Early Animal Origin of BACE1 APP/Aβ Proteolytic Function
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylogenetic Analysis
2.2. Expression Constructs
2.3. Cell Culture and Transfection
2.4. Collection of Conditioned Media and ELISA
2.5. Statistics
3. Results
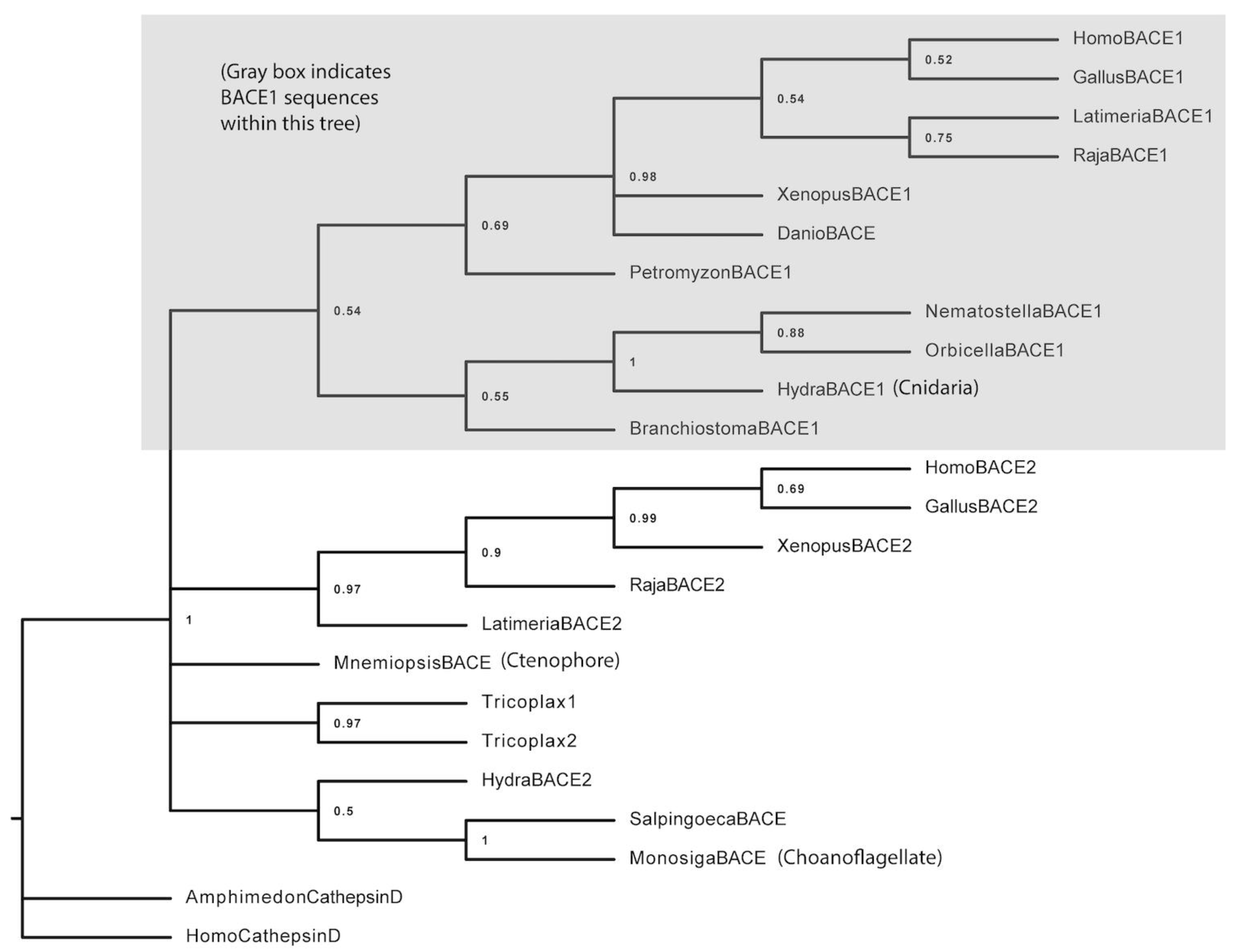
3.1. BACE Phylogenetic Analysis
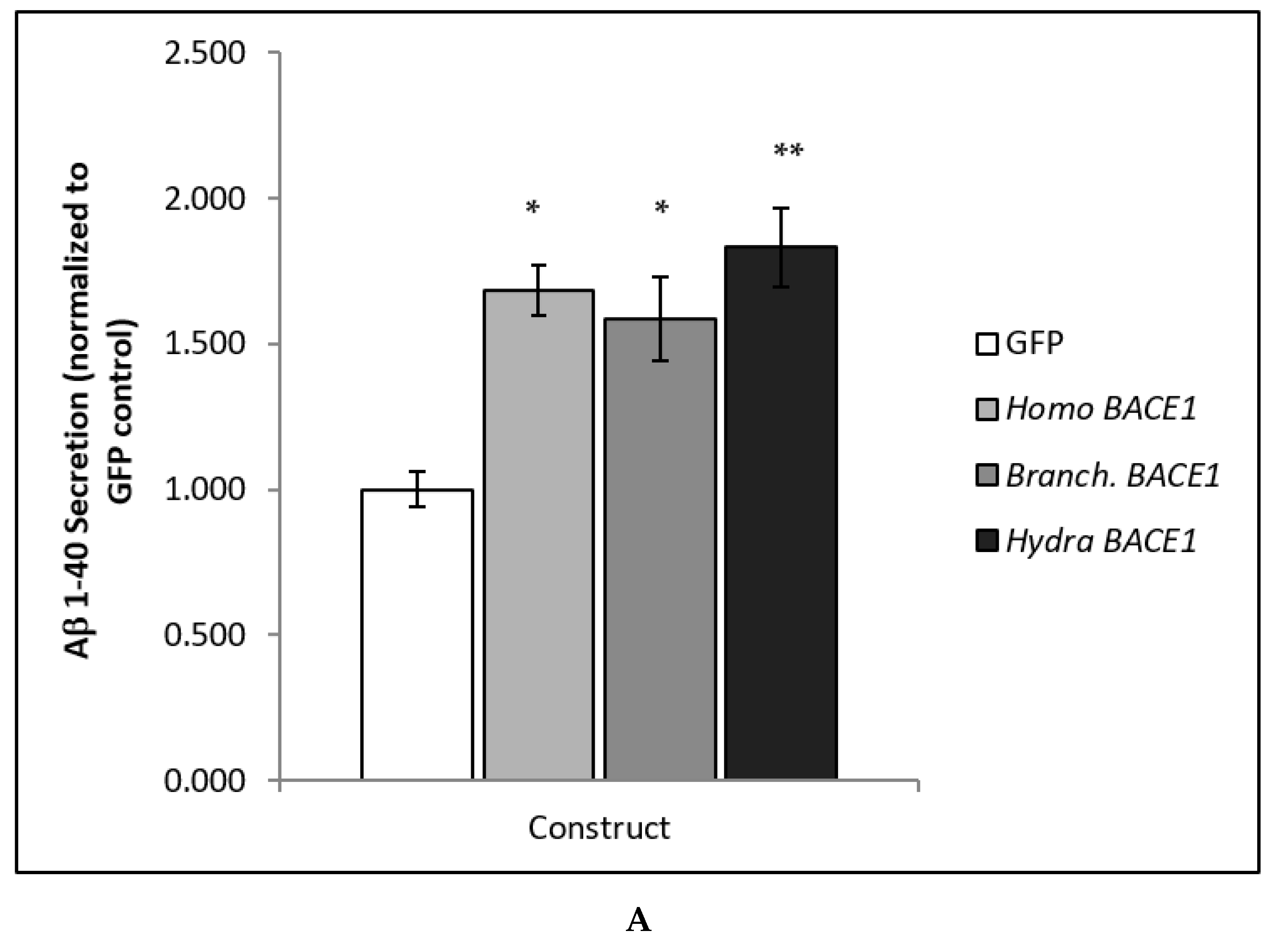
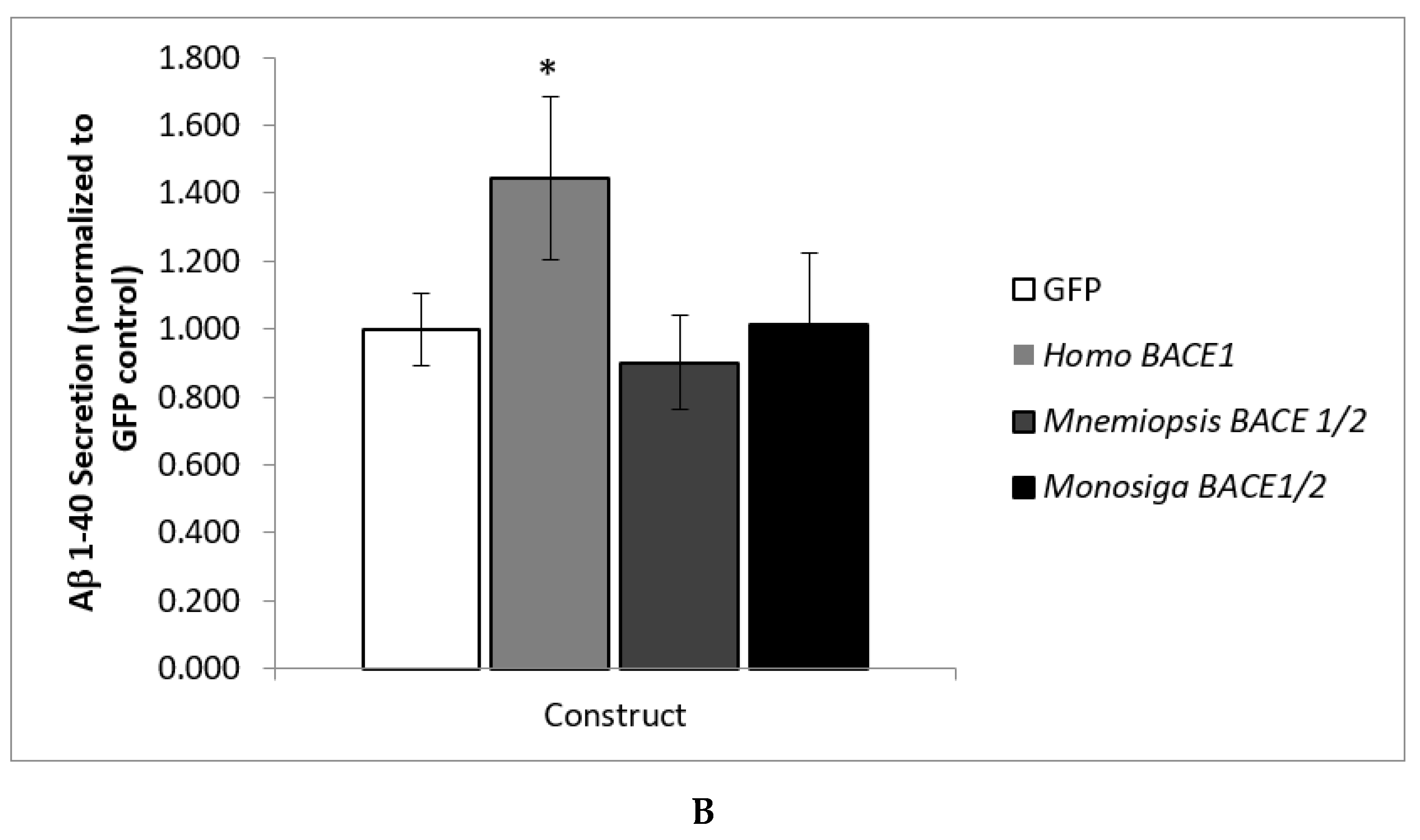
3.2. Analysis of BACE Functional Activity
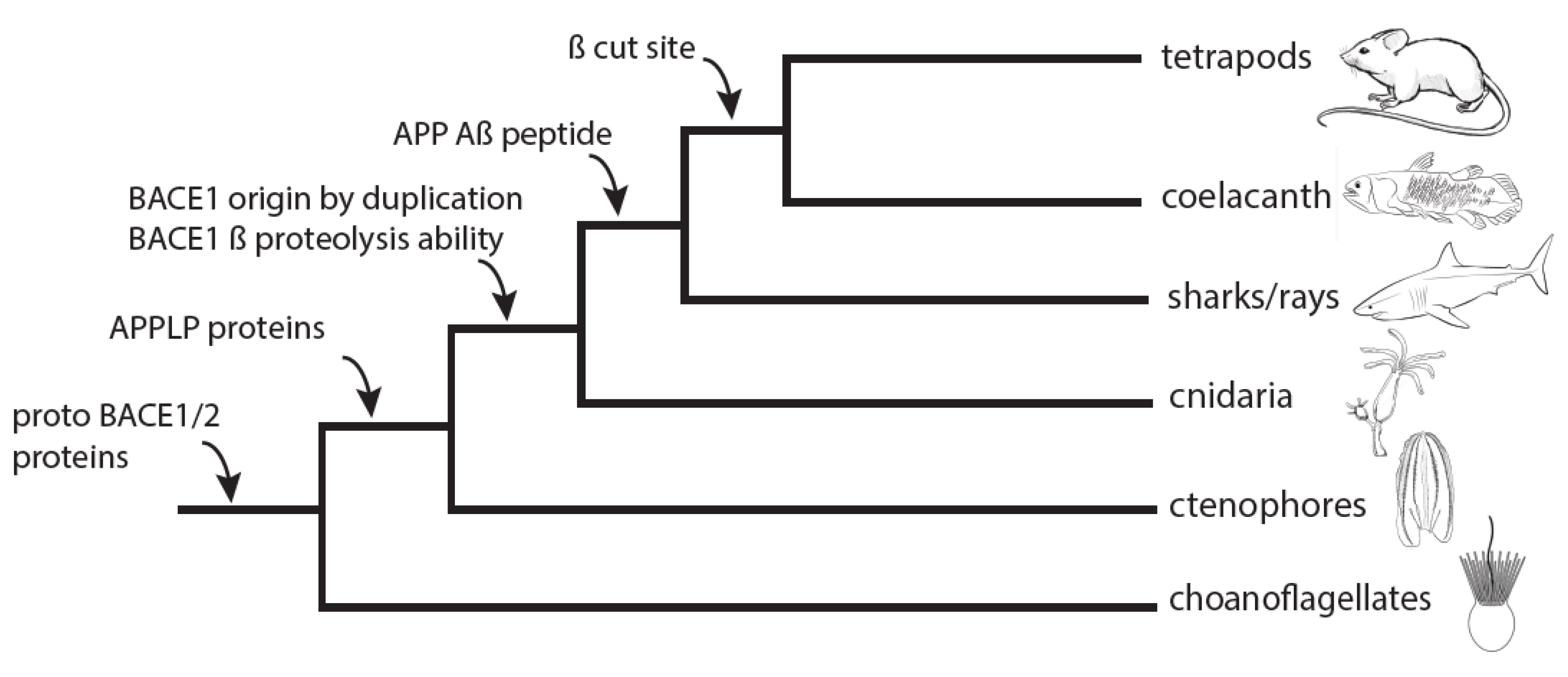
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics 2015, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzheimers Dis. 2010, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Li, G.; Zhang, Y.; Wu, Y.; Song, W. Modifications and Trafficking of APP in the Pathogenesis of Alzheimer’s Disease. Front. Mol. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.R.; Holler, C.J.; Webb, R.L.; Li, F.; Beckett, T.L.; Murphy, M.P. BACE1 and BACE2 Enzymatic Activities in Alzheimer’s Disease. J. Neurochem. 2010, 112, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.; Frigon, N.L.; Games, D.; Hu, K.; et al. BACE Knockout Mice Are Healthy despite Lacking the Primary β-Secretase Activity in Brain: Implications for Alzheimer’s Disease Therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hicks, C.W.; He, W.; Wong, P.; Macklin, W.B.; Trapp, B.D.; Yan, R. Bace1 Modulates Myelination in the Central and Peripheral Nervous System. Nat. Neurosci. 