Lysophosphatidylcholine Acetyltransferase 2 (LPCAT2) Influences the Gene Expression of the Lipopolysaccharide Receptor Complex in Infected RAW264.7 Macrophages, Depending on the E. coli Lipopolysaccharide Serotype
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture
2.2. Gene Manipulation
2.3. Bacterial Infection
2.4. RNA Purification and Quantification
2.5. Primer Design
2.6. Reverse Transcriptase-Real Time Quantitative Polymerase Chain Reaction
2.7. Relative Quantitation and Statistical Analysis
3. Results
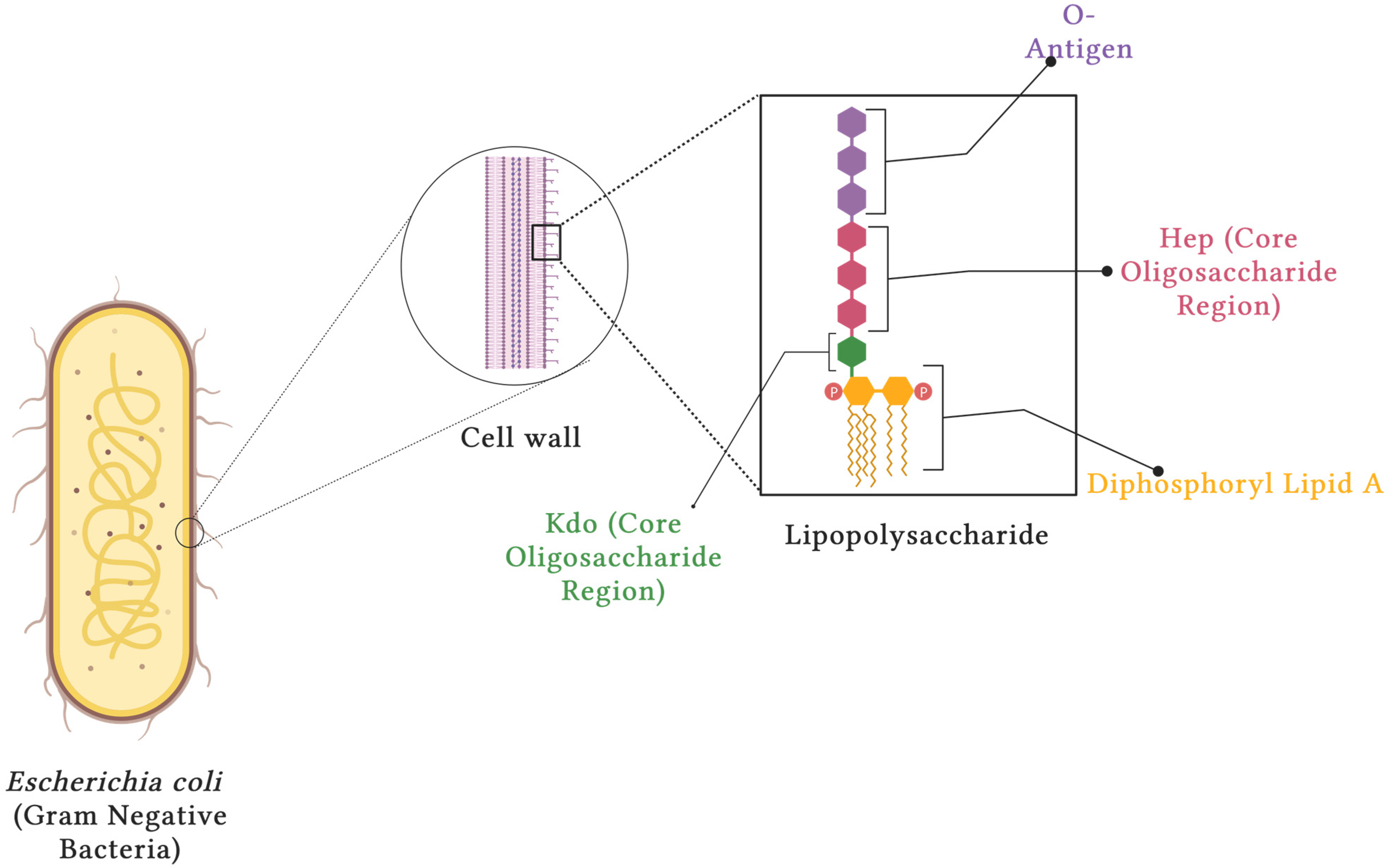
3.1. Structure of LPS
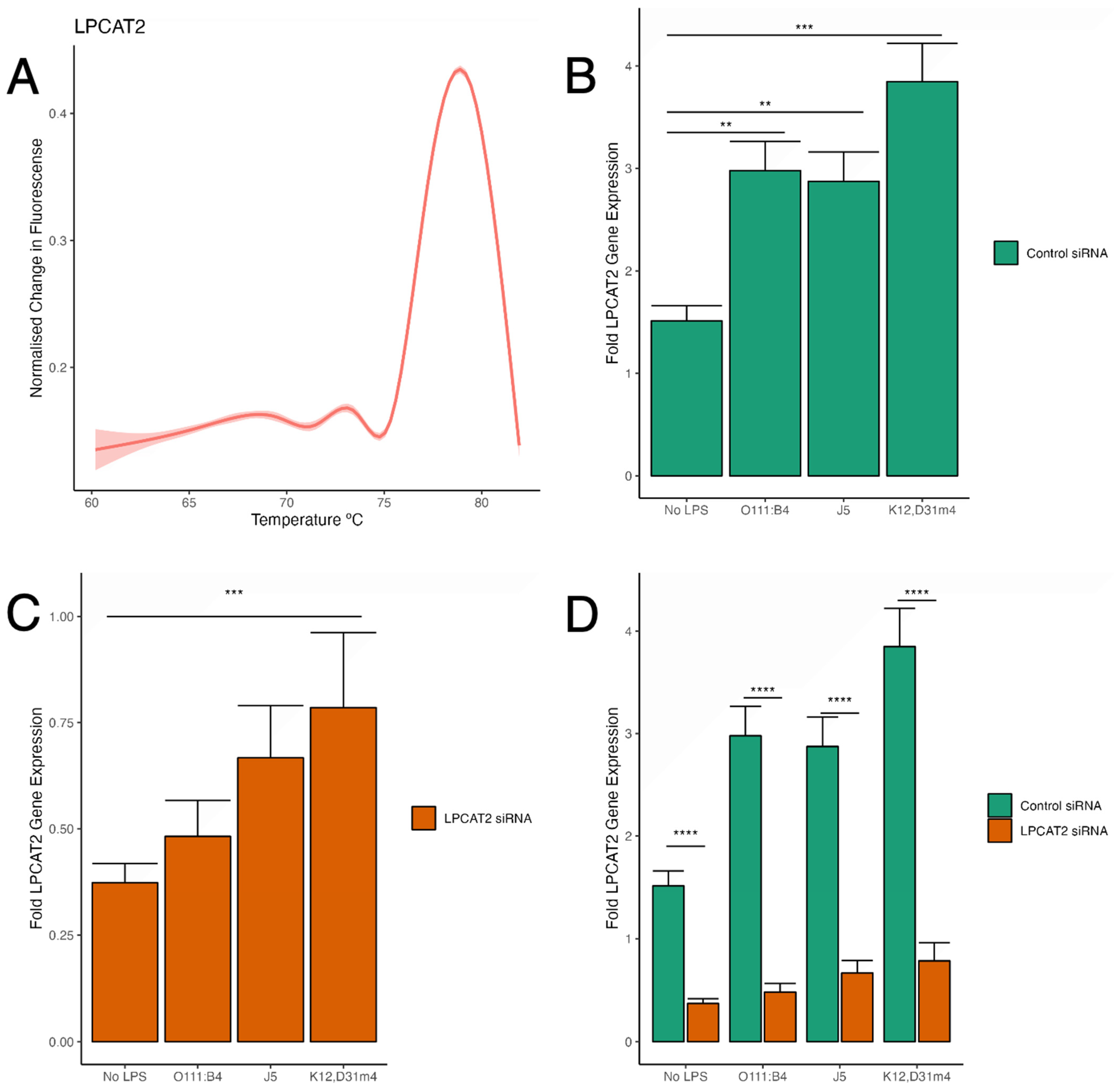
3.2. Analysis of LPCAT2 Transcription after Gene Silencing in Both Infected and Non-Infected RAW264.7 Macrophages
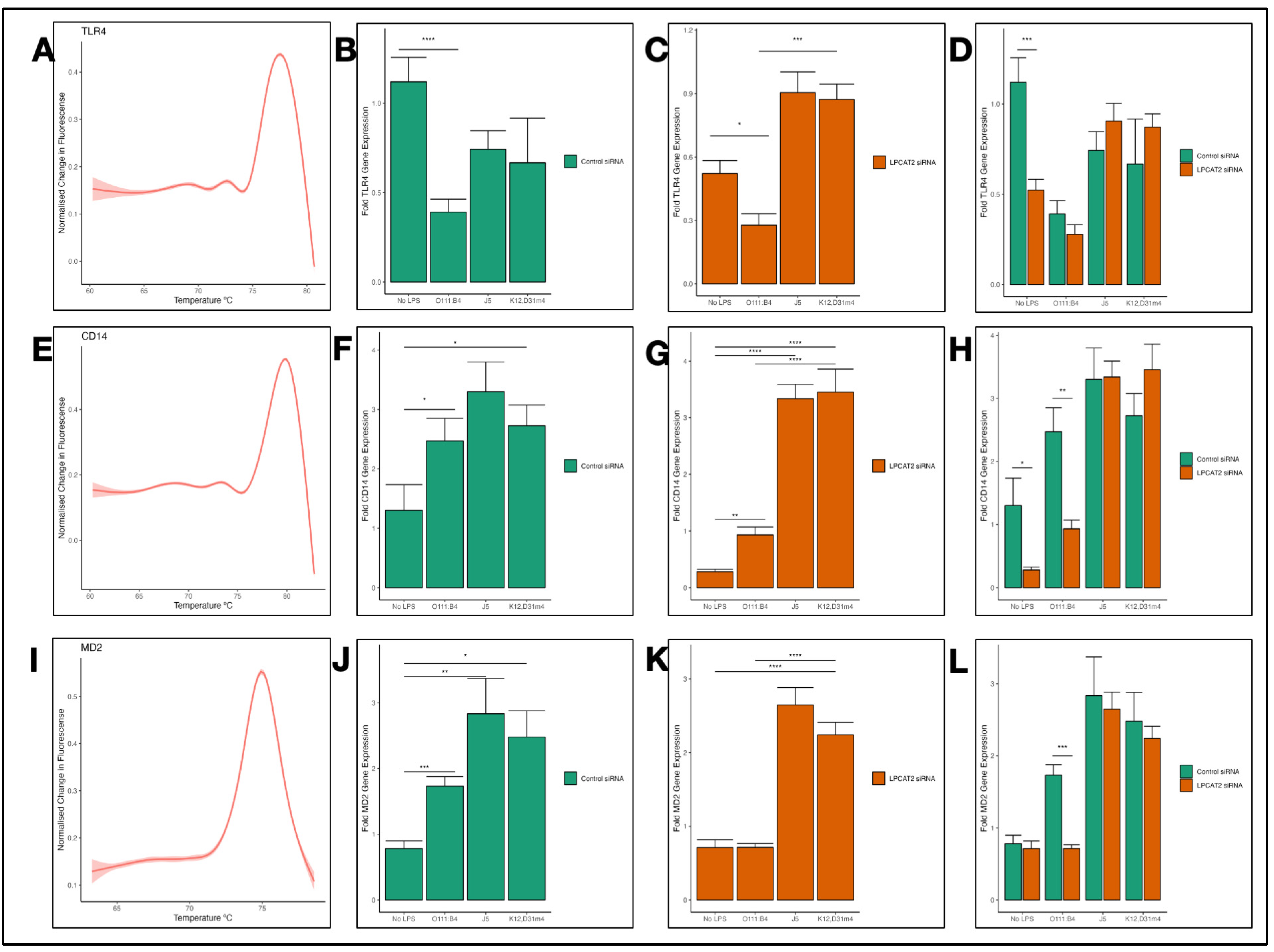
