The TRPV6 Calcium Channel and Its Relationship with Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. General Function and Structure of TRPV6
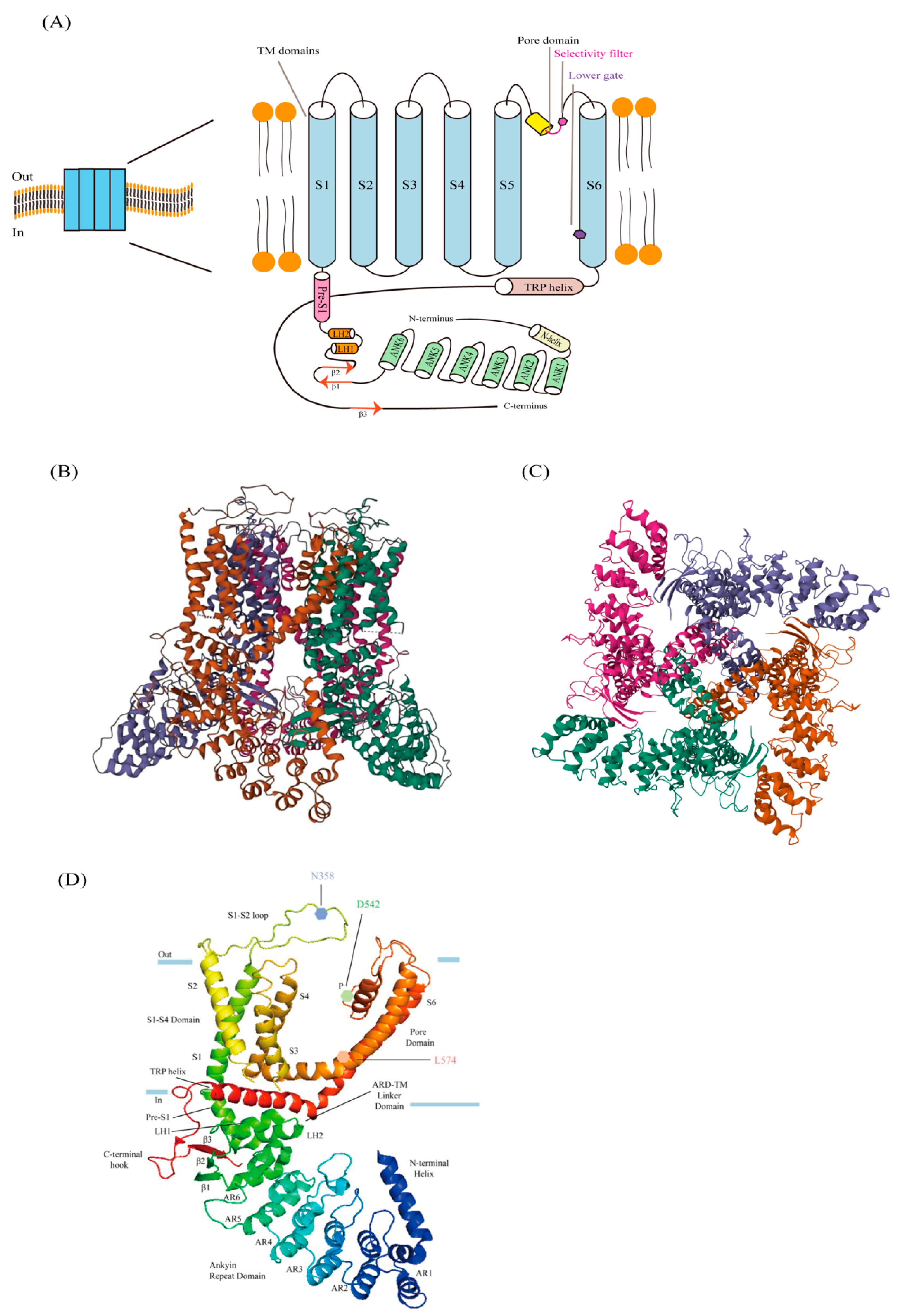
2.1. Structure of TRPV6
2.2. Ion-Conducting Pore
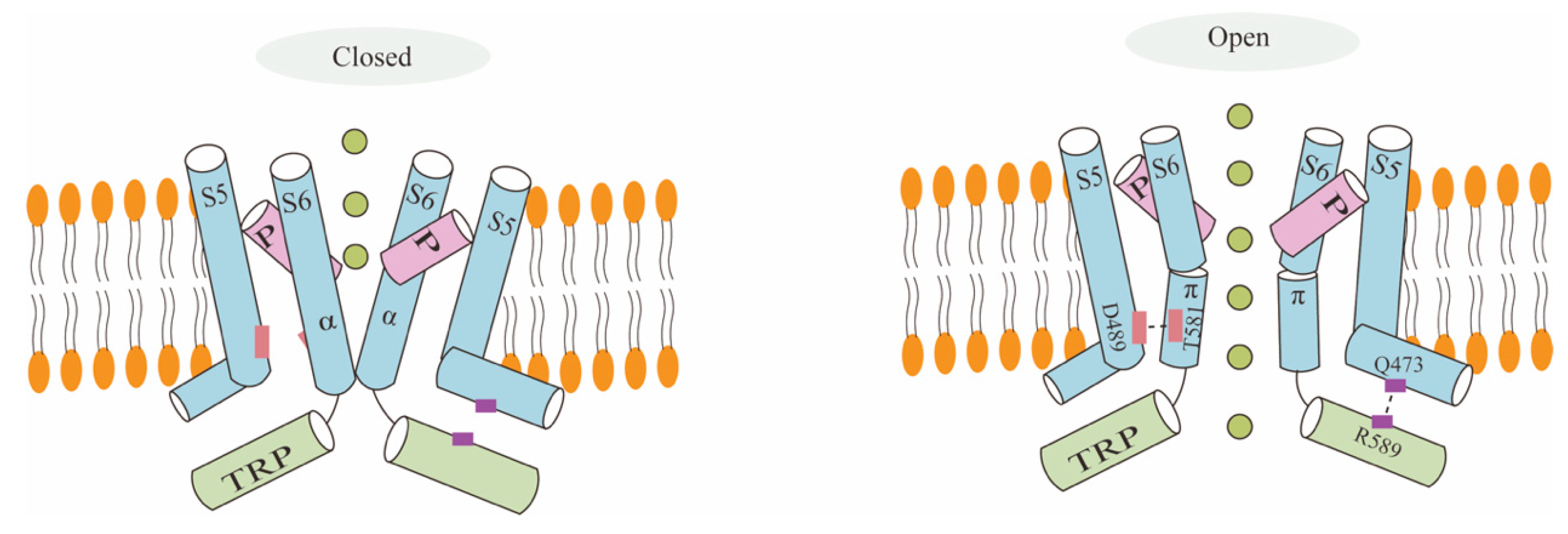
2.3. TRPV6 Channel Gating Mechanism
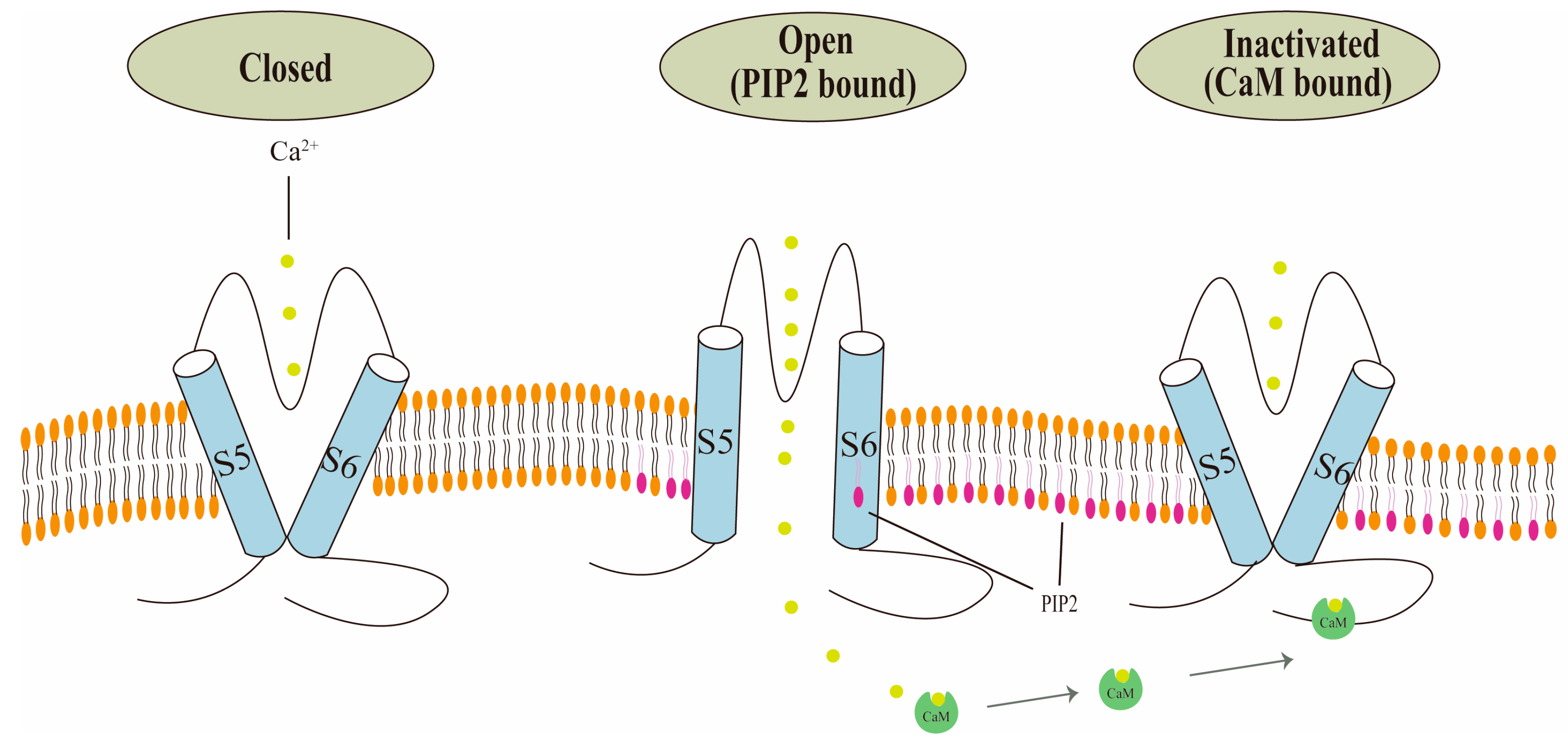
2.4. Regulation by Calmodulin (CaM) and Phosphatidylinositol 4,5-Bisphosphate (PIP2)
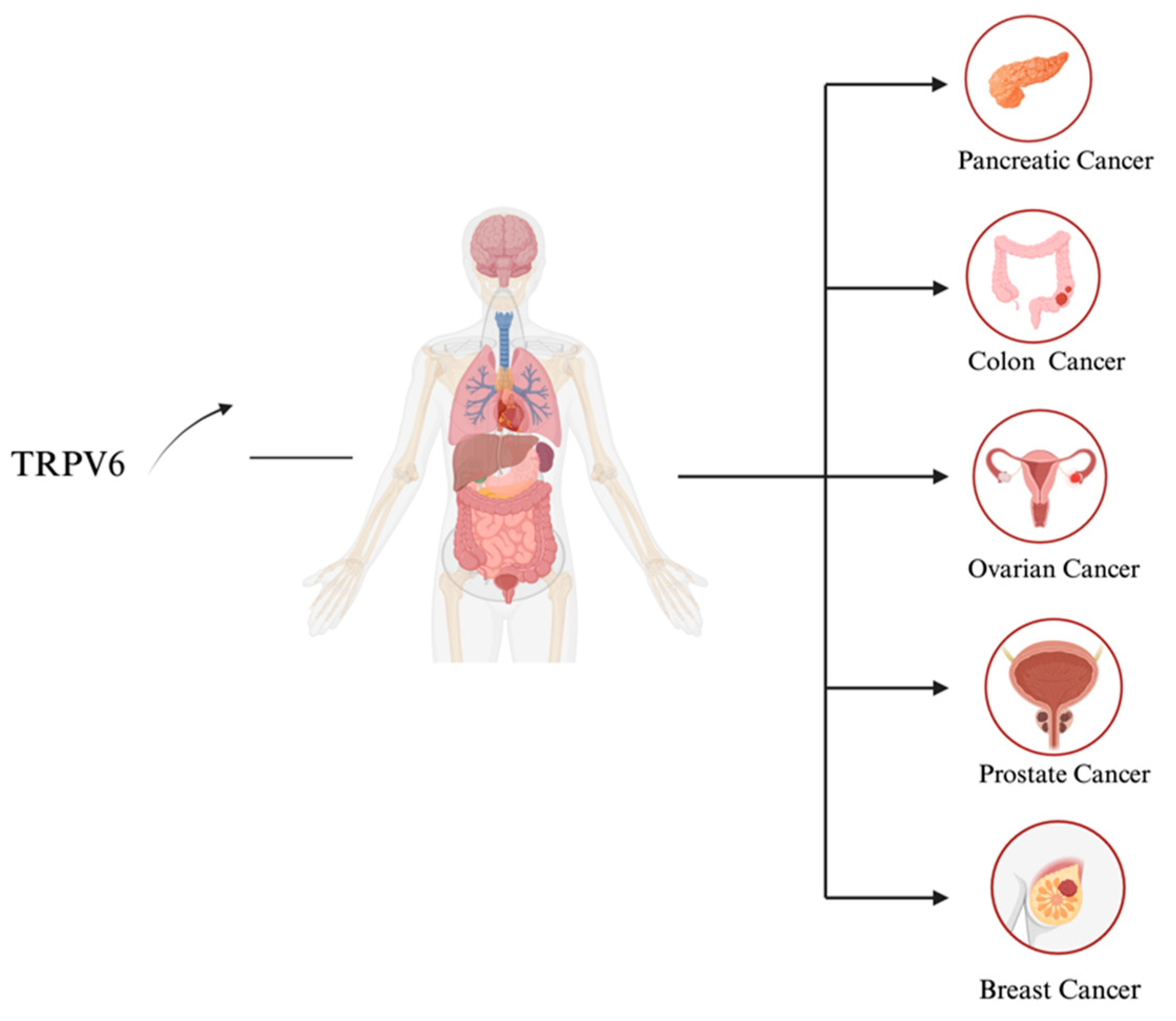
3. TRPV6 Expression in Cancer
3.1. Calcium and Cancer
3.2. TRPV6 as an Oncochannel and its Mechanism of Action
3.3. Prostate Cancer
3.4. Breast Cancer
3.5. Ovarian Cancer
3.6. Pancreatic Cancer
3.7. Colon Cancer
4. Mechanism of TRPV6 Channel Expression Control in Cancer
4.1. TRPV6 Transcription Control by Vitamin D3 Receptor (VDR)
4.2. TRPV6 Transcription Control by Androgen Receptor (AR)
4.3. TRPV6 Transcriptional Control by Estrogen Receptor
4.4. TRPV6 Transcriptional Control by PPARα
4.5. TRPV6 Transcriptional Control by Other Transcriptional Factors
5. Pharmacology of TRPV6
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montell, C.; Birnbaumer, L.; Flockerzi, V. The TRP channels, a remarkably functional family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Seebohm, G.; Schreiber, J.A. Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation. Cell. Physiol. Biochem. 2021, 55 (Suppl. S3), 108–130. [Google Scholar]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Nilius, B.; Vennekens, R.; Prenen, J.; Hoenderop, J.G.J.; Bindels, R.J.M.; Droogmans, G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J. Physiol. 2000, 527 Pt 2, 239–248. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Vennekens, R.; Müller, D.; Prenen, J.; Droogmans, G.; Bindels, R.J.; Nilius, B. Function and expression of the epithelial Ca(2+) channel family: Comparison of mammalian ECaC1 and 2. J. Physiol. 2001, 537 Pt 3, 747–761. [Google Scholar] [CrossRef]
- Brown, E.M. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 1991, 71, 371–411. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. 1), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Beggs, M.R.; Lee, J.J.; Busch, K.; Raza, A.; Dimke, H.; Weissgerber, P.; Engel, J.; Flockerzi, V.; Alexander, R.T. TRPV6 and Cav1.3 Mediate Distal Small Intestine Calcium Absorption Before Weaning. