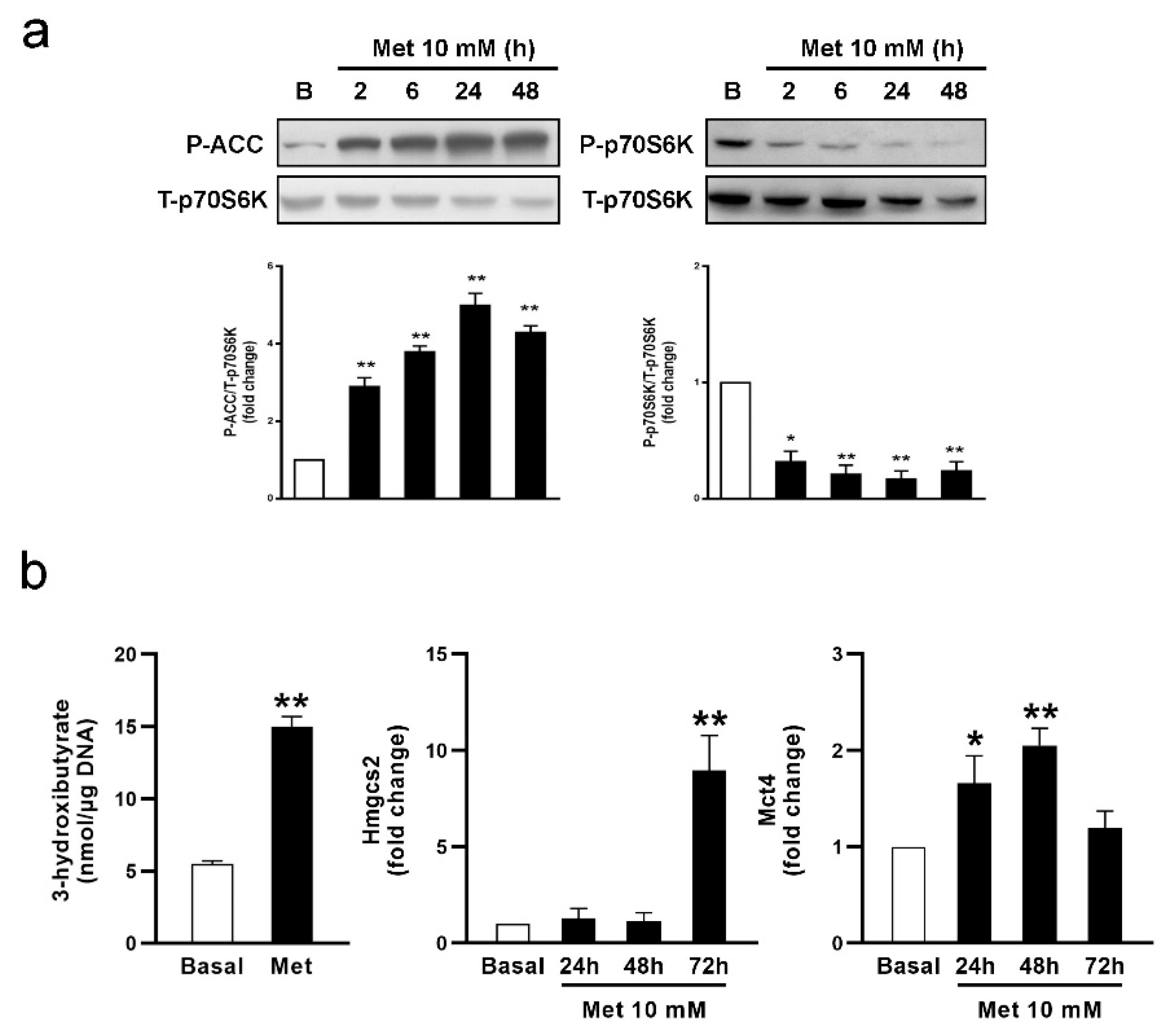
3.1. Metformin Affects FA Oxidation and Ketone Body Production in SC Cultures
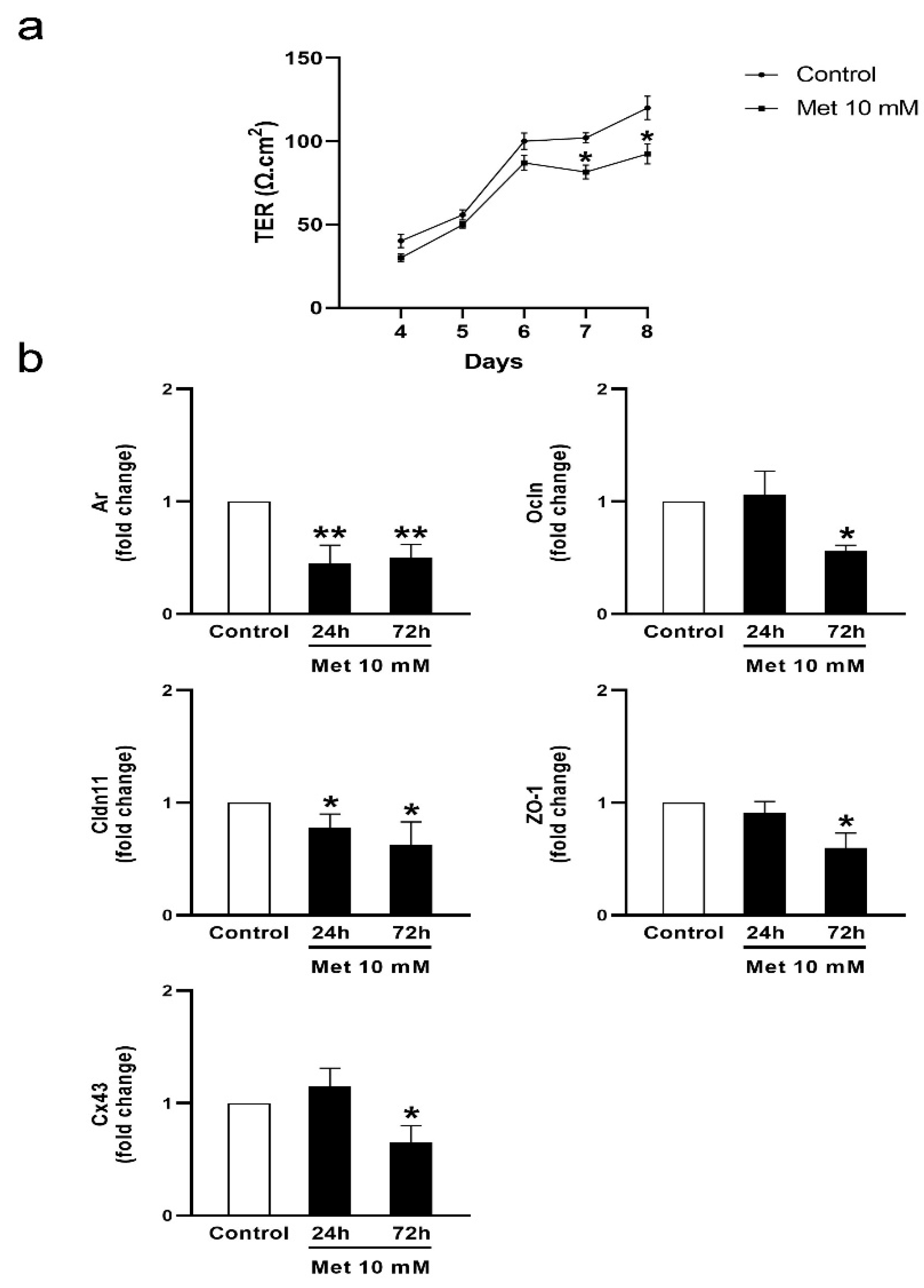
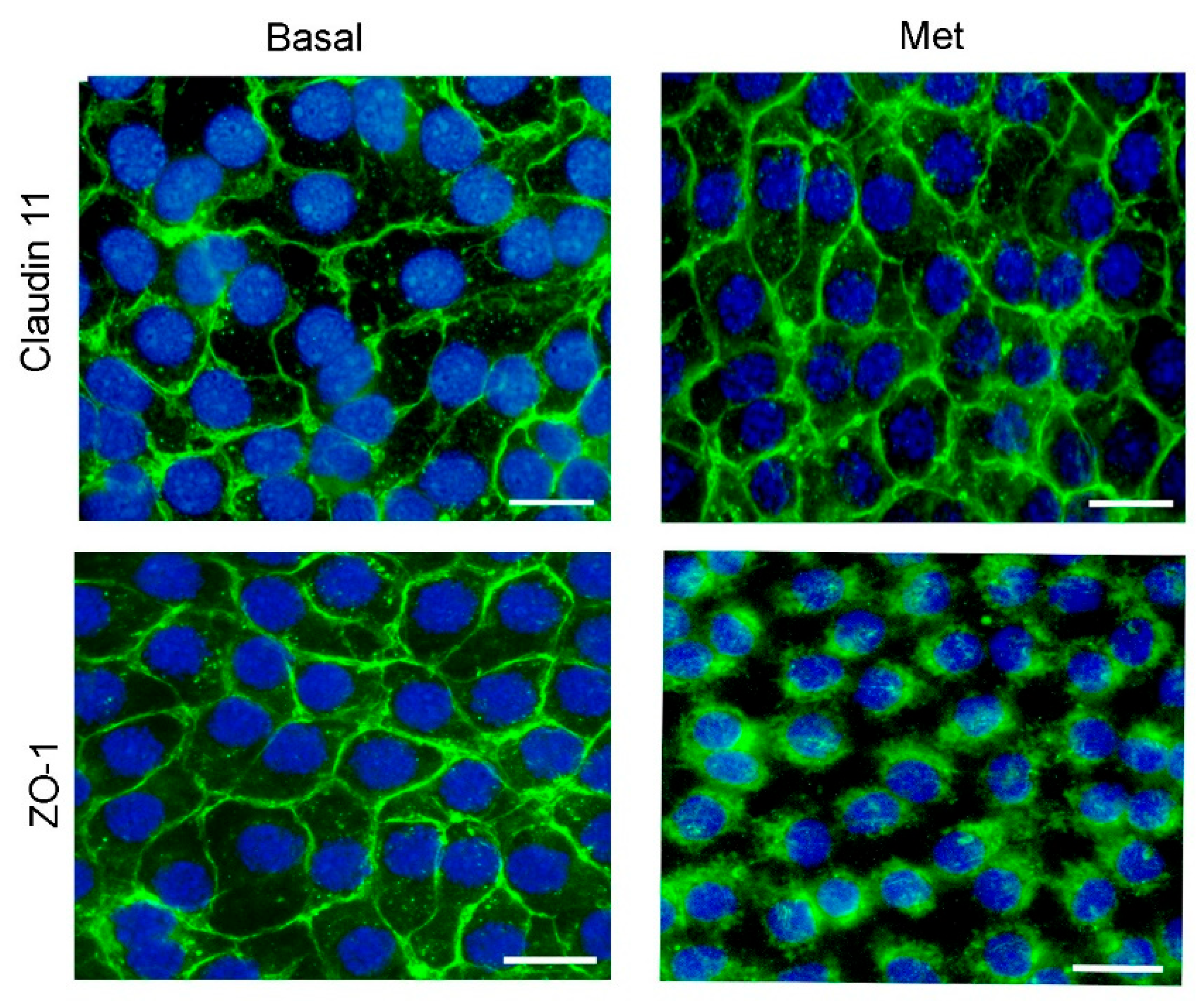
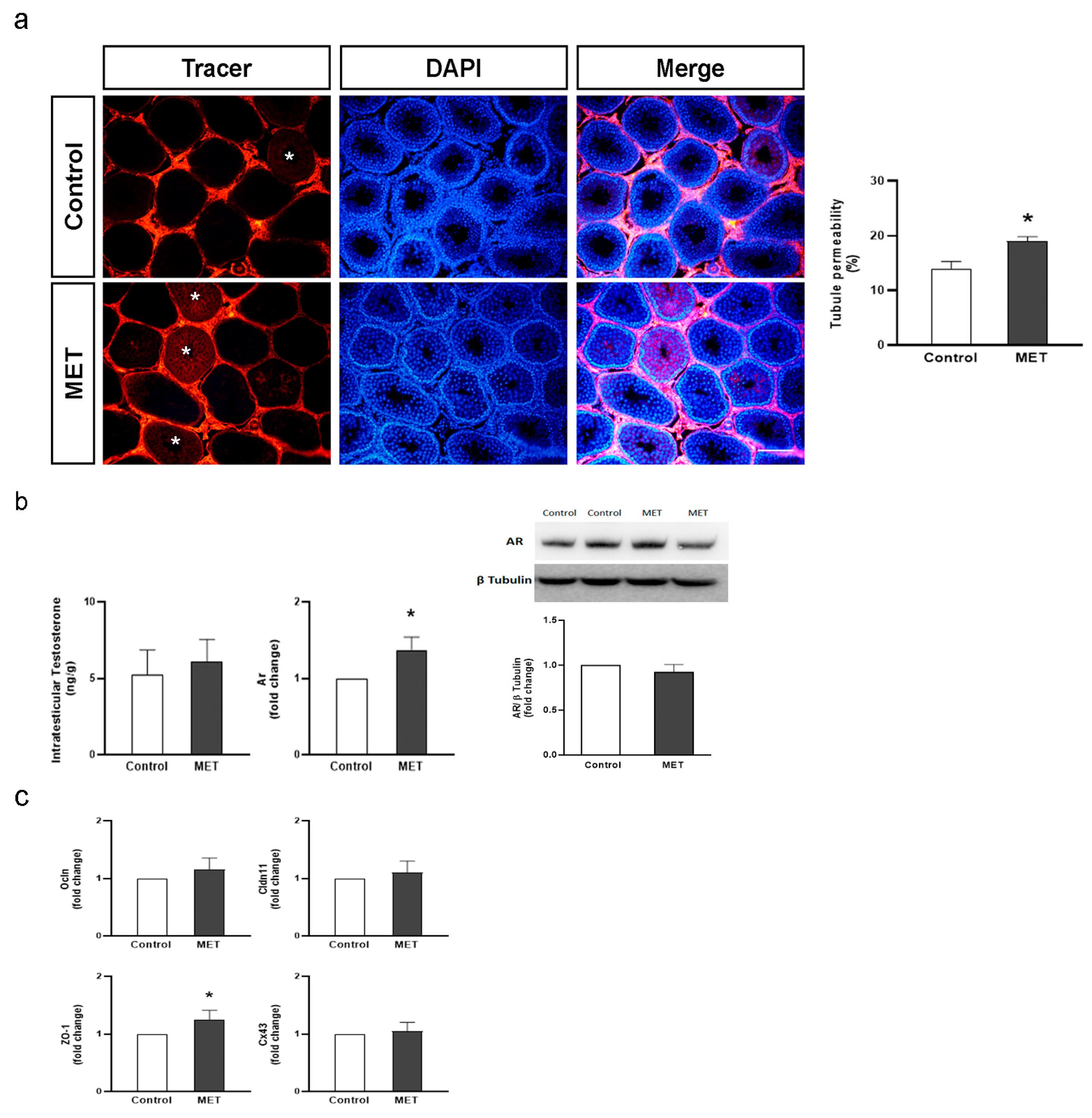
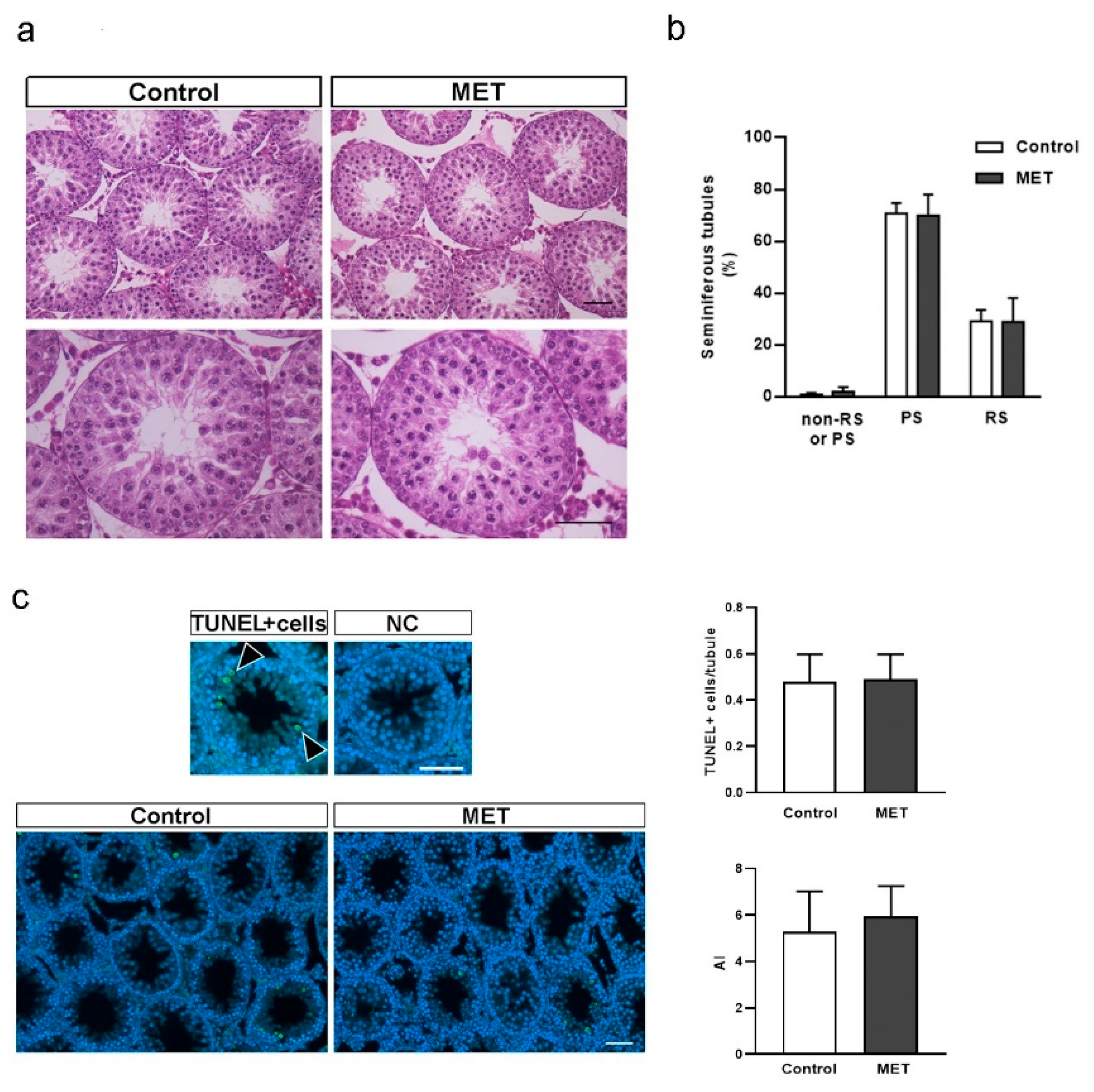
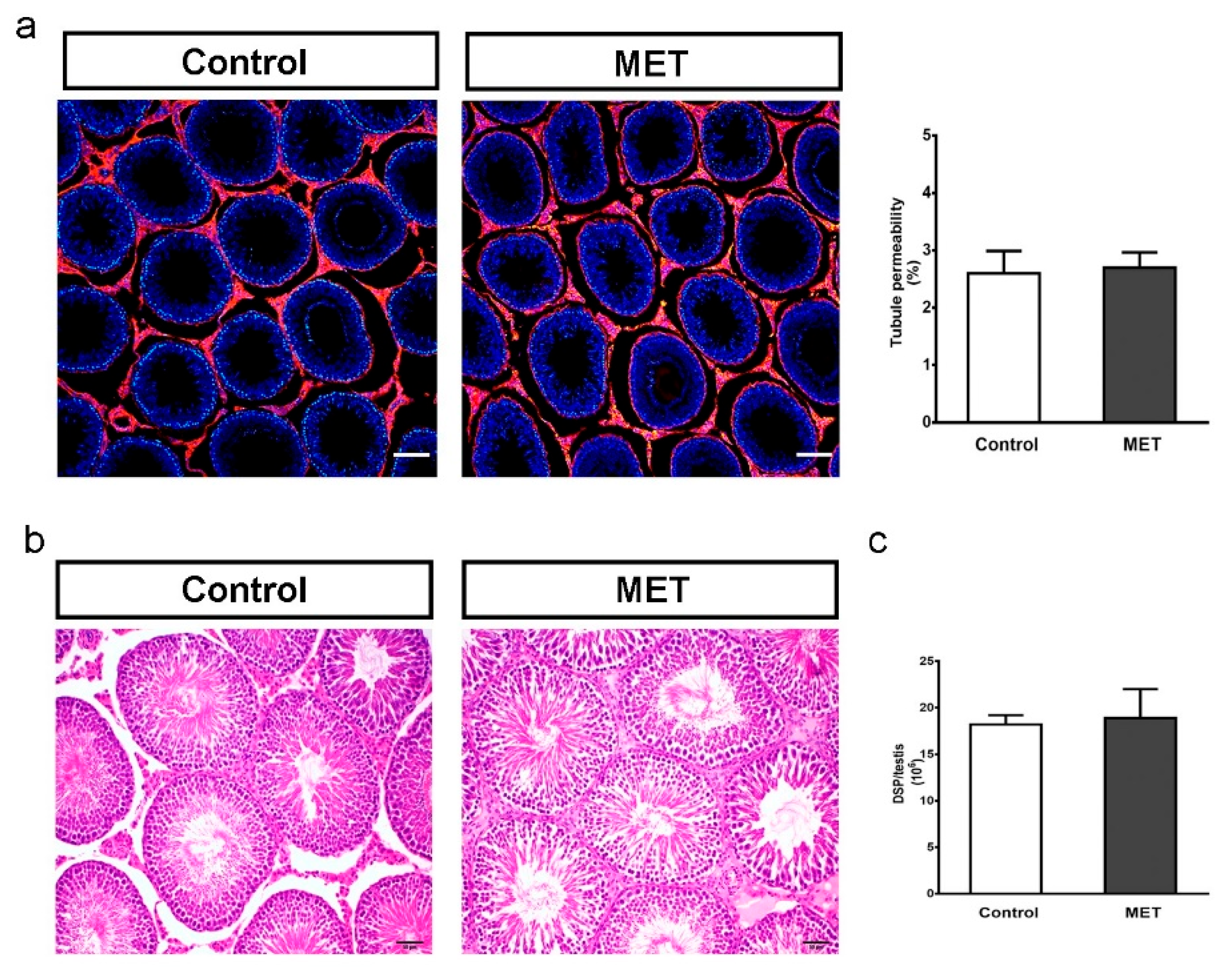
3.2. Metformin Alters the Formation of Cell Junctions in Cultured SCs and Increases BTB Permeability in Juvenile Male Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell Dev. Biol. 2016, 59, 166–173. [Google Scholar] [CrossRef]
- Pérez, C.V.; Sobarzo, C.M.; Jacobo, P.V.; Pellizzari, E.H.; Cigorraga, S.B.; Denduchis, B.; Lustig, L. Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: Effect of interleukin 6 on Sertoli cell tight junctions. Biol. Reprod. 2012, 87, 122. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Zhang, X.; Zhang, Y.; Gu, J.; Chen, M.; Zhang, Z.; Wang, X.; Wang, S.L. Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol. Sci. 2013, 135, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert. Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Fritz, I.B. Metabolism of glucose by Sertoli cells in culture. Biol. Reprod. 1981, 24, 1032–1041. [Google Scholar] [CrossRef]
- Jutte, N.H.; Grootegoed, J.A.; Rommerts, F.F.; van der Molen, H.J. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J. Reprod. Fertil. 1981, 62, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Hall, P.F. Metabolism of round spermatids from rats: Lactate as the preferred substrate. Biol. Reprod. 1982, 26, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Jutte, N.H.; Eikvar, L.; Levy, F.O.; Hansson, V. Metabolism of palmitate in cultured rat Sertoli cells. J. Reprod. Fertil. 1985, 73, 497–503. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, H.; Wu, H.; Chen, Y.; Han, D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 2009, 137, 469–479. [Google Scholar] [CrossRef]