3.3. Differential Transcriptional Effect of Silencing LPCAT2 Gene on TLR4, CD14, and MD2 Gene Expression, Depending on LPS Serotype
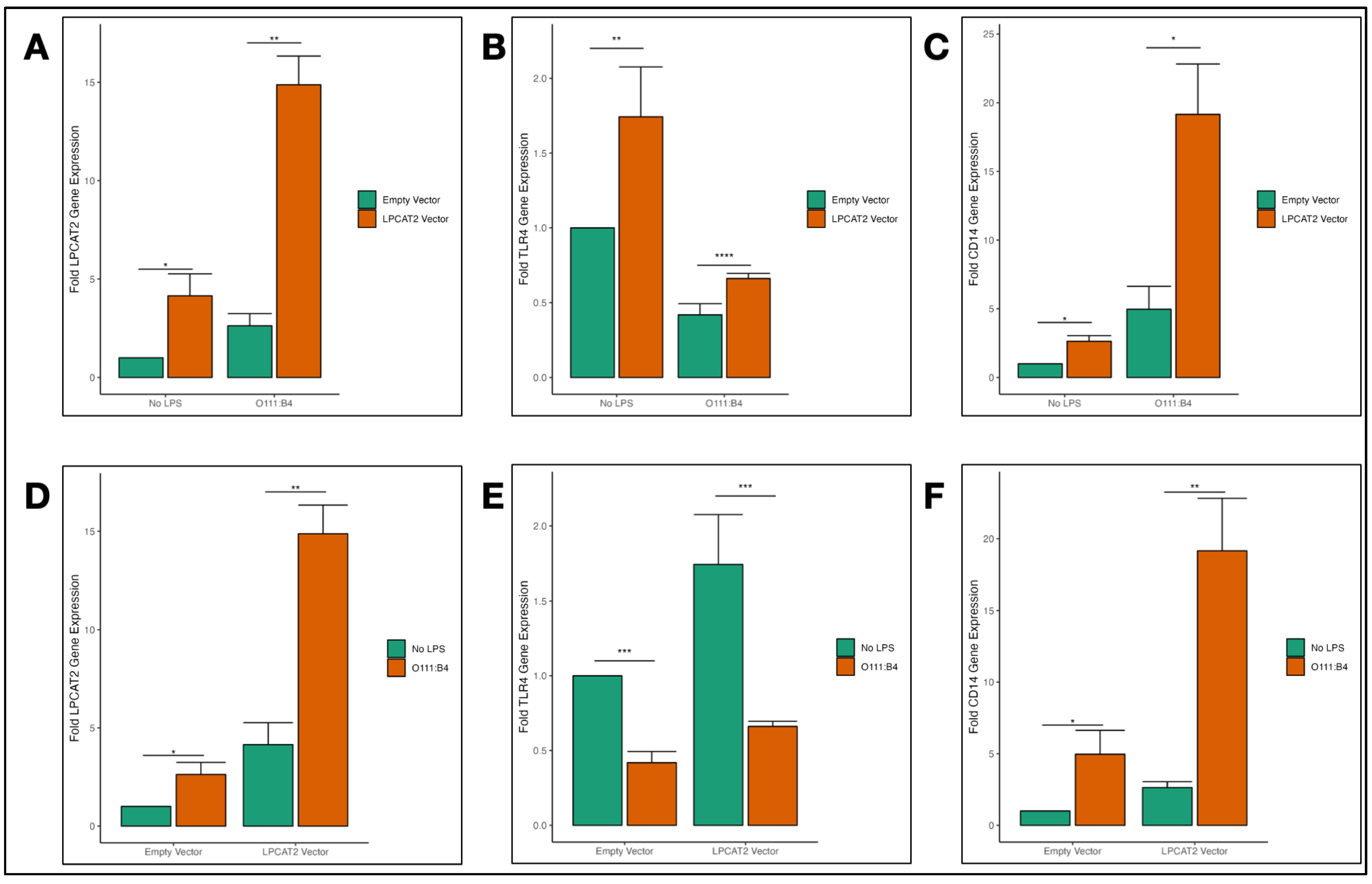
3.4. Overexpression of LPCAT2 in RAW264.7 Macrophages and Transcriptional Effect on TLR4 and CD14 Gene Expression
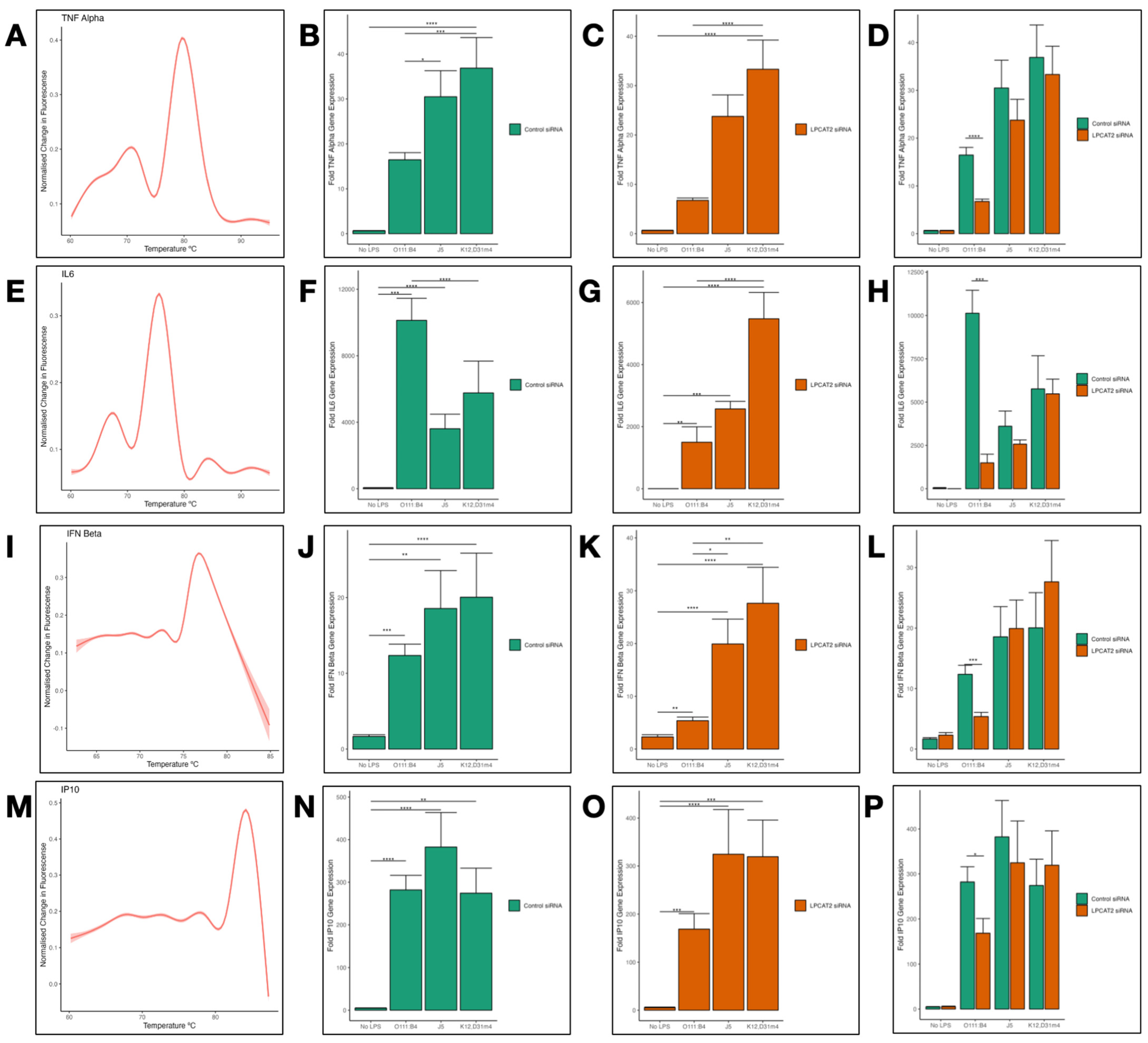
3.5. Differential Transcriptional Effect of Silencing LPCAT2 Gene on Tumor Necrosis Factor (TNFα), Interleukin 6 (IL6), Interferon Beta (IFNß), and Interferon Gamma-Inducible Protein 10 (IP10) Gene Expression, Depending on LPS Serotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taye, Z.W.; Abebil, Y.A.; Akalu, T.Y.; Tessema, G.M.; Taye, E.B. Incidence and determinants of nosocomial infection among hospital admitted adult chronic disease patients in University of Gondar Comprehensive Specialized Hospital, North–West Ethiopia, 2016–2020. Front. Public Health 2023, 11, 1087407. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Tainter, R.C. Escherichia coli Infection. 13 July 2023; [Online]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564298/ (accessed on 28 March 2024).