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Liu, X.; O’Neill, D.; Beggs, M.R.; Weissgerber, P.; Flockerzi, V.; Chen, X.Z.; Dimke, H.; Alexander, R.T. Activation of the calcium sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. J. Clin. Investig. Insight 2019, 5, e128013. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Fecher-Trost, C.; Weissgerber, P.; Wissenbach, U. TRPV6 channels. Handb. Exp. Pharmacol. 2014, 222, 359–384. [Google Scholar] [PubMed]
- Lehen’kyi, V.; Raphaël, M.; Prevarskaya, N. The role of the TRPV6 channel in cancer. J. Physiol. 2012, 590, 1369–1376. [Google Scholar] [CrossRef]
- Haustrate, A.; Mihalache, A.; Cordier, C.; Gosset, P.; Prevarskaya, N.; Lehen’kyi, V. A Novel Anti-TRPV6 Antibody and Its Application in Cancer Diagnosis In Vitro. Int. J. Mol. Sci. 2022, 24, 419. [Google Scholar] [CrossRef]
- McGoldrick, L.L.; Singh, A.K.; Saotome, K.; Yelshanskaya, M.V.; Twomey, E.C.; Grassucci, R.A.; Sobolevsky, A.I. Opening of the human epithelial calcium channel TRPV6. Nature 2018, 553, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Singh, A.K.; Yelshanskaya, M.V.; Sobolevsky, A.I. Crystal structure of the epithelial calcium channel TRPV6. Nature 2016, 534, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Yelshanskaya, M.V.; Nadezhdin, K.D.; Kurnikova, M.G.; Sobolevsky, A.I. Structure and function of the calcium-selective TRP channel TRPV6. J. Physiol. 2021, 599, 2673–2697. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Boros, S.; Chang, Q.; Bindels, R.J.; Hoenderop, J.G. The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol. Dial. Transplant. 2008, 23, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Khattar, V.; Wang, L.; Peng, J.B. Calcium selective channel TRPV6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef] [PubMed]
- Owsianik, G.; Talavera, K.; Voets, T.; Nilius, B. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 2006, 68, 685–717. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Janssens, A.; Droogmans, G.; Nilius, B. Outer pore architecture of a Ca2+-selective TRP channel. J. Biol. Chem. 2004, 279, 15223–15230. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Saotome, K.; Sobolevsky, A.I. Swapping of transmembrane domains in the epithelial calcium channel TRPV6. Sci. Rep. 2017, 7, 10669. [Google Scholar] [CrossRef]