- Riera, M.F.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E907–E914. [Google Scholar] [CrossRef]
- Rindone, G.M.; Dasso, M.E.; Centola, C.L.; Pellizzari, E.H.; Camberos, M.D.C.; Toneatto, J.; Galardo, M.N.; Meroni, S.B.; Riera, M.F. Sertoli cell adaptation to glucose deprivation: Potential role of AMPK in the regulation of lipid metabolism. J. Cell Biochem. 2023, 124, 716–730. [Google Scholar] [CrossRef]
- Regueira, M.; Rindone, G.M.; Galardo, M.N.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B.; Riera, M.F. Germ cells regulate 3-hydroxybutyrate production in rat Sertoli cells. Gen. Comp. Endocrinol. 2017, 248, 5–15. [Google Scholar] [CrossRef]
- Koga, M.; Tanaka, H.; Yomogida, K.; Nozaki, M.; Tsuchida, J.; Ohta, H.; Nakamura, Y.; Masai, K.; Yoshimura, Y.; Yamanaka, M.; et al. Isolation and characterization of a haploid germ cell-specific novel complementary deoxyribonucleic acid; testis-specific homologue of succinyl CoA:3-Oxo acid CoA transferase. Biol. Reprod. 2000, 63, 1601–1609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, H.; Takahashi, T.; Iguchi, N.; Kitamura, K.; Miyagawa, Y.; Tsujimura, A.; Matsumiya, K.; Okuyama, A.; Nishimune, Y. Ketone bodies could support the motility but not the acrosome reaction of mouse sperm. Int. J. Androl. 2004, 27, 172–177. [Google Scholar] [CrossRef]
- Orth, J.M. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat. Rec. 1982, 203, 485–492. [Google Scholar] [CrossRef]
- Mok, K.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis 2011, 1, 270–280. [Google Scholar] [CrossRef]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.A.; Campo, S.M.; Ropelato, M.G.; Bergadá, I. Hormonal Changes in Childhood and Puberty. In Puberty; Kumanov, P., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Chapter 3; pp. 23–37. [Google Scholar] [CrossRef]