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, I.; Vanderleyden, J. O-antigen structural variation: Mechanisms and possible roles in animal/plant–microbe interactions. FEMS Microbiol. Rev. 2002, 26, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Somerville, J.E., Jr.; Cassiano, L.; Bainbridge, B.; Cunningham, M.D.; Darveau, R.P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Investig. 1996, 97, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pulido, D.; Garcia-Mayoral, M.F.; Moussaoui, M.; Velazquez, D.; Torrent, M.; Bruix, M.; Boix, E. Structural basis for endotoxin neutralization by the eosinophil cationic protein. FEBS J. 2016, 283, 4176–4191. [Google Scholar] [CrossRef] [PubMed]
- Qimron, U.; Marintcheva, B.; Tabor, S.; Richardson, C.C. Genomewide screens for Escherichia coli genes affecting growth of T7 bacteriophage. Proc. Natl. Acad. Sci. USA 2006, 103, 19039–19044. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.E.; Yethon, J.A.; Whitfield, C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 1998, 30, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2009, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Fu, T.-M.; Li, J.; Wu, H. Structural Biology of Innate Immunity. Annu. Rev. Immunol. 2015, 33, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Ingolfsson, H.I.; Melo, M.N.; van Eerden, F.J.; Arnarez, C.; Lopez, C.A.; Wassenaar, T.A.; Periole, X.; de Vries, A.H.; Tieleman, P.D.; Marrink, S.J. Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Robert, E. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Rajendran, L.; Simons, K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005, 118, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Abate, W.; Alrammah, H.; Kiernan, M.; Tonks, A.J.; Jackson, S.K. Lysophosphatidylcholine acyltransferase 2 (LPCAT2) co-localises with TLR4 and regulates macrophage inflammatory gene expression in response to LPS. Sci. Rep. 2020, 10, 10355. [Google Scholar] [CrossRef] [PubMed]
- Poloamina, V.I.; Abate, W.; Fejer, G.; Jackson, S.K. Possible regulation of Toll-like receptor 4 by lysine acetylation through LPCAT2 activity in RAW264.7 cells. Biosci. Rep. 2022, 42, BSR20220251. [Google Scholar] [CrossRef] [PubMed]
- Tarui, M.; Shindou, H.; Kumagai, K.; Nagano, T.; Nagase, T.; Shimizu, T. Selective inhibitors of a PAF biosynthetic enzyme lysophosphatidylcholine acyltransferase 2. J. Lipid Res. 2014, 55, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Raschke, W.C.; Baird, S.; Ralph, P.; Nakoinz, I. Functional macrophage cell lines transformed by abelson leukemia virus. Cell 1978, 15, 261–267. [Google Scholar] [CrossRef]
- Rutledge, H.R.; Jiang, W.; Yang, J.; Warg, L.A.; Schwartz, D.A.; Pisetsky, D.S.; Yang, V.I. Gene expression profiles of RAW264.7 macrophages stimulated with preparations of LPS differing in isolation and purity. Innate Immun. 2011, 18, 80–88. [Google Scholar] [CrossRef]
- Hambleton, J.; Weinstein, S.L.; Lem, L.; DeFranco, A.L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Zipper, H.; Brunner, H.; Bernhagen, J.; Vitzthum, F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004, 32, e103. [Google Scholar] [CrossRef]
- Keyserling, H.V.; Bergmann, T.; Wiesel, M.; Kaufmann, A.M. The use of melting curves as a novel approach for validation of real-time PCR instruments. BioTechniques 2011, 51, 179–184. [Google Scholar] [CrossRef]
- Downey, N. Integrated DNA Technologies. 2 February 2023. [Online]. Available online: https://eu.idtdna.com/pages/education/decoded/article/interpreting-melt-curves-an-indicator-not-a-diagnosis (accessed on 28 March 2024).