- Peng, J.B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.A. TRPV5 and TRPV6 Calcium-Selective Channels. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; Chapter 13. [Google Scholar]
- Cai, R.; Liu, X.; Zhang, R.; Hofmann, L.; Zheng, W.; Amin, M.R.; Wang, L.; Hu, Q.; Peng, J.B.; Michalak, M.; et al. Autoinhibition of TRPV6 Channel and Regulation by PIP2. iScience 2020, 23, 101444. [Google Scholar] [CrossRef]
- Niemeyer, B.A.; Bergs, C.; Wissenbach, U.; Flockerzi, V.; Trost, C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc. Natl. Acad. Sci. USA 2001, 98, 3600–3605. [Google Scholar] [CrossRef]
- Cao, C.; Zakharian, E.; Borbiro, I.; Rohacs, T. Interplay between calmodulin and phosphatidylinositol 4,5-bisphosphate in Ca2+-induced inactivation of transient receptor potential vanilloid 6 channels. J. Biol. Chem. 2013, 288, 5278–5290. [Google Scholar] [CrossRef]
- Singh, A.K.; McGoldrick, L.L.; Twomey, E.C.; Sobolevsky, A.I. Mechanism of calmodulin inactivation of the calcium-selective TRP channel TRPV6. Sci. Adv. 2018, 4, eaau6088. [Google Scholar] [CrossRef]
- Bate, N.; Caves, R.E.; Skinner, S.P.; Goult, B.T.; Basran, J.; Mitcheson, J.S.; Vuister, G.W. A Novel Mechanism for Calmodulin-Dependent Inactivation of Transient Receptor Potential Vanilloid 6. Biochemistry 2018, 57, 2611–2622. [Google Scholar] [CrossRef]
- Raphaël, M.; Lehen’kyi, V.; Vandenberghe, M.; Beck, B.; Khalimonchyk, S.; Abeele, F.V.; Farsetti, L.; Germain, E.; Bokhobza, A.; Mihalache, A.; et al. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc. Natl. Acad. Sci. USA 2014, 111, E3870–E3879. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Bernardes, L.B.; Trevisan, G. TRP channels in cancer pain. Eur. J. Pharmacol. 2021, 904, 174185. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as A Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals. 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Kärki, T.; Tojkander, S. TRPV Protein Family—From Mechanosensing to Cancer Invasion. Biomolecules 2021, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Galluzzi, L.; Pinton, P. Ca2+ Fluxes and Cancer. Mol. Cell. 2020, 78, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Rokhlin, O.; Taghiyev, A.F.; Bayer, K.U.; Bumcrot, D.; Kotelianski, V.E.; Glover, R.A.; Cohen, M.B. Calcium/Calmodulin-Dependent Kinase II Plays an Important Role in Prostate Cancer Cell Survival. Cancer Biol. Ther. 2007, 6, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. NF-ΚB Directly Regulates Fas Transcription to Modulate Fas-Mediated Apoptosis and Tumor Suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef]
