Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant Materials and Experimental Design
2.3. Measurement of Fresh Weight and Oil Yield
2.4. GC-MS Analyses and Identification of Components
2.5. Antioxidant Potential
2.6. Antimicrobial Assay
2.6.1. Tested Microorganisms
2.6.2. Disk Diffusion Method
2.6.3. Minimal Inhibition Concentration
2.6.4. In Situ Analyses on the Vegetable
2.7. Biofilm Development Study
Crystal Violet Assay
2.8. Insecticidal Activity
2.9. Statistical Analysis
3. Results
3.1. Fresh Weight
3.2. Essential Oil Content
3.3. Essential Oil Yield
3.4. Essential Oil Composition
3.5. Antioxidant Activity
3.6. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. essential oils: Chemical profiles/phytochemical screening, antimicrobial activity and O/W nano-emulsion formulations. Pharmaceutics 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Bastami, A.; Amirnia, R.; Sayyed, R.Z.; El Enshasy, H.A. The effect of mycorrhizal fungi and organic fertilizers on quantitative and qualitative traits of two important Satureja species. Agronomy 2021, 11, 1285. [Google Scholar] [CrossRef]
- Vilmosh, N.; Georgieva-Kotetarova, M.; Dimitrova, S.; Zgureva, M.; Atanassova, P.K.; Hrischev, P.I.; Kostadinova, I. Composition and chronic toxicity of dry methanol-aqueous extract of wild-growing Satureja montana. Folia Med. 2023, 65, 482–489. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hussien, M.S. Effect of nitrogen and phosphorus application on herb and essential oil composition of Satureja montana L. ‘carvacrol’ chemotype. J. Chem. Pharm. Res. 2016, 8, 119–128. [Google Scholar]
- Hudz, N.; Makowicz, E.; Shanaida, M.; Biało, M.; Jasicka-Misiak, I.; Yezierska, O.; Svydenko, L.; Wieczorek, P.P. Phytochemical evaluation of tinctures and essential oil obtained from Satureja montana Herb. Molecules 2020, 25, 4763. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in biological activities of Satureja montana subsp. montana and subsp. variegata based on different extraction methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef]
- Dordevic, N.; Karabegovic, I.; Cvetkovic, D.; Šojic, B.; Savic, D.; Danilovic, B. Assessment of chitosan coating enriched with free and nanoencapsulated Satureja montana L. essential oil as a novel tool for beef preservation. Foods 2022, 11, 2733. [Google Scholar] [CrossRef] [PubMed]
- Said-Al Ahl, H.A.H.; Sabra, A.S.; Alataway, A.; Astatkie, T.; Mahmoud, A.A.; Bloem, E. Biomass production and essential oil composition of Thymus vulgaris in response to water stress and harvest time. J. Essent. Oil Res. 2018, 31, 63–68. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Özgüven, M.; Hassanpouraghdam, M.B. Planting-date and cutting-time affect the growth and essential oil composition of Mentha × piperita and Mentha arvensis. Ind. Crop. Prod. 2021, 170, 113790. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Abdou, M.A.A. Impact of water stress and phosphorus fertilizer on fresh herb and essential oil content of dragonhead. Int. Agrophysics 2009, 23, 403–407. [Google Scholar]
- Said-Al Ahl, H.A.H.; Omer, E.A.; Naguib, N.Y. Effect of water stress and nitrogen fertilizer on herb and essential oil of oregano. Int. Agrophysics 2009, 23, 269–275. [Google Scholar]