- Morimoto, R.; Shindou, H.; Oda, Y.; Shimizu, T. Phosphorylation of Lysophosphatidylcholine Acyltransferase 2 at Ser34 Enhances Platelet-activating Factor Production in Endotoxin-stimulated Macrophages. J. Biol. Chem. 2010, 285, 29857–29862. [Google Scholar] [CrossRef]
- Murano, H.; Inoue, S.; Miyazake, O.; Hanawa, T.; Minegishi, Y.; Sato, K.; Kobayashi, M.; Sato, M.; Nemoto, T.; Nishiwaki, M.; et al. Lysophosphatidylcholine Acyltransferase 2 Promotes Cigarette Smoke Induced Emphysema Via Platelet-activating Factor. ATS J. 2023, 207, A5721. [Google Scholar]
- Watanabe, H.; Miyake, K.; Matsuoka, T.; Kojima, R.; Sakurai, D.; Masuyama, K.; Yamagata, Z. LPCAT2 Methylation, a Novel Biomarker for the Severity of Cedar Pollen Allergic Rhinitis in Japan. Am. J. Rhinol. Allergy 2020, 35, 631–639. [Google Scholar] [CrossRef]
- Ma, X.; Chen, L.; He, Y.; Zhao, L.; Yu, W.; Xie, Y.; Yu, Y.; Yu, X.; Zheng, Y.; Li, R.; et al. Targeted lipidomics reveals phospholipids and lysophospholipids as biomarkers for evaluating community-acquired pneumonia. Ann. Transl. Med. 2022, 10, 395. [Google Scholar] [CrossRef]
- Long, N.P.; Anh, N.K.; Yen, N.T.H.; Phat, N.K.; Park, S.; Thu, V.T.A.; Cho, Y.; Shin, J.; Oh, J.Y.; Kim, D.H. Comprehensive lipid and lipid-related gene investigations of host immune responses to characterize metabolism-centric biomarkers for pulmonary tuberculosis. Sci. Rep. 2022, 12, 13395. [Google Scholar] [CrossRef]
- Fujihara, M.; Muroi, M.; Tanamoto, K.-I.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef]
- Zanoni, I.; Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef]
- Visintin, A.; Iliev, D.B.; Monks, B.G.; Halmen, K.A.; Golenblock, D.T. MD-2. Immunobiology 2006, 211, 437–447. [Google Scholar] [CrossRef]
- Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Huber, M.; Kalis, C.; Keck, S.; Galanos, C.; et al. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 2005, 6, 565–570. [Google Scholar] [CrossRef]
- Haugen, T.S.; Nakstad, B.; Skjonsberg, O.H.; Lyberg, T. CD14 Expression and Binding of Lipopolysaccharide to Alveolar Macrophages and Monocytes. Inflammation 1998, 22, 521–532. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Musikacharoen, T.; Ogawa, T.; Yoshikai, Y. Gene Expressions of Toll-Like Receptor 2, But Not Toll-Like Receptor 4, Is Induced by LPS and Inflammatory Cytokines in Mouse Macrophages. J. Immunol. 2000, 165, 5767–5772. [Google Scholar] [CrossRef]
- Ren, W.; Hu, L.; Hua, F.; Jin, J.; Wang, Y.; Zhu, L. Myeloid differentiation protein 2 silencing decreases LPS-induced cytokine production and TLR4/MyD88 pathway activity in alveolar macrophages. Immunol. Lett. 2011, 141, 94–101. [Google Scholar] [CrossRef]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef]
- Zhao, J.; He, D.; Su, Y.; Berdyshev, E.; Chun, J.; Natarajan, V.; Zhao, Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Lung Cell. Mol. Physiol. 2011, 301, L547–L556. [Google Scholar] [CrossRef]
- Lauener, R.P.; Goyert, S.M.; Geha, S.R.; Vercelli, D. Interleukin 4 down-regulates the expression of CD14 in normal human monocytes. Eur. J. Immunol. 1990, 20, 2375–2381. [Google Scholar] [CrossRef]
- Abreu, M.T.; Vora, P.; Faure, E.; Thomas, L.S.; Arnold, E.T.; Arditi, M. Decreased Expression of Toll-Like Receptor-4 and MD-2 Correlates with Intestinal Epithelial Cell Protection Against Dysregulated Proinflammatory Gene Expression in Response to Bacterial Lipopolysaccharide. J. Immunol. 2001, 167, 1609–1616. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptor downstream signaling. Arthritis Res. Ther. 2004, 7, 12–19. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science 2001, 301, 640–643. [Google Scholar] [CrossRef]
- Gohda, J.; Matsumura, T.; Inoue, J.-i. TNFR-Associated Factor (TRAF) 6 Is Essential for MyD88-Dependent Pathway but Not Toll/IL-1 Receptor Domain-Containing Adaptor-Inducing IFN-β (TRIF)-Dependent Pathway in TLR Signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef]