- Sée, V.; Rajala, N.K.M.; Spiller, D.G.; White, M.R.H. Calcium-Dependent Regulation of the Cell Cycle via a Novel MAPK–NF-ΚB Pathway in Swiss 3T3 Cells. J. Cell Biol. 2004, 166, 661–672. [Google Scholar] [CrossRef]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.; Wu, J.; et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007, 22, 274–285. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.A. TRPV6. Handb. Exp. Pharmacol. 2007, 179, 221–234. [Google Scholar]
- Kogel, A.; Fecher-Trost, C.; Wissenbach, U.; Flockerzi, V.; Schaefer, M. Ca2+ transport via TRPV6 is regulated by rapid internalization of the channel. Cell Calcium 2022, 106, 102634. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Amantini, C. TRPV channels in tumor growth and progression. Adv. Exp. Med. Biol. 2011, 704, 947–967. [Google Scholar] [PubMed]
- Lehen’kyi, V.; Prevarskaya, N. Oncogenic TRP channels. Adv. Exp. Med. Biol. 2011, 704, 929–945. [Google Scholar] [PubMed]
- Huber, S.M. Oncochannels. Cell Calcium 2013, 53, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.C.; Wissenbach, U.; Niemeyer, B.A.; Strauß, B.; Philipp, S.E.; Flockerzi, V.; Hoth, M. TRPV6 potentiates calcium-dependent cell proliferation. Cell Calcium 2006, 39, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lehen’kyi, V.; Flourakis, M.; Skryma, R.; Prevarskaya, N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene 2007, 26, 7380–7385. [Google Scholar] [CrossRef]
- Bolanz, K.A.; Hediger, M.A.; Landowski, C.P. The role of TRPV6 in breast carcinogenesis. Mol. Cancer Ther. 2008, 7, 271–279. [Google Scholar] [CrossRef]
- König, A.; Fernandez-Zapico, M.E.; Ellenrieder, V. Primers on molecular pathways—The NFAT transcription pathway in pancreatic cancer. Pancreatology 2010, 10, 416–422. [Google Scholar] [CrossRef]
- Marangoni, F.; Murooka, T.T.; Manzo, T.; Kim, E.Y.; Carrizosa, E.; Elpek, N.M.; Mempel, T.R. The transcription factor NFAT exhibits signal memory during serial T cell interactions with antigen-presenting cells. Immunity 2013, 38, 237–249. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Sana, I.; Mantione, M.E.; Angelillo, P.; Muzio, M. Role of NFAT in Chronic Lymphocytic Leukemia and Other B-Cell Malignancies. Front. Oncol. 