2006, 9, 1520–1525. [Google Scholar] [CrossRef]
- Laird, F.M.; Cai, H.; Savonenko, A.V.; Farah, M.H.; He, K.; Melnikova, T.; Wen, H.; Chiang, H.-C.; Xu, G.; Koliatsos, V.E.; et al. BACE1, a Major Determinant of Selective Vulnerability of the Brain to Amyloid-Beta Amyloidogenesis, Is Essential for Cognitive, Emotional, and Synaptic Functions. J. Neurosci. 2005, 25, 11693–11709. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; et al. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef]
- Wessels, A.M.; Tariot, P.N.; Zimmer, J.A.; Selzler, K.J.; Bragg, S.M.; Andersen, S.W.; Landry, J.; Krull, J.H.; Downing, A.M.; Willis, B.A.; et al. Efficacy and Safety of Lanabecestat for Treatment of Early and Mild Alzheimer Disease: The AMARANTH and DAYBREAK-ALZ Randomized Clinical Trials. JAMA Neurol. 2020, 77, 199–209. [Google Scholar] [CrossRef]
- Moore, D.B.; Gillentine, M.A.; Botezatu, N.M.; Wilson, K.A.; Benson, A.E.; Langeland, J.A. Asynchronous Evolutionary Origins of Aβ and BACE1. Mol. Biol. Evol. 2014, 31, 696–702. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Rokas, A. Embracing Uncertainty in Reconstructing Early Animal Evolution. Curr. Biol. 2017, 27, R1081–R1088. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Braun, E.L. Phylogenetic Analyses of Sites in Different Protein Structural Environments Result in Distinct Placements of the Metazoan Root. Biology 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, X.-X.; Evans, B.; Dunn, C.W.; Rokas, A. Rooting the Animal Tree of Life. Mol. Biol. Evol. 2021, 38, 4322–4333. [Google Scholar] [CrossRef] [PubMed]
- Ros-Rocher, N.; Pérez-Posada, A.; Leger, M.M.; Ruiz-Trillo, I. The Origin of Animals: An Ancestral Reconstruction of the Unicellular-to-Multicellular Transition. Open Biol. 2021, 11, 200359. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Balan, A.G.; Myers, B.J.; Maganti, J.L.; Moore, D.B. ER-Targeted Bcl-2 and Inhibition of ER-Associated Caspase-12 Rescue Cultured Immortalized Cells from Ethanol Toxicity. Alcohol 2010, 44, 553–563. [Google Scholar] [CrossRef]
- Willem, M.; Garratt, A.N.; Novak, B.; Citron, M.; Kaufmann, S.; Rittger, A.; DeStrooper, B.; Saftig, P.; Birchmeier, C.; Haass, C. Control of Peripheral Nerve Myelination by the Beta-Secretase BACE1. Science 2006, 314, 664–666. [Google Scholar] [CrossRef]
