Correction: Satam et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997
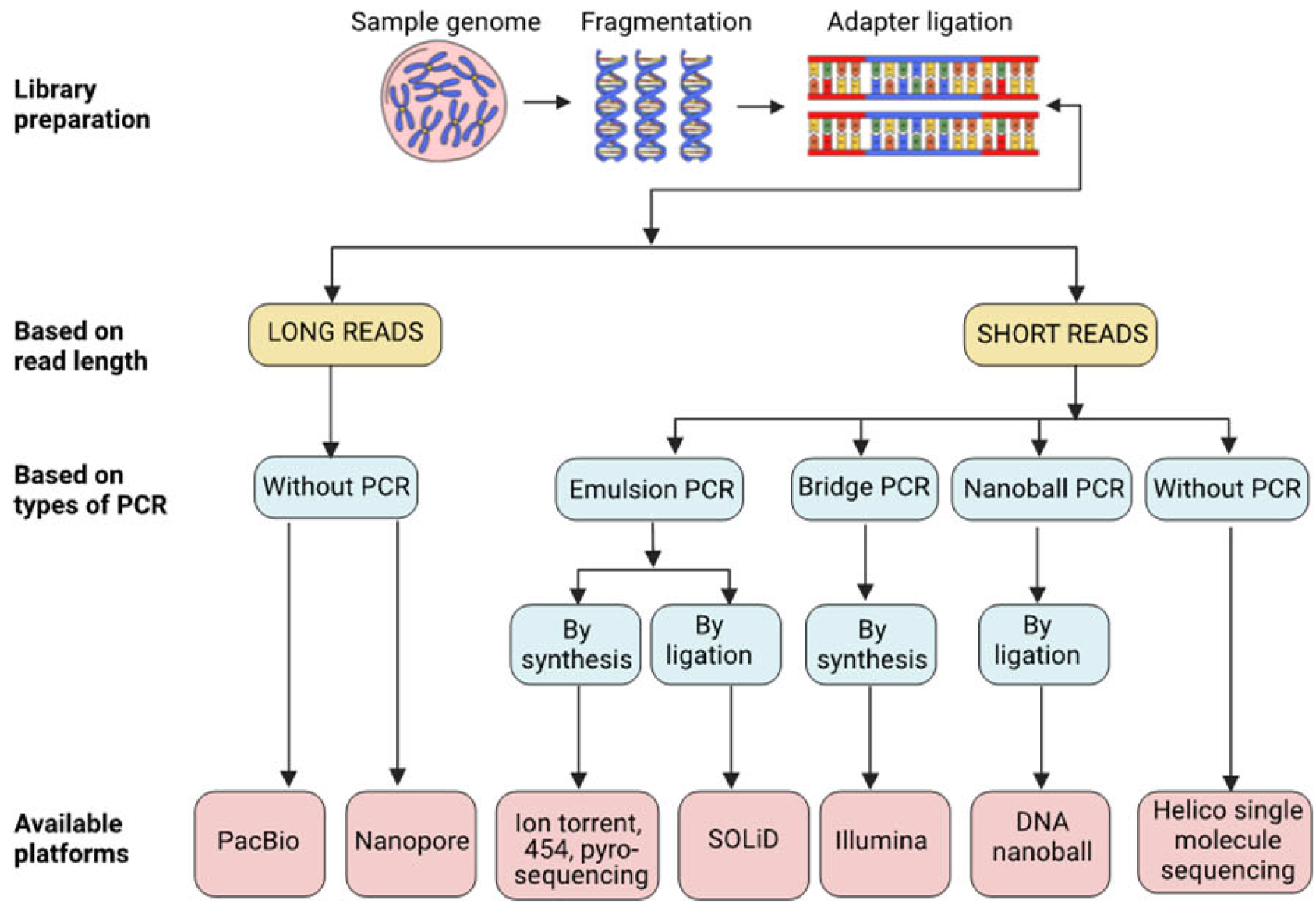
| Sr No. | Platform | Use | Sequencing Technology | Amplification Type | Principle | Read Length (bp) | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 454 pyrosequencing | Short read sequencing | Seq by synthesis | Emulsion PCR | Detection of pyrophosphate released during nucleotide incorporation. | 400–1000 | May contain deletion and insertion sequencing errors due to inefficient determination of homopolymer length. | [18–20] |
| 2 | Ion Torrent | Short read sequencing | Seq by synthesis | Emulsion PCR | Ion semiconductor sequencing principle detecting H+ ion generated during nucleotide incorporation. | 200–400 | When homopolymer sequences are sequenced, it may lead to loss in signal strength. | [19–21] |
| 3 | Illumina | Short read sequencing | Seq by synthesis | Bridge PCR | Solid-phase sequencing on immobilized surface leveraging clonal array formation using proprietary reversible terminator technology for rapid and accurate large-scale sequencing using single labeled dNTPs, which is added to the nucleic acid chain. | 36–300 | In case of sample overloading, the sequencing may result in overcrowding or overlapping signals, thus spiking the error rate up to 1%. | [19,20,22] |
| 4 | SOLiD | Short read sequencing | Seq by ligation | Emulsion PCR | An enzymatic method of sequencing using DNA ligase. 8-Mer probes with a hydroxyl group at 3′ end and a fluorescent tag (unique to each base A, T, G, C) at 5′ end are used in ligation reaction. | 75 | This platform displays substitution errors and may also under-represent GC-rich regions. Their short reads also limit their wider applications. | [20,23] |
| 5 | DNA nanoball sequencing | Short read sequencing | Seq by ligation | Amplification by Nanoball PCR | Splint oligo hybridization with post-PCR amplicon from libraries helps in the formation of circles. This circular ssDNA acts as the DNA template to generate a long string of DNA that self-assembles into a tight DNA nanoball. These are added to the aminosilane (positively charged)-coated flow cell to allow patterned binding of the DNA nanoballs. The fluorescently tagged bases are incorporated into the DNA strand, and the release of the fluorescent tag is captured using imaging techniques. | 50–150 | Multiple PCR cycles are needed with a more exhaustive workflow. This, combined with the output of short-read sequencing, can be a possible limitation. | [24,25] |
| 6 | Helicos single-molecule sequencing | Short-read sequencing | Seq by synthesis | Without Amplification | Poly-A-tailed short 100–200 bp fragmented genomic DNA is sequenced on poly-T oligo-coated flow cells using fluorescently labeled 4 dNTPS. The signal released upon adding each nucleotide is captured. | 35 | Highly sensitive instrumentation required. As the sequence length increases, the percentage of strands that can be utilized decreases. | [26,27] |