2021, 11, 651057. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Toker, A. NFAT proteins: Emerging roles in cancer progression. Nat. Rev. Cancer 2009, 9, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Aoki, Y.; Badri, L.; Walker, N.M.; Manning, C.M.; Lagstein, A.; Fearon, E.R.; Lama, V.N. Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis. J. Clin. Investig. 2017, 127, 1517–1530. [Google Scholar] [CrossRef]
- Gómez, J.; Martínez, A.C.; González, A.; García, A.; Rebollo, A. The Bcl-2 gene is differentially regulated by IL-2 and IL-4: Role of the transcription factor NF-AT. Oncogene 1998, 17, 1235–1243. [Google Scholar] [CrossRef][Green Version]
- Morgan, M.P.; Cooke, M.M.; Christopherson, P.A.; Westfall, P.R.; McCarthy, G.M. Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol. Carcinog. 2001, 32, 111–117. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.A.; Fixemer, T.; Schneidewind, A.; Trost, C.; Cavalie, A.; Reus, K.; Meese, E.; Bonkhoff, H.; Flockerzi, V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J. Biol. Chem. 2001, 276, 19461–19468. [Google Scholar] [CrossRef]
- Kessler, T.; Wissenbach, U.; Grobholz, R.; Flockerzi, V. TRPV6 alleles do not influence prostate cancer progression. BMC Cancer 2009, 9, 380. [Google Scholar] [CrossRef]
- Bödding, M.; Fecher-Trost, C.; Flockerzi, V. Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J. Biol. Chem. 2003, 278, 50872–50879. [Google Scholar] [CrossRef]
- Thebault, S.; Flourakis, M.; Vanoverberghe, K.; Vandermoere, F.; Roudbaraki, M.; Lehen’kyi, V.; Slomianny, C.; Beck, B.; Mariot, P.; Bonnal, J.-L.; et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006, 66, 2038–2047. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.; Himmerkus, N.; Fixemer, T.; Bonkhoff, H.; Flockerzi, V. TRPV6 and prostate cancer: Cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem. Biophys. Res. Commun. 2004, 322, 1359–1363. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kołodziejski, P.A.; Mergler, S.; Khajavi, N.; Nowak, K.W.; Strowski, M.Z. TRPV6 modulates proliferation of human pancreatic neuroendocrine BON-1 tumour cells. Biosci. Rep. 2016, 36, e00372. [Google Scholar] [CrossRef]
- Pigozzi, D.; Ducret, T.; Tajeddine, N.; Gala, J.L.; Tombal, B.; Gailly, P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium 2006, 39, 401–415. [Google Scholar] [CrossRef]