- Skubij, N.; Dzida, K. Essential oil composition of summer savory (Satureja hortensis L.) cv. Saturn depending on nitrogen nutrition and plant development phases in raw material cultivated for industrial use. Ind. Crop. Prod. 2019, 135, 260–270. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Mahmoud, A.A.; Astatkie, T.; Said-Al Ahl, H.A.H. Growth and essential oil composition responses of parsley cultivars to phosphorus fertilization and harvest date. J. Plant Nutr. 2018, 42, 2395–2405. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Estahbanati, M.N.; Rezaei, M.; Tafazoli, E.; Delavar, H. Foliar application of diammonium phosphate increases essential oil content and changes its compositions in Mexican marigold (Tagetes minuta L.). J. Essent. Oil-Bear. Plants 2012, 15, 244–249. [Google Scholar] [CrossRef]
- Letchamo, W.; Xu, H.L.; Gosselin, A. Variations in photosynthesis and essential oil in thyme. J. Plant Physiol. 1995, 147, 29–37. [Google Scholar] [CrossRef]
- Damjanovic-Vratnica, B.; Perovic, A.; Sukovic, D.; Perovic, S. Effect of vegetation cycle on chemical content and antibacterial activity of Satureja montana L. Arch. Biol. Sci. 2011, 63, 1173–1179. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Guenther, G. The Essential Oils VIII, E.D. Robert; Nastrand Comp. Inc.: Toronto, ON, Canada; New York, NY, USA; London, UK, 1961. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Quadruple Mass Spectroscopy, 4th ed.; Allured Publishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea essential oil chemical composition and biological activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical composition and biological activity of Salvia officinalis essential oil. Acta Hortic. Regiotect. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Ninou, E.; Cook, C.M.; Papathanasiou, F.; Aschonitis, V.; Avdikos, I.; Tsivelikas, A.L.; Stefanou, S.; Ralli, P.; Mylonas, I. Nitrogen effects on the essential oil and biomass production of field grown Greek oregano (Origanum vulgare subsp. hirtum) Populations. Agronomy 2021, 11, 1722. [Google Scholar] [CrossRef]
- Malaka, M.J.; Araya, N.A.; Soundy, P.; du Plooy, C.P.; Araya, H.T.; Van Rensburg, W.S.J.; Watkinson, E.; Levember, E.; Wadiwala, E.; Amoo, S.O. Biomass, essential oil yield, and composition of marjoram as influenced by interactions of different agronomic practices under controlled conditions. Plants 2023, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Muetasam Jafr, S.; Rahimi, A.R.; Hashemi, M.; Rokhzadi, A. Influence of N, K, and seaweed extract fertilization on biomass, photosynthetic pigments, and essential oil of Thymus vulgaris: Optimization study by response surface methodology. Agronomy 2022, 12, 3222. [Google Scholar] [CrossRef]
- Abd-Allah, W.H. Effect of chemical fertilization and biofertilization on growth and productivity of savory (Satureja hortensis L.) plants. Egypt. J. Desert Res. 2015, 65, 101–123. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Mengel, K.; Kirkby, E.A. Principles of plant nutrition. Ann. Bot. 2004, 93, 479–480. [Google Scholar] [CrossRef]
- Dunkić, V.; Bezić, N.; Vuko, E.; Cukrov, D. Antiphytoviral activity of Satureja montana L. spp. variegata (Host) P. W. Ball Essential oil and phenol compounds on CMV and TMV. Molecules 2010, 15, 6713–6721. [Google Scholar] [CrossRef]
- Thomson, C.; Marschner, H.; Römheld, V. Effect of nitrogen fertilizer form on pH of the bulk soil and rhizosphere, and on the growth, phosphorus, and micronutrient uptake of bean. J. Plant Nutr. 1993, 16, 493–506. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, F.; Wong, M.H. Effect of nitrogen form and phosphorus source on the growth, nutrient uptake and rhizosphere soil property of Camellia sinensis L. Plant Soil 2000, 223, 65–73. [Google Scholar] [CrossRef]