- Tavares, R.S.; Escada-Rebelo, S.; Silva, A.F.; Sousa, M.I.; Ramalho-Santos, J.; Amaral, S. Antidiabetic therapies and male reproductive function: Where do we stand? Reproduction 2018, 155, R13–R37. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Jones, K.L.; Arslanian, S.; Peterokova, V.A.; Park, J.S.; Tomlinson, M.J. Effect of metformin in pediatric patients with type 2 diabetes: A randomized controlled trial. Diabetes Care 2002, 25, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, A.; Umpaichitra, V.; Chin, V.L.; Perez-Colon, S. Metformin Use in Children and Adolescents with Prediabetes. Pediatr. Clin. N. Am. 2017, 64, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Onge, E.S.; Miller, S.A.; Motycka, C.; DeBerry, A. A review of the treatment of type 2 diabetes in children. J. Pediatr. Pharmacol. Ther. 2015, 20, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Martins, A.D.; Vaz, C.V.; Correia, S.; Moreira, P.I.; Oliveira, P.F.; Socorro, S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014, 171, 1033–1042. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Mohamed, M. Metformin mitigates impaired testicular lactate transport/utilization and improves sexual behavior in streptozotocin-induced diabetic rats. Arch. Physiol. Biochem. 2021, 127, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Luo, D.; Xu, X.; Sun, M.; Su, X.; Tian, Z.; Zhang, M.; Yu, C.; Guan, Q. Metformin Improves Fertility in Obese Males by Alleviating Oxidative Stress-Induced Blood-Testis Barrier Damage. Oxid. Med. Cell Longev. 2019, 2019, 9151067. [Google Scholar] [CrossRef] [PubMed]