- Zhuang, L.; Peng, J.B.; Tou, L.; Takanaga, H.; Adam, R.M.; Hediger, M.A.; Freeman, M.R. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab. Investig. 2002, 82, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, C.; Wie, J.; Kim, E.; Kim, B.J.; Ha, K.; Cho, N.H.; Kim, I.G.; Jeon, J.H.; So, I. Reciprocal positive regulation between TRPV6 and NUMB in PTEN-deficient prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 447, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell. Physiol. Biochem. 2001, 28, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.A.; Simpson, P.T.; Bassett, J.J.; Lee, J.M.; Da Silva, L.; Reid, L.E.; Song, S.; Parat, M.-O.; Lakhani, S.R.; Kenny, P.A.; et al. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor–negative breast cancer. Mol. Cancer Ther. 2012, 11, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.M.; Huang, Y.Y.; Tian, T.; Li, X.Y.; Tang, Y.B. Knockdown of Chloride Channel-3 Inhibits Breast Cancer Growth In Vitro and In Vivo. J. Breast Cancer 2018, 21, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, L.; Liu, X.; Michalak, M.; Tang, J.; Peng, J.B.; Chen, X.Z. Auto-inhibitory intramolecular S5/S6 interaction in the TRPV6 channel regulates breast cancer cell migration and invasion. Commun. Biol. 2021, 4, 990. [Google Scholar] [CrossRef] [PubMed]
- Kärki, T.; Rajakylä, E.K.; Acheva, A.; Tojkander, S. TRPV6 calcium channel directs homeostasis of the mammary epithelial sheets and controls epithelial mesenchymal transition. Sci. Rep. 2020, 10, 14683. [Google Scholar] [CrossRef]
- Cho, K.R.; Shih, I.M. Ovarian cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef]
- Xue, H.; Wang, Y.; MacCormack, T.J.; Lutes, T.; Rice, C.; Davey, M.; Dugourd, D.; Ilenchuk, T.T.; Stewart, J.M. Inhibition of Transient Receptor Potential Vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J. Cancer 2018, 9, 3196–3207. [Google Scholar] [CrossRef]
- Bowen, C.V.; DeBay, D.; Ewart, H.S.; Gallant, P.; Gormley, S.; Ilenchuk, T.T.; Iqbal, U.; Lutes, T.; Martina, M.; Mealing, G.; et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS ONE 2013, 8, e58866. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.H.; Yang, E.K. RNA interference mediated suppression of TRPV6 inhibits the progression of prostate cancer in vitro by modulating cathepsin B and MMP9 expression. Investig. Clin. Urol. 2021, 62, 447–454. [Google Scholar] [CrossRef]