- Rittig, M.G.; Kaufmann, A.; Robins, A.; Shaw, B.; Sprenger, H.; Gemsa, D.; Foulongne, V.; Rouot, B.; Dornand, J. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J. Leukoc. Biol. 2003, 74, 1045–1055. [Google Scholar] [CrossRef]
- Kelly, N.M.; Young, L.; Cross, A.S. Differential induction of tumor necrosis factor by bacteria expressing rough and smooth lipopolysaccharide phenotypes. Infect. Immun. 1991, 59, 4491–4496. [Google Scholar] [CrossRef]
- Duenas, A.I.; Orduna, A.; Crespo, M.S.; Garcia-Rodriguez, C. Interaction of endotoxins with Toll-like receptor 4 correlates with their endotoxic potential and may explain the proinflammatory effect of Brucella spp. LPS. Int. Immunol. 2004, 16, 1467–1475. [Google Scholar] [CrossRef]
- Zanoni, I.; Bodio, C.; Broggi, A.; Ostuni, R.; Caccia, M.; Collini, M.; Venkatesh, A.; Spreafico, R.; Capuano, G.; Granucci, F. Similarities and differences of innate immune responses elicited by smooth and rough LPS. Immunol. Lett. 2011, 142, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pupo, E.; Lindner, B.; Brade, H.; Schromm, A.B. Intact rough- and smooth-form lipopolysaccharides from Escherichia coli separated by preparative gel electrophoresis exhibit differential biologic activity in human macrophages. FEBS J. 2012, 280, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Triantafilou, K.; Fernandez, N. Rough and smooth forms of fluorescein-labelled bacterial endotoxin exhibit CD14/LBP dependent and independent binding that is influenced by endotoxin concentration. Eur. J. Biochem. 2000, 267, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Toll-like Receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Turse, J.E.; Ficht, T.A. Evidence of Brucella abortus OPS dictating uptake and restricting NF-κB activation in murine macrophages. Microbes Infect. 2008, 10, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Lopez-Enriquez, S.; Martin, M.E.; Montserrat-de la Paz, S. High-density lipoproteins and immune response: A review. Int. J. Biol. Macromol. 2022, 195, 117–123. [Google Scholar] [CrossRef]
- Hamann, L.; Alexander, C.; Stamme, C.; Zahringer, U.; Schumann, R.R. Acute-Phase Concentrations of Lipopolysaccharide (LPS)-Binding Protein Inhibit Innate Immune Cell Activation by Different LPS Chemotypes via Different Mechanisms. Infect. Immun. 2005, 73, 193–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poloamina, V.I.; Alrammah, H.; Abate, W.; Avent, N.D.; Fejer, G.; Jackson, S.K. Lysophosphatidylcholine Acetyltransferase 2 (LPCAT2) Influences the Gene Expression of the Lipopolysaccharide Receptor Complex in Infected RAW264.7 Macrophages, Depending on the E. coli Lipopolysaccharide Serotype. Biology 2024, 13, 314. https://doi.org/10.3390/biology13050314
Poloamina VI, Alrammah H, Abate W, Avent ND, Fejer G, Jackson SK. Lysophosphatidylcholine Acetyltransferase 2 (LPCAT2) Influences the Gene Expression of the Lipopolysaccharide Receptor Complex in Infected RAW264.7 Macrophages, Depending on the E. coli Lipopolysaccharide Serotype. Biology. 2024; 13(5):314. https://doi.org/10.3390/biology13050314
Chicago/Turabian StylePoloamina, Victory Ibigo, Hanaa Alrammah, Wondwossen Abate, Neil D. Avent, Gyorgy Fejer, and Simon K. Jackson. 2024. "Lysophosphatidylcholine Acetyltransferase 2 (LPCAT2) Influences the Gene Expression of the Lipopolysaccharide Receptor Complex in Infected RAW264.7 Macrophages, Depending on the E. coli Lipopolysaccharide Serotype" Biology 13, no. 5: 314. https://doi.org/10.3390/biology13050314
APA StylePoloamina, V. I., Alrammah, H., Abate, W., Avent, N. D., Fejer, G., & Jackson, S. K. (2024). Lysophosphatidylcholine Acetyltransferase 2 (LPCAT2) Influences the Gene Expression of the Lipopolysaccharide Receptor Complex in Infected RAW264.7 Macrophages, Depending on the E. coli Lipopolysaccharide Serotype. Biology, 13(5), 314. https://doi.org/10.3390/biology13050314