- Lima, V.T.; Vieira, M.C.; Formagio, A.S.N.; Zárate, N.A.H.; Cardoso, C.A.L.; Gonçalves, W.V.; Aran, H.D.V.R.; Carnevali, T.O. Chicken manure and phosphorus influence on biomass production and chemical composition of the essential oil of Ocimum kilimandscharicum. J. Agri. Sci. 2020, 12, 77. [Google Scholar] [CrossRef]
- Peng, L.-C.; Ng, L.-T. Impacts of nitrogen and phosphorus fertilization on biomass, polyphenol contents, and essential oil yield and composition of Vitex negundo Linn. Agriculture 2022, 12, 859. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of fertilization in selected phytometric features and contents of bioactive compounds in dry matter of two varieties of basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of organic and mineral fertilization on yield and selected quality parameters for dried herbs of two varieties of oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Mehanna, H.M.; Ramadan, M.F. Impact of water regime and phosphorus fertilization and their interaction on the characteristics of rapeseed (Brassica napus) and fatty acid profile of extracted oil. Commun. Biometry Crop. Sci. 2016, 11, 64–76. [Google Scholar]
- Nooshkam, A.; Hosseini, N.M.; Hadian, J.; Jahansooz, M.R.; Khavazi, K.; Salehnia, A.N.; Hedayatpour, S. Study the effects of biological and chemical fertilizers on quantitative and qualitative characteristics of savory species (Satureja khuzestanica Jamzad). J. Crop Prod. 2016, 8, 87–103. [Google Scholar]
- Abbaszadeh, B.; Sefidkon, F.; Haghighi, M.L.; Hajiabadi, E.K. The Effect of planting time and planting density on yield and essential oil of Satureja sahendica Bornm. J. Med. Plants By-Prod. 2014, 3, 141–146. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Martínez-Force, E.; Mandrioli, M.; Toschi, T.G.; Monti, A. Shifting sowing of camelina from spring to autumn enhances the oil quality for bio-based applications in response to temperature and seed carbon stock. Ind. Crop. Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Sahzabi, A.A.; Ashoorabadi, E.S.; Shiranirad, A.H.; Bohlool; Farahani, H.A. The methods of nitrogen application influence on essential oil yield and water use efficiency of summer savory (Satureja hortensis L.). Int. J. Hortic. Floric. 2010, 7, 52–56. [Google Scholar]
- Pirzad, A.; Mashmoul, A.; Hassani, A. Effect of chemical and biological phosphorus on the yield of summer savory (Satureja hortensis L.). J. Med. Plants By-Prod. 2016, 1, 33–38. [Google Scholar]
- Mehanna, H.M.; Said-Al Ahl, H.A.H.; Mursy, M.H.; Ngezimana, W.; Mudau, F.N. Yield and essential oil response in coriander to water stress and phosphorus fertilizer application. J. Essent. Oil-Bear. Plants 2015, 18, 82–92. [Google Scholar]
- Mihajilov-Krstev, T.; Radnović, D.; Kitić, D.; Jovanović, V.; Mitić, V.; Stojanović-Radić, Z.; Zlatković, B. Chemical composition, antimicrobial, antioxidative and anticholinesterase activity of Satureja montana L. ssp montana essential oil. Open Life Sci. 2014, 9, 668–677. [Google Scholar] [CrossRef]
- Ibraliu, A.; Dhillon, B.S.; Faslia, N.; Stich, B. Variability of essential oil composition in Albanian accessions of Satureja montana L. J. Med. Plants. Res. 2010, 4, 1359–1364. [Google Scholar]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricini, D.; Epifano, F.; Genovese, S.; Curini, M. Chemical composition and antifungal activity of the essential oil of Satureja montana from central Italy. Chem. Nat. Comp. 2007, 43, 622–624. [Google Scholar] [CrossRef]