- Jiang, Y.; Gou, H.; Zhu, J.; Tian, S.; Yu, L. Lidocaine inhibits the invasion and migration of TRPV6-expressing cancer cells by TRPV6 downregulation. Oncol. Lett. 2016, 12, 1164–1170. [Google Scholar]
- Song, H.; Dong, M.; Zhou, J.; Sheng, W.; Li, X.; Gao, W. Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer. Oncol. Rep. 2018, 39, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Seok, J.; Kang, G.H.; Lim, K.M.; Cho, S.G. The role of NUMB/NUMB isoforms in cancer stem cells. BMB Rep. 2021, 54, 335–343. [Google Scholar] [CrossRef]
- Saldías, M.P.; Maureira, D.; Orellana-Serradell, O.; Silva, I.; Lavanderos, B.; Cruz, P.; Torres, C.; Cáceres, M.; Cerda, O. TRP Channels Interactome as a Novel Therapeutic Target in Breast Cancer. Front. Oncol. 2021, 11, 621614. [Google Scholar] [CrossRef]
- Peleg, S.; Sellin, J.H.; Wang, Y.; Freeman, M.R.; Umar, S. Suppression of aberrant transient receptor potential cation channel, subfamily V, member 6 expression in hyperproliferative colonic crypts by dietary calcium. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G593–G601. [Google Scholar] [CrossRef]
- Arbabian, A.; Iftinca, M.; Altier, C.; Singh, P.P.; Isambert, H.; Coscoy, S. Mutations in calmodulin binding domains of TRPV4/6 channels confer invasive properties to colon adenocarcinoma cells. Channels 2020, 14, 101–109. [Google Scholar] [CrossRef]
- Ishizawa, M.; Akagi, D.; Yamamoto, J.; Makishima, M. 1α, 25-Dihydroxyvitamin D3 enhances TRPV6 transcription through p38 MAPK activation and GADD45 expression. J. Steroid Biochem. Mol. Biol. 2017, 172, 55–61. [Google Scholar] [CrossRef]
- Dai, W.; Bai, Y.; Hebda, L.; Zhong, X.; Liu, J.; Kao, J.; Duan, C. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014, 21, 568–581. [Google Scholar] [CrossRef]
- Balesaria, S.; Sangha, S.; Walters, J.R. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1193–G1197. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Burnstein, K.L. GADD45gamma: A new vitamin D-regulated gene that is antiproliferative in prostate cancer cells. Endocrinology 2010, 151, 4654–4664. [Google Scholar] [CrossRef] [PubMed]