- Regueira, M.; Riera, M.F.; Galardo, M.N.; Camberos, M.C.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. FSH and bFGF regulate the expression of genes involved in Sertoli cell energetic metabolism. Gen. Comp. Endocrinol. 2015, 222, 124–133. [Google Scholar] [CrossRef]
- Gorga, A.; Rindone, G.M.; Centola, C.L.; Sobarzo, C.; Pellizzari, E.H.; Camberos, M.D.C.; Cigorraga, S.B.; Riera, M.F.; Galardo, M.N.; Meroni, S.B. In vitro effects of glyphosate and Roundup on Sertoli cell physiology. Toxicol. Vitro 2020, 62, 104682. [Google Scholar] [CrossRef]
- Gorga, A.; Rindone, G.M.; Centola, C.L.; Sobarzo, C.; Pellizzari, E.H.; Camberos, M.D.C.; Marin-Briggiler, C.I.; Cohen, D.J.; Riera, M.F.; Galardo, M.N.; et al. Low Doses of Glyphosate/Roundup Alter Blood-Testis Barrier Integrity in Juvenile Rats. Front. Endocrinol. 2021, 12, 615678. [Google Scholar] [CrossRef]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Sobarzo, C.; Scarcelli, R.; Denduchis, B.; Lustig, L.; Cigorraga, S.B.; Meroni, S.B. Adenosine regulates Sertoli cell function by activating AMPK. Mol. Cell Endocrinol. 2010, 330, 49–58. [Google Scholar] [CrossRef]
- Chandel, N.S.; Avizonis, D.; Reczek, C.R.; Weinberg, S.E.; Menz, S.; Neuhaus, R.; Christian, S.; Haegebarth, A.; Algire, C.; Pollak, M. Are Metformin Doses Used in Murine Cancer Models Clinically Relevant? Cell Metab. 2016, 23, 569–570. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Rindone, G.M.; Gorga, A.; Pellizzari, E.H.; Camberos, M.D.C.; Galardo, M.N.; Da Ros, V.G.; Buffone, M.G.; Meroni, S.B.; Riera, M.F. Postnatal metformin treatment alters rat Sertoli cell proliferation and daily sperm production. Andrology 2021, 9, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Musutova, M.; Elkalaf, M.; Klubickova, N.; Koc, M.; Povysil, S.; Rambousek, J.; Volckaert, B.; Duska, F.; Trinh, M.D.; Kalous, M.; et al. The Effect of Hypoxia and Metformin on Fatty Acid Uptake, Storage, and Oxidation in L6 Differentiated Myotubes. Front. Endocrinol. 