- Zhu, K.; Xiang, X.; Filser, S.; Marinković, P.; Dorostkar, M.M.; Crux, S.; Neumann, U.; Shimshek, D.R.; Rammes, G.; Haass, C.; et al. Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibition Impairs Synaptic Plasticity via Seizure Protein 6. Biol. Psychiatry 2018, 83, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Barão, S.; Laga, M.; Bockstael, K.; Borgers, M.; Gijsen, H.; Annaert, W.; Moechars, D.; Mercken, M.; Gevaert, K.; et al. The Neural Cell Adhesion Molecules L1 and CHL1 Are Cleaved by BACE1 Protease in Vivo. J. Biol. Chem. 2012, 287, 25927–25940. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.D.; Babu-Khan, S.; Loeloff, R.; Louis, J.C.; Curran, E.; Citron, M.; Vassar, R. Expression Analysis of BACE2 in Brain and Peripheral Tissues. J. Biol. Chem. 2000, 275, 20647–20651. [Google Scholar] [CrossRef] [PubMed]
- Yan, R. Physiological Functions of the β-Site Amyloid Precursor Protein Cleaving Enzyme 1 and 2. Front. Mol. Neurosci. 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, G.; Song, W. BACE2, as a Novel APP Theta-Secretase, Is Not Responsible for the Pathogenesis of Alzheimer’s Disease in Down Syndrome. FASEB J. 2006, 20, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hay, S.O.; Sahara, T.; McBride, M.; Kang, D.; Leissring, M.A. Identification of BACE2 as an Avid SS-Amyloid-Degrading Protease. Mol. Neurodegener. 2012, 7, 46. [Google Scholar] [CrossRef]