| 7 | PacBio Onso system | Short-read sequencing | Seq by binding | Optional PCR | Sequencing by binding (SBB) chemistry uses native nucleotides and scarless incorporation under optimized conditions for binding and extension (https://www.pacb.com/technology/sequencing-by-binding/, accessed on 1 July 2023). | 100–200 | The higher cost compared to other sequencing platforms. | |
| 8 | PacBio Single-molecule real-time sequencing (SMRT) technology | Long-read sequencing | Seq by synthesis | Without PCR | The SMRT sequencing employs SMRT Cell, housing numerous small wells known as zero-mode waveguides (ZMWs). Individual DNA molecules are immobilized within these wells, emitting light as the polymerase incorporates each nucleotide, allowing real-time measurement of nucleotide incorporation | average 10,000–25,000 | The higher cost compared to other sequencing platforms. | [28,29] |
| 9 | Nanopore DNA sequencing | Long-read sequencing | Sequence detection through electrical impedance | Without PCR | The method relies on the linearization of DNA or RNA molecules and their capability to move through a biological pore called “nanopores”, which are eight nanometers wide. Electrophoretic mobility allows the passage of linear nucleic acid strand, which in turn is capable of generating a current signal. | average 10,000–30,000 | The error rate can spike up to 15%, especially with low-complexity sequences. Compared to short-read sequencers, it has a lower read accuracy. | [5,19,30] |
Reference
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Correction: Satam et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. Biology 2024, 13, 286. https://doi.org/10.3390/biology13050286
Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, Thakare RP, Banday S, Mishra AK, Das G, et al. Correction: Satam et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. Biology. 2024; 13(5):286. https://doi.org/10.3390/biology13050286
Chicago/Turabian StyleSatam, Heena, Kandarp Joshi, Upasana Mangrolia, Sanober Waghoo, Gulnaz Zaidi, Shravani Rawool, Ritesh P. Thakare, Shahid Banday, Alok K. Mishra, Gautam Das, and et al. 2024. "Correction: Satam et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997" Biology 13, no. 5: 286. https://doi.org/10.3390/biology13050286
APA StyleSatam, H., Joshi, K., Mangrolia, U., Waghoo, S., Zaidi, G., Rawool, S., Thakare, R. P., Banday, S., Mishra, A. K., Das, G., & Malonia, S. K. (2024). Correction: Satam et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. Biology, 13(5), 286. https://doi.org/10.3390/biology13050286