- Mirjana, S.; Nada, B. Chemical composition and antimicrobial variability of Satureja montana L. essential oils produced during ontogenesis. J. Essent. Oil Res. 2004, 16, 387–391. [Google Scholar] [CrossRef]
- Piccaglia, R.; Dellacecca, V.; Marotti, M.; Giovanelli, E. Agronomic factors affecting the yields and the essential oil composition of peppermint (Mentha piperita L.). Acta Hortic. 1993, 344, 29–40. [Google Scholar] [CrossRef]
- Baydar, H. The effects of different harvest dates on essential oil content and essential oil composition in Origanum minutiflorum O. Schwarz et. P.H. Davis. Akdeniz Univ. J. Fac. Agricult. 2005, 18, 175–178. [Google Scholar]
- Saeb, K.; Gholamrezaee, S. Variation of essential oil composition of Melissa officinalis L. leaves during different stages of plant growth. Asian Pac. J. Trop. Biomed. 2012, 2, S547–S549. [Google Scholar] [CrossRef]
- Türkmen, M.; Kara, M.; Maral, H.; Soylu, S. Determination of chemical component of essential oil of plants grown at different altitudes and antifungal activity against Sclerotinia sclerotiorum. J. Food Process. Preserv. 2022, 46, e15787. [Google Scholar] [CrossRef]
- Massoud, H.Y.A.; Abd El-Shafy, M.K.; El-Eraky, M. Influence of planting dates and distances on growth and essential oil productivity of Rosmarinus officinalis L. plant. J. Agric. Sci. Mansoura Univ. 2007, 32, 2937–2955. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed.; Lavoisier and Publishing: Paris, France, 1999. [Google Scholar]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour. Technol. 2004, 93, 307–311. [Google Scholar] [CrossRef]
- Baranauskienne, R.; Venskutonis, P.R.; Viskelis, P.; Dambrausiene, E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hussein, M.S.; Abd El-Kader, A.A. Effect of nitrogen fertilizer and/or some foliar application on growth, herb yield, essential oil and chemical composition of dragonhead. J. Med. Food Plants 2010, 2, 12–28. [Google Scholar]
- Bakhtiari, M.; Mozafari, H.; Karimzadeh Asl, K.; Sani, B.; Mirza, M. Bio-organic and inorganic fertilizers modify leaf nutrients, essential oil properties, and antioxidant capacity in medic savory (Satureja macrantha). J. Biol. Res. 2020, 93, 8477. [Google Scholar] [CrossRef]
- Mohammadi, S.M.; Sefidkon, F.; Asadi-Sanam, S.; Kalatejari, S. The changes of carvacrol content and essential oil yield of Satureja khuzestanica Jamzad in response to different fertilizer sources. Flavour. Fragr. J. 2023, 38, 37–48. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Yang, D.; Du, X.; Liang, X.; Han, R.; Liang, Z.; Liu, Y.; Liu, F.; Zhao, J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef]
- Kulić, M.; Drakul, D.; Sokolović, D.; Kordić-Bojinović, J.; Milovanović, S.; Blagojević, D. Essential oil of Satureja montana L. from Herzegovina: Assessment of composition, antispasmodic, and antidiarrheal effects. Rec. Nat. Prod. 2023, 17, 3. [Google Scholar]
- Bojović, D.; Šoškić, M.; Tadić, V. Comparative study of chemical composition of the essential oils from Satureja cuneifolia Ten. and Satureja montana L., lamiaceae collected at National Park Lovćen, Montenegro. Stud. Univ. Babeș-Bolyai Chem. 2018, 63, 167–180. [Google Scholar] [CrossRef]
- Čopra-Janićijević, A.; Vidic, D.; Maksimović, M. Chemical composition of the essential oil and headspace of Satureja montana L. Nat. Volatiles Essent. Oils 2020, 7, 22–34. [Google Scholar] [CrossRef]