2018, 9, 616. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Lord, S.R.; Collins, J.M.; Cheng, W.C.; Haider, S.; Wigfield, S.; Gaude, E.; Fielding, B.A.; Pinnick, K.E.; Harjes, U.; Segaran, A.; et al. Transcriptomic analysis of human primary breast cancer identifies fatty acid oxidation as a target for metformin. Br. J. Cancer 2020, 122, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fulgencio, J.P.; Kohl, C.; Girard, J.; Pegorier, J.P. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem. Pharmacol. 2001, 62, 439–446. [Google Scholar] [CrossRef]
- Zabielski, P.; Chacinska, M.; Charkiewicz, K.; Baranowski, M.; Gorski, J.; Blachnio-Zabielska, A.U. Effect of metformin on bioactive lipid metabolism in insulin-resistant muscle. J. Endocrinol. 2017, 233, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Cheng, K.; Hao, M. Metformin Inhibits TGF-beta1-Induced Epithelial-to-Mesenchymal Transition via PKM2 Relative-mTOR/p70s6k Signaling Pathway in Cervical Carcinoma Cells. Int. J. Mol. Sci. 2016, 17, 2000. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Hardie, D.G.; Pan, D.A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002, 30, 1064–1070. [Google Scholar] [CrossRef]
- Serra, D.; Casals, N.; Asins, G.; Royo, T.; Ciudad, C.J.; Hegardt, F.G. Regulation of mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase protein by starvation, fat feeding, and diabetes. Arch. Biochem. Biophys. 1993, 307, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hegardt, F.G. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: A control enzyme in ketogenesis. Biochem. J. 1999, 338 Pt 3, 558–569. [Google Scholar] [CrossRef]