- Jutkowitz, E.; Kane, R.L.; Gaugler, J.E.; MacLehose, R.F.; Dowd, B.; Kuntz, K.M. Societal and Family Lifetime Cost of Dementia: Implications for Policy. J. Am. Geriatr. Soc. 2017, 65, 2169–2175. [Google Scholar] [CrossRef]
- Xu, J.; Murphy, S.L.; Kockanek, K.D.; Arias, E. Mortality in the United States, 2018. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langeland, J.A.; Baumann, L.; DeYoung, E.M.; Varella, R.A.; Mwenda, N.; Aguirre, A.; Moore, D.B. Early Animal Origin of BACE1 APP/Aβ Proteolytic Function. Biology 2024, 13, 320. https://doi.org/10.3390/biology13050320
Langeland JA, Baumann L, DeYoung EM, Varella RA, Mwenda N, Aguirre A, Moore DB. Early Animal Origin of BACE1 APP/Aβ Proteolytic Function. Biology. 2024; 13(5):320. https://doi.org/10.3390/biology13050320
Chicago/Turabian StyleLangeland, James A., Lillian Baumann, Eva M. DeYoung, Raphaela Angelina Varella, Nkatha Mwenda, Alejandro Aguirre, and D. Blaine Moore. 2024. "Early Animal Origin of BACE1 APP/Aβ Proteolytic Function" Biology 13, no. 5: 320. https://doi.org/10.3390/biology13050320
APA StyleLangeland, J. A., Baumann, L., DeYoung, E. M., Varella, R. A., Mwenda, N., Aguirre, A., & Moore, D. B. (2024). Early Animal Origin of BACE1 APP/Aβ Proteolytic Function. Biology, 13(5), 320. https://doi.org/10.3390/biology13050320