- Hassanein, H.D.; Said-AL Ahl, H.A.H.; Abdelmohsen, M.M. Antioxidant polyphenolic constituents of Satureja montana L. grown. in Egypt. Int J. Pharm. Pharm. 2014, 6, 578–581. [Google Scholar]
- Kustrak, D.; Kuftinec, J.; Blazevic, N.; Maffei, M. Comparison of the essential oil composition of two subspecies of Satureja montana. J. Essent. Oil Res. 1996, 8, 7–13. [Google Scholar] [CrossRef]
- Abdelrazik, T.M.; Sabra, A.S.; Astatkie, T.; Hegazy, M.H.; Grulova, D.; Said-Al Ahl, H.A.H. Response of growth, essential oil content and its constituent’s of Plectranthus amboinicus to iron and/or urea foliar application under saline irrigation. Int. J. Pharm. Pharm. Sci. 2016, 8, 223–231. [Google Scholar]
- Marin, M.; Novaković, M.; Tešević, V.; Vučković, I.; Milojević, N.; Vuković-Gačić, B.; Marin, P.D. Antioxidative, antibacterial and antifungal activity of the essential oil of wild-growing Satureja montana L. from Dalmatia, Croatia. Flavour Fragr. J. 2012, 27, 216–223. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Cilkiz, M.A. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2015, 54, 375412. [Google Scholar]
- Hajdari, A.; Mustafa, B.; Kaçiku, A.; Xhavit, M.; Brigitte, L.; Alban, I.; Gjoshe, S.; Johannes, N. Chemical composition of the essential oil, total phenolics, total flavonoids and antioxidant activity of methanolic extracts of Satureja montana L. Rec. Nat. Prod. 2016, 10, 750–760. [Google Scholar]
- Tumbas, V.; Djilas, S. Antioxidative and antiproliferative effects of Satureja montana L. extracts. J. BUON 2004, 9, 443–449. [Google Scholar]
- Lin, C.W.; Yu, C.W.; Wu, S.C.; Yih, K.H. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J. Food Drug Anal. 2009, 1, 9. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Gan, C.; Terrado, E.; Sanz, M.A.; Ballestero, D.; Langa, E. Antibiotic properties of Satureja montana L. hydrolate in bacteria and fungus of clinical interest and its impact in non-target environmental microorganisms. Sci. Rep. 2022, 12, 18460. [Google Scholar] [CrossRef] [PubMed]
- Dunkić, V.; Kremer, D.; Dragojević Müller, I.; Stabentheiner, E.; Kuzmić, S.; Jurišić Grubešić, R.; Vujić, L.; Kosalec, I.; Randić, M.; Srěcec, S.; et al. Chemotaxonomic and micromorphological traits of Satureja montana L. and S. subspicata Vis. (Lamiaceae). Chem. Biodivers. 2012, 9, 2825–2842. [Google Scholar] [CrossRef] [PubMed]
- Parsaei, P.; Bahmani, M.; Naghdi, N.; Asadi-Samani, M.; Rafieian-Kopaei, M. A review of therapeutic and pharmacological effects of thymol. Der Pharm. Lett. 2016, 8, 150–154. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbahshs, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Ćebović, T.; Vidović, S.; Jokić, S. Evaluation of anticancer activity of Satureja montana supercritical and spray-dried extracts on Ehrlich’s ascites carcinoma bearing mice. Plants 2020, 9, 1532. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Šolić, M.E.; Jerković-Mujkić, A.; Bešta, R. Chemical composition and antioxidant and antimicrobial activity of two Satureja essential oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Carramiñana, J.J.; Rota, C.; Burillo, J.; Herrera, A. Antibacterial efficiency of Spanish Satureja montana essential oil against Listeria monocytogenes among natural flora in Minced Pork. J. Food Prot. 2008, 71, 502–508. [Google Scholar] [CrossRef]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F.; et al. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Menghini, L.; Mariani, F.; Pagiotti, R.; Menghini, A.; Fatichenti, F. Antimicrobial properties of essential oil of Satureja montana L. on pathogenic and spoilage yeasts. Biotechnol. Lett. 2000, 22, 1007–1010. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media, efficacy, synergisticpotential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Ziaee, M.; Krutmuang, P. Acaricidal, insecticidal, and nematicidal efficiency of essential oils isolated from the Satureja genus. Int. J. Environ. Res. Public Health 2021, 18, 6050. [Google Scholar] [CrossRef]