- Halestrap, A.P. The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Tartarin, P.; Moison, D.; Guibert, E.; Dupont, J.; Habert, R.; Rouiller-Fabre, V.; Frydman, N.; Pozzi, S.; Frydman, R.; Lecureuil, C.; et al. Metformin exposure affects human and mouse fetal testicular cells. Hum. Reprod. 2012, 27, 3304–3314. [Google Scholar] [CrossRef]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar] [CrossRef]
- Wang, R.S.; Yeh, S.; Chen, L.M.; Lin, H.Y.; Zhang, C.; Ni, J.; Wu, C.C.; di Sant’Agnese, P.A.; deMesy-Bentley, K.L.; Tzeng, C.R.; et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 2006, 147, 5624–5633. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef]
- Risley, M.S.; Tan, I.P.; Roy, C.; Saez, J.C. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J. Cell Sci. 1992, 103 Pt 1, 81–96. [Google Scholar] [CrossRef]
- Mazaud-Guittot, S.; Meugnier, E.; Pesenti, S.; Wu, X.; Vidal, H.; Gow, A.; Le Magueresse-Battistoni, B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol. Reprod. 2010, 82, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Simon, L.; Meling, D.D.; Cyr, D.G.; Gutstein, D.E.; Fishman, G.I.; Guillou, F.; Cooke, P.S. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 2007, 76, 804–812. [Google Scholar] [CrossRef]
- McCabe, M.J.; Foo, C.F.; Dinger, M.E.; Smooker, P.M.; Stanton, P.G. Claudin-11 and occludin are major contributors to Sertoli cell tight junction function, in vitro. Asian J. Androl. 2016, 18, 620–626. [Google Scholar] [CrossRef]
- Siu, E.R.; Wong, E.W.; Mruk, D.D.; Sze, K.L.; Porto, C.S.; Cheng, C.Y. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: A study using the cadmium model. Endocrinology 2009, 150, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B.P.; Pollanen, P.; Zupp, J.L. Development of the blood-testis barrier and changes in vascular permeability at puberty in rats. Int. J. Androl. 1988, 11, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Willems, A.; De Gendt, K.; Allemeersch, J.; Smith, L.B.; Welsh, M.; Swinnen, J.V.; Verhoeven, G. Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int. J. Androl. 2010, 33, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Sluka, P.; Foo, C.F.; Stanton, P.G. Claudin-11 expression and localization is regulated by androgens in rat Sertoli cells in vitro. Reproduction 2007, 133, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Segretain, D.; Fiorini, C.; Decrouy, X.; Defamie, N.; Prat, J.R.; Pointis, G. A proposed role for ZO-1 in targeting connexin 43 gap junctions to the endocytic pathway. Biochimie 2004, 86, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef]
- Chung, N.P.; Mruk, D.; Mo, M.Y.; Lee, W.M.; Cheng, C.Y. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol. Reprod. 2001, 65, 1340–1351. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Stanton, P.G.; Chen, J.L.; Olcorn, J.S.; Haverfield, J.T.; Qian, H.; Walton, K.L.; Gregorevic, P.; Harrison, C.A. Activin signaling regulates Sertoli cell differentiation and function. Endocrinology 2012, 153, 6065–6077. [Google Scholar] [CrossRef]
- Wong, C.H.; Mruk, D.D.; Lui, W.Y.; Cheng, C.Y. Regulation of blood-testis barrier dynamics: An in vivo study. J. Cell Sci. 2004, 117, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.V.; Pellizzari, E.H.; Cigorraga, S.B.; Galardo, M.N.; Naito, M.; Lustig, L.; Jacobo, P.V. IL17A impairs blood-testis barrier integrity and induces testicular inflammation. Cell Tissue Res. 2014, 358, 885–898. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hu, L.; Li, H. Exogenous leptin affects sperm parameters and impairs blood-testis barrier integrity in adult male mice. Reprod. Biol. Endocrinol. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.C.; Khoueiry, R.; Quanico, J.; Acloque, H.; Guerquin, M.J.; Bertoldo, M.J.; Chevaleyre, C.; Ramé, C.; Fournier, I.; Salzet, M.; et al. In Utero Exposure to Metformin Reduces the Fertility of Male Offspring in Adulthood. Front. Endocrinol. 2021, 12, 750145. [Google Scholar] [CrossRef] [PubMed]