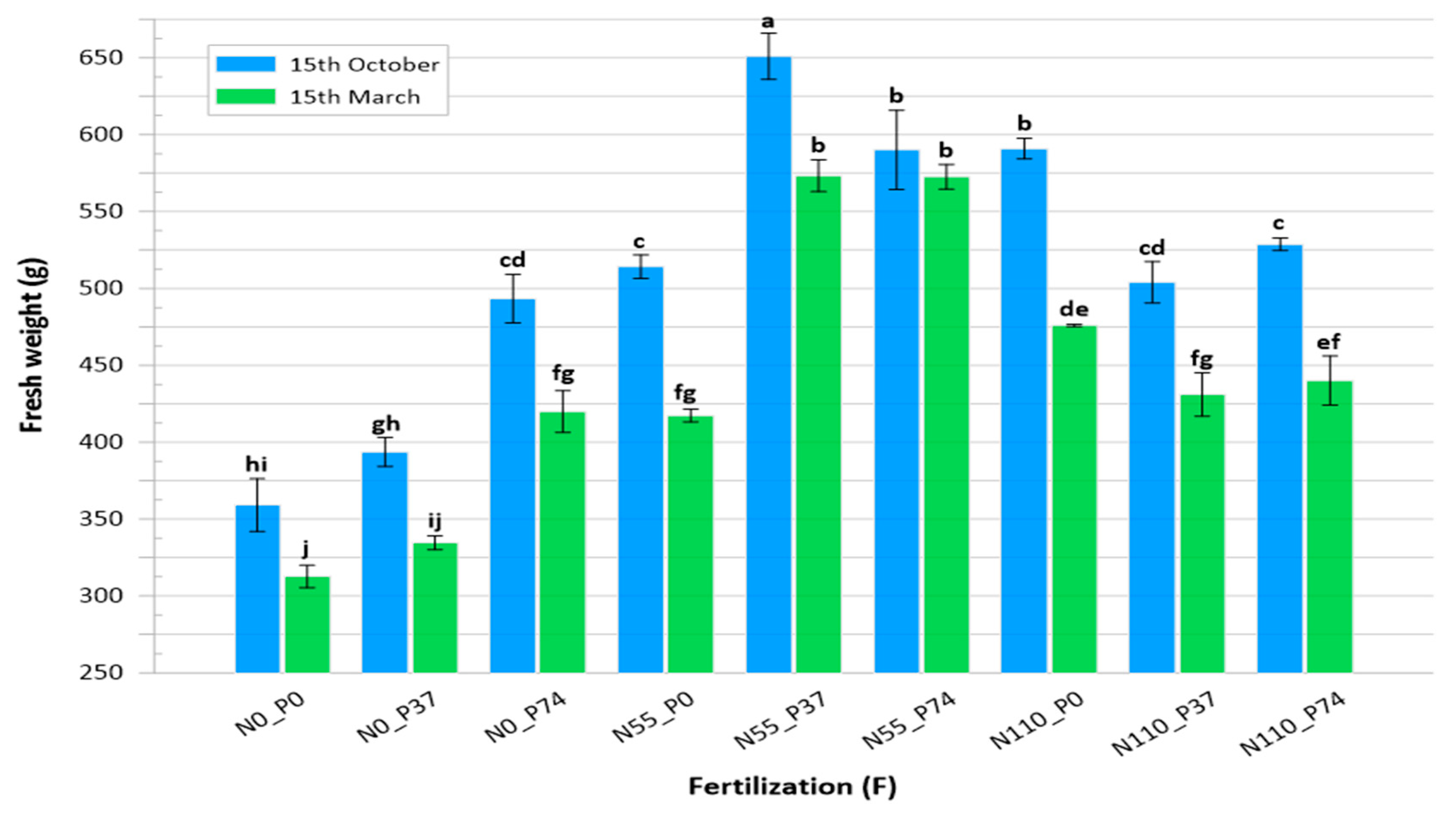
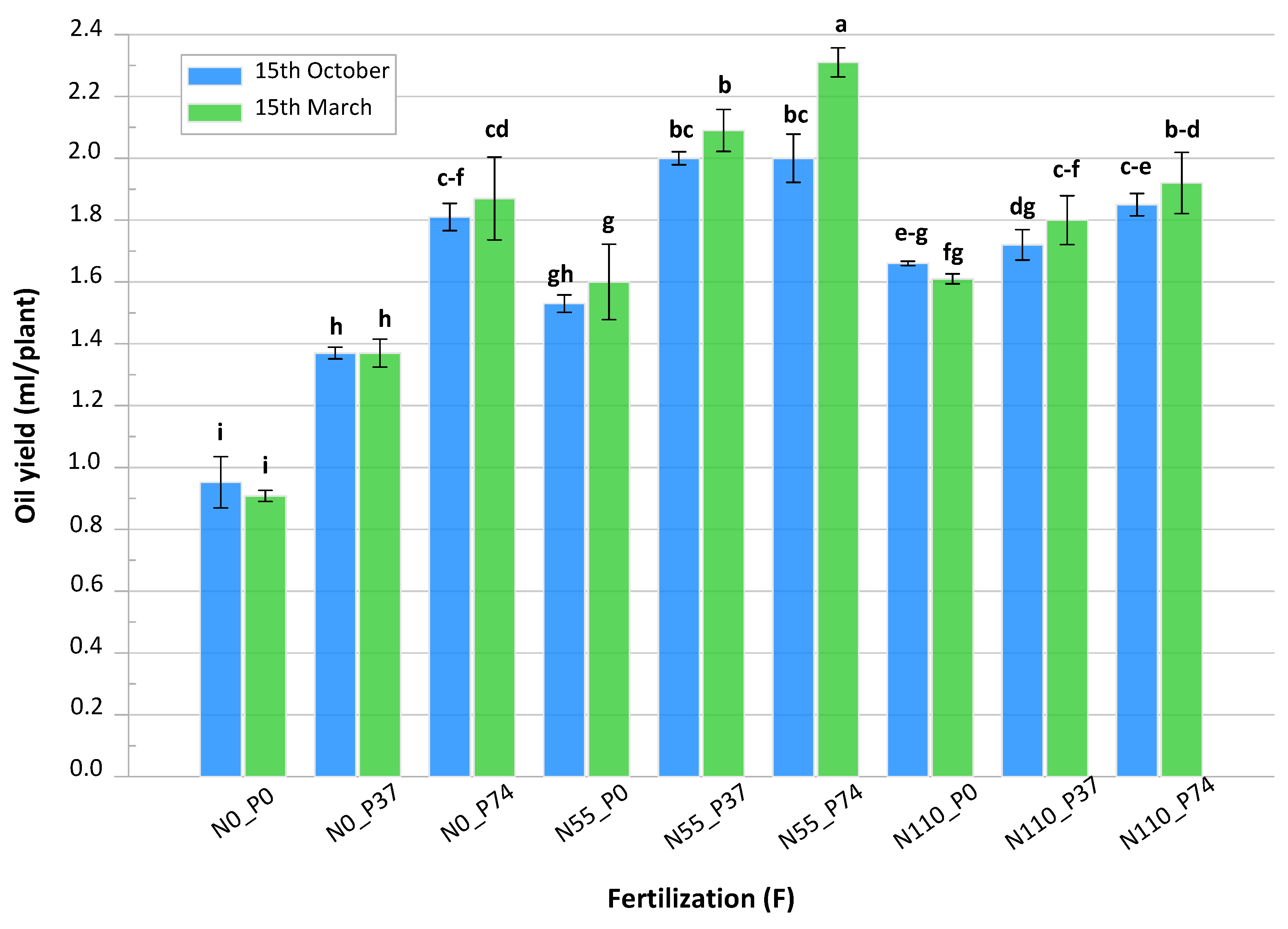
| Fertilization (F) | Cut | |||||
|---|---|---|---|---|---|---|
| 1st Cut | 2nd Cut | |||||
| Date of Sowing (D) | ||||||
| October | March | Mean | October | March | Mean | |
| N0_P0 | 140.5 ± 8.73 f–h | 102.5 ± 8.12 i | 121.5 ± 22.2 F | 218.6 ± 9.19 f | 210.2 ± 0.884 f | 214.4 ± 7.43 F |
| N0_P37 | 145.8 ± 4.92 fg | 112.7 ± 1.70 ij | 129.2 ± 18.5 F | 247.9 ± 5.23 e | 222.0 ± 3.01 ef | 235.0 ± 14.7 E |
| N0_P74 | 185.9 ± 5.28 e | 118.2 ± 9.81 ij | 151.5 ± 38.3 E | 307.6 ± 10.6 cd | 302.7 ± 4.40 cd | 305.1 ± 7.72 CD |
| N55_P0 | 205.4 ± 7.95 c–e | 128.7 ± 5.84 g–i | 167.0 ± 42.5 D | 308.8 ± 6.29 cd | 288.7 ± 4.03 d | 298.7 ± 12.0 D |
| N55_P37 | 245.4 ± 7.33 a | 199.5 ± 9.41 c–e | 222.4 ± 26.3 A | 405.6 ± 14.5 a | 373.9 ± 1.58 b | 389.7 ± 19.6 A |
| N55_P74 | 214.2 ± 10.0 bc | 192.1 ± 2.07 de | 203.2 ± 13.7 B | 375.9 ± 22.2 b | 380.5 ± 8.00 ab | 378.2 ± 15.2 A |
| N110_P0 | 228.3 ± 3.52 ab | 150.8 ± 4.31 f | 189.5 ± 42.6 C | 362.7 ± 7.00 b | 325.2 ± 4.18 c | 343.9 ± 21.1 B |
| N110_P37 | 196.5 ± 3.69 c–e | 120.9 ± 4.43 h–j | 158.7 ± 41.5 DE | 307.6 ± 10.4 cd | 310.1 ± 9.78 cd | 308.8 ± 9.12 CD |
| N110_P74 | 206.6 ± 1.53 cd | 127.2 ± 8.08 g–i | 166.9 ± 43.8 D | 322.1 ± 2.83 c | 313.0 ± 8.18 cd | 317.5 ± 7.41 C |
| Mean | 196.5 ± 33.9 A | 139.0 ± 33.9 B | 167.8 ± 44.4 | 317.4 ± 57.7 A | 302.9 ± 56.1 B | 310.2 ± 56.8 |
| Fertilization (F) | Cut | |||||
|---|---|---|---|---|---|---|
| 1st Cut | 2nd Cut | |||||
| Date of Sowing (D) | ||||||
| October | March | Mean | October | March | Mean | |
| N0_P0 | 0.238 ± 0.018 h | 0.267 ± 0.008 g | 0.253 ± 0.020 E | 0.282 ± 0.008 i | 0.302 ± 0.013 g–i | 0.292 ± 0.014 G |
| N0_P37 | 0.288 ± 0.008 fg | 0.365 ± 0.009 c | 0.327 ± 0.043 C | 0.383 ± 0.014 cd | 0.433 ± 0.012 ab | 0.408 ± 0.030 AB |
| N0_P74 | 0.333 ± 0.003 d | 0.438 ± 0.003 a | 0.386 ± 0.058 A | 0.387 ± 0.006 cd | 0.458 ± 0.008 a | 0.423 ± 0.040 A |
| N55_P0 | 0.283 ± 0.006 fg | 0.370 ± 0.009 c | 0.327 ± 0.048 C | 0.307 ± 0.012 f–i | 0.387 ± 0.046 cd | 0.347 ± 0.053 EF |
| N55_P37 | 0.272 ± 0.008 fg | 0.332 ± 0.003 d | 0.302 ± 0.033 D | 0.330 ± 0.009 e–h | 0.383 ± 0.008 cd | 0.357 ± 0.030 DE |
| N55_P74 | 0.325 ± 0.009 d | 0.397 ± 0.006 b | 0.361 ± 0.040 B | 0.345 ± 0.005 d–g | 0.408 ± 0.015 bc | 0.377 ± 0.036 CD |
| N110_P0 | 0.265 ± 0.005 g | 0.315 ± 0.010 de | 0.290 ± 0.028 D | 0.290 ± 0.000 hi | 0.350 ± 0.005 d–f | 0.320 ± 0.033 F |
| N110_P37 | 0.318 ± 0.003 de | 0.405 ± 0.005 b | 0.362 ± 0.048 B | 0.357 ± 0.006 de | 0.423 ± 0.010 a–c | 0.390 ± 0.037 BC |
| N110_P74 | 0.297 ± 0.015 ef | 0.410 ± 0.010 b | 0.353 ± 0.063 B | 0.383 ± 0.003 cd | 0.447 ± 0.010 ab | 0.415 ± 0.035 AB |
| Mean | 0.291 ± 0.031 B | 0.367 ± 0.052 A | 0.329 ± 0.057 | 0.340 ± 0.040 B | 0.399 ± 0.050 A | 0.370 ± 0.054 |
| Fertilization (F) | Cut | |||||
|---|---|---|---|---|---|---|
| 1st Cut | 2nd Cut | |||||
| Date of Sowing (D) | ||||||
| October | March | Mean | October | March | Mean | |
| N0_P0 | 0.336 ± 0.044 hi | 0.274 ± 0.023 i | 0.305 ± 0.046 E | 0.616 ± 0.039 h | 0.634 ± 0.027 h | 0.625 ± 0.031 G |
| N0_P37 | 0.420 ± 0.004 f–h | 0.411 ± 0.015 gh | 0.415 ± 0.011 D | 0.949 ± 0.017 g | 0.960 ± 0.034 g | 0.954 ± 0.025 F |
| N0_P74 | 0.619 ± 0.016 b–d | 0.484 ± 0.097 e–g | 0.551 ± 0.097 C | 1.187 ± 0.029 d–f | 1.387 ± 0.037 bc | 1.287 ± 0.114 CD |
| N55_P0 | 0.582 ± 0.011 c–e | 0.476 ± 0.027 fg | 0.529 ± 0.061 C | 0.947 ± 0.017 g | 1.120 ± 0.144 ef | 1.034 ± 0.132 EF |
| N55_P37 | 0.665 ± 0.005 a–c | 0.661 ± 0.037 a–c | 0.663 ± 0.024 B | 1.339 ± 0.018 b–d | 1.434 ± 0.031 ab | 1.386 ± 0.056 AB |
| N55_P74 | 0.696 ± 0.037 ab | 0.762 ± 0.004 a | 0.729 ± 0.043 A | 1.299 ± 0.089 b–d | 1.553 ± 0.044 a | 1.426 ± 0.152 A |
| N110_P0 | 0.605 ± 0.015 b–d | 0.474 ± 0.015 fg | 0.540 ± 0.073 C | 1.052 ± 0.020 fg | 1.138 ± 0.029 ef | 1.095 ± 0.052 E |
| N110_P37 | 0.625 ± 0.017 b–d | 0.490 ± 0.024 e–g | 0.558 ± 0.077 C | 1.097 ± 0.037 e–g | 1.313 ± 0.057 b–d | 1.205 ± 0.126 D |
| N110_P74 | 0.613 ± 0.035 b–d | 0.522 ± 0.045 d–f | 0.567 ± 0.061 C | 1.235 ± 0.018 c–e | 1.399 ± 0.060 ab | 1.317 ± 0.098 BC |
| Mean | 0.573 ± 0.115 A | 0.506 ± 0.138 B | 0.540 ± 0.130 | 1.080 ± 0.218 B | 1.215 ± 0.279 A | 1.148 ± 0.257 |
| Fertilization (F) | Carvacrol | Thymol | p-Cymene | γ-Terpinene | β-Caryophyllene | Linalool | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of Sowing (D) | ||||||||||||
| October | March | October | March | October | March | October | March | October | March | October | March | |
| N0_P0 | 65.32 | 65.41 | 2.56 | 1.97 | 9.77 | 9.93 | 6.33 | 6.56 | 1.41 | 1.46 | 1.92 | 1.90 |
| N0_P37 | 67.32 | 68.04 | 2.89 | 2.97 | 7.89 | 7.75 | 5.03 | 4.87 | 1.43 | 2.22 | 0.74 | 0.75 |
| N0_P74 | 67.89 | 68.89 | 3.69 | 3.61 | 5.81 | 5.91 | 4.23 | 3.66 | 1.31 | 1.13 | 0.91 | 0.95 |
| N55_P0 | 65.45 | 65.79 | 2.67 | 1.99 | 9.68 | 8.67 | 5.24 | 5.98 | 1.33 | 1.68 | 1.80 | 1.81 |
| N55_P37 | 70.78 | 70.88 | 3.74 | 3.72 | 4.98 | 4.9 | 3.89 | 3.38 | 2.93 | 2.60 | 0.78 | 0.72 |
| N55_P74 | 72.31 | 73.05 | 3.88 | 3.98 | 4.77 | 4.85 | 3.80 | 3.32 | 2.74 | 2.54 | 0.65 | 1.01 |
| N110_P0 | 65.97 | 66.99 | 2.96 | 2.59 | 8.81 | 8.3 | 5.21 | 5.38 | 2.39 | 2.34 | 0.78 | 1.45 |
| N110_P37 | 75.44 | 75.92 | 3.89 | 4.07 | 3.79 | 3.58 | 3.10 | 2.19 | 2.81 | 2.09 | 0.90 | 0.98 |
| N110_P74 | 76.67 | 77.35 | 5.87 | 4.76 | 3.56 | 3.18 | 2.96 | 2.11 | 1.40 | 2.11 | 0.56 | 0.90 |
| Microorganism | Inhibition Zone | ATB |
|---|---|---|
| Gram-positive bacteria | ||
| Micrococcus luteus CCM 732 | 8.67 ± 0.58 a | 29.67 ± 0.58 ab |
| Listeria monocytogenes CCM 4699 | 8.33 ± 0.57 ab | 30.33 ± 0.57 a |
| Staphylococcus aureus CCM 3953 | 9.33 ± 0.58 a | 28.33 ± 0.58 b |
| Gram-negative bacteria | ||
| Escherichia coli CCM 3953 | 6.67 ± 0.57 bc | 28.67 ± 0.57 ab |
| Salmonella enterica subsp. enterica CCM 3807 | 6.33 ± 0.58 cd | 29.33 ± 0.58 ab |
| Yersinia enterocolitica CCM 7204T | 5.67 ± 0.59 cde | 29.67 ± 0.58 ab |
| Yeasts | ||
| Candida albicans CCM 8186 | 4.33 ± 0.58 e | 28.33 ± 0.58 b |
| Candida glabrata CCM 8270 | 5.33 ± 0.58 cde | 29.67 ± 0.58 ab |
| Candida krusei CCM 8271 | 4.67 ± 0.58 de | 29.33 ± 0.59 ab |
| Candida tropicalis CCM 8223 | 4.33 ± 0.59 e | 29.67 ± 0.59 ab |
| Microorganism | MIC50 | MIC90 |
|---|---|---|
| Gram-positive bacteria | ||
| Micrococcus luteus CCM 732 | 3.34 ± 0.21 g | 3.78 ± 0.10 g |
| Listeria monocytogenes CCM 4699 | 2.42 ± 0.23 h | 2.73 ± 0.14 h |
| Staphylococcus aureus CCM 3953 | 2.19 ± 0.14 h | 2.35 ± 0.10 h |