| Cell Viability (% Basal) | |
|---|---|
| Basal | 100 |
| Met 24 h | 99 ± 3 |
| Met 48 h | 106 ± 5 |
| Met 72 h | 105 ± 4 |
| Control | MET | |
|---|---|---|
| Animal weight (g) | 120.6 ± 12 | 123.4 ± 5.7 |
| Testis weight (mg) | 362 ± 83 | 372 ± 76 |
| Control | MET | |
|---|---|---|
| Body weight (g) | 461.0 ± 8.5 | 463.3 ± 11.0 |
| Testis weight (g) | 1.8 ± 0.2 | 1.7 ± 0.2 |
| Epididymal weight (g) | 0.63 ± 0.05 | 0.61 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rindone, G.M.; Dasso, M.E.; Centola, C.L.; Sobarzo, C.M.; Galardo, M.N.; Meroni, S.B.; Riera, M.F. Effect of Metformin on Sertoli Cell Fatty Acid Metabolism and Blood–Testis Barrier Formation. Biology 2024, 13, 330. https://doi.org/10.3390/biology13050330
Rindone GM, Dasso ME, Centola CL, Sobarzo CM, Galardo MN, Meroni SB, Riera MF. Effect of Metformin on Sertoli Cell Fatty Acid Metabolism and Blood–Testis Barrier Formation. Biology. 2024; 13(5):330. https://doi.org/10.3390/biology13050330
Chicago/Turabian StyleRindone, Gustavo Marcelo, Marina Ercilia Dasso, Cecilia Lucia Centola, Cristian Marcelo Sobarzo, María Noel Galardo, Silvina Beatriz Meroni, and María Fernanda Riera. 2024. "Effect of Metformin on Sertoli Cell Fatty Acid Metabolism and Blood–Testis Barrier Formation" Biology 13, no. 5: 330. https://doi.org/10.3390/biology13050330
APA StyleRindone, G. M., Dasso, M. E., Centola, C. L., Sobarzo, C. M., Galardo, M. N., Meroni, S. B., & Riera, M. F. (2024). Effect of Metformin on Sertoli Cell Fatty Acid Metabolism and Blood–Testis Barrier Formation. Biology, 13(5), 330. https://doi.org/10.3390/biology13050330