| Gram-negative bacteria | ||
| Escherichia coli CCM 3953 | 13.16 ± 0.06 e | 13.37 ± 0.18 e |
| Salmonella enterica subsp. enterica CCM 3807 | 17.35 ± 0.16 d | 17.63 ± 0.15 d |
| Yersinia enterocolitica CCM 7204T | 18.43 ± 0.28 c | 18.65 ± 0.14 c |
| Yeasts | ||
| Candida albicans CCM 8186 | 22.18 ± 0.25 b | 22.35 ± 0.07 b |
| Candida glabrata CCM 8270 | 23.43 ± 0.10 a | 23.74 ± 0.22 a |
| Candida krusei CCM 8271 | 22.24 ± 0.08 b | 22.43 ± 0.10 b |
| Candida tropicalis CCM 8223 | 23.24 ± 0.18 a | 23.52 ± 0.13 a |
| Biofilm forming bacteria (BFB) | ||
| Salmonella enterica | 4.56 ± 0.25 f | 4.79 ± 0.20 f |
| Food Model | Microorganisms | Concentration of EO (μg/mL) | |||
|---|---|---|---|---|---|
| Kohlrabi | 62.5 | 125 | 250 | 500 | |
| Gram-positive | Micrococcus luteus | 85.34 ± 2.40 b | 65.48 ± 1.71 bc | 56.67 ± 1.66 b | 44.76 ± 1.05 abc |
| Listeria monocytogenes | 83.67 ± 0.89 b | 63.56 ± 2.18 c | 55.48 ± 1.08 b | 42.43 ± 0.95 c | |
| Staphylococcus aureus | 86.46 ± 1.30 b | 67.43 ± 1.00 b | 53.25 ± 0.91 b | 46.65 ± 0.85 a | |
| Gram-negative | Escherichia coli | 93.38 ± 0.83 a | 77.43 ± 1.50 a | 63.65 ± 1.67 a | 43.54 ± 0.99 bc |
| Salmonella enterica | 94.45 ± 1.16 a | 75.45 ± 1.18 a | 65.45 ± 1.27 a | 44.96 ± 0.78 ab | |
| Yersinia enterocolitica | 96.37 ± 1.30 a | 74.43 ± 1.08 a | 66.48 ± 1.28 a | 46.69 ± 0.89 a | |
| Yeasts | Candida albicans | 76.56 ± 1.62 c | 55.47 ± 1.00 d | 34.54 ± 0.49 cd | 15.67 ± 0.58 de |
| Candida glabrata | 76.43 ± 0.98 c | 54.18 ± 1.08 d | 32.28 ± 0.82 d | 16.65 ± 0.45 de | |
| Candida krusei | 75.76 ± 0.88 c | 56.78 ± 0.88 d | 35.65 ± 1.75 cd | 17.54 ± 1.20 d | |
| Candida tropicalis | 73.43 ± 0.72 c | 57.77 ± 0.86 d | 36.56 ± 1.08 c | 14.76 ± 0.58 e | |
| Concentration (%) | Number of Living Individuals | Number of Dead Individuals | Insecticidal Activity (%) |
|---|---|---|---|
| 100 | 0 | 100 | 100.00 ± 0.00 |
| 50 | 20 | 80 | 80.00 ± 0.00 |
| 25 | 30 | 70 | 70.00 ± 0.00 |
| 12.5 | 40 | 60 | 60.00 ± 0.00 |
| 6.25 | 60 | 40 | 40.00 ± 0.00 |
| 3.125 | 90 | 10 | 10.00 ± 0.00 |
| Control group | 100 | 0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said-Al Ahl, H.A.H.; Kačániova, M.; Mahmoud, A.A.; Hikal, W.M.; Čmiková, N.; Szczepanek, M.; Błaszczyk, K.; Al-Balawi, S.M.; Bianchi, A.; Smaoui, S.; et al. Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates. Biology 2024, 13, 328. https://doi.org/10.3390/biology13050328
Said-Al Ahl HAH, Kačániova M, Mahmoud AA, Hikal WM, Čmiková N, Szczepanek M, Błaszczyk K, Al-Balawi SM, Bianchi A, Smaoui S, et al. Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates. Biology. 2024; 13(5):328. https://doi.org/10.3390/biology13050328
Chicago/Turabian StyleSaid-Al Ahl, Hussein A. H., Miroslava Kačániova, Abeer A. Mahmoud, Wafaa M. Hikal, Natália Čmiková, Małgorzata Szczepanek, Karolina Błaszczyk, Siham M. Al-Balawi, Alessandro Bianchi, Slim Smaoui, and et al. 2024. "Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates" Biology 13, no. 5: 328. https://doi.org/10.3390/biology13050328
APA StyleSaid-Al Ahl, H. A. H., Kačániova, M., Mahmoud, A. A., Hikal, W. M., Čmiková, N., Szczepanek, M., Błaszczyk, K., Al-Balawi, S. M., Bianchi, A., Smaoui, S., & Tkachenko, K. G. (2024). Phytochemical Characterization and Biological Activities of Essential Oil from Satureja montana L., a Medicinal Plant Grown under the Influence of Fertilization and Planting Dates. Biology, 13(5), 328. https://doi.org/10.3390/biology13050328