- Dodeller, F.; Schulze-Koops, H. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res. Ther. 2006, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Gkika, D.; Prevarskaya, N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim. Biophys. Acta 2009, 1793, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Bolanz, K.A.; Kovacs, G.G.; Landowski, C.P.; Hediger, M.A. Tamoxifen inhibits TRPV6 Activity via estrogen receptor–independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol. Cancer Res. 2009, 7, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2018, 29, 75–108. [Google Scholar] [CrossRef]
- Cui, M.; Li, Q.; Johnson, R.; Fleet, J.C. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J. Bone Miner. Res. 2012, 27, 2097–2107. [Google Scholar] [CrossRef]
- Neuberger, A.; Sobolevsky, A.I. Molecular pharmacology of the onco-TRP channel TRPV6. Channels 2023, 17, 2266669. [Google Scholar] [CrossRef]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. Erratum to: First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 397. [Google Scholar] [CrossRef] [PubMed]
- Simonin, C.; Awale, M.; Brand, M.; van Deursen, R.; Schwartz, J.; Fine, M.; Kovacs, G.; Häfliger, P.; Gyimesi, G.; Sithampari, A.; et al. Optimization of TRPV6 Calcium Channel Inhibitors Using a 3D Ligand-Based Virtual Screening Method. Angew. Chem. Int. Ed Engl. 2015, 54, 14748–14752. [Google Scholar] [CrossRef]
- Pope, L.; Lolicato, M.; Minor, D.L., Jr. Polynuclear Ruthenium Amines Inhibit K2P Channels via a “Finger in the Dam” Mechanism. Cell Chem. Biol. 2020, 27, 511–524.e4. [Google Scholar] [CrossRef]
- Neuberger, A.; Nadezhdin, K.D.; Sobolevsky, A.I. Structural mechanisms of TRPV6 inhibition by ruthenium red and econazole. Nat. Commun. 2021, 12, 6284. [Google Scholar] [CrossRef]
- Jegal, H.G.; Park, H.J.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Koo, D.B. Ruthenium red improves blastocyst developmental competence by regulating mitochondrial Ca2+ and mitochondrial functions in fertilized porcine oocytes in vitro. J. Reprod. Dev. 2020, 66, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Montalbetti, N.; Simonin, A.; Danko, T.; Balazs, B.; Zsembery, A.; Hediger, M.A. Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB). Cell Calcium 2012, 52, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Dellenbach, P.; Thomas, J.L.; Guerin, V.; Ochsenbein, E.; Contet-Audonneau, N. Topical treatment of vaginal candidosis with sertaconazole and econazole sustained-release suppositories. Int. J. Gynecol. Obstet. 2000, 71 (Suppl. 1), S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yang, R.; Li, H.; Ke, K.; Luo, C.; Yang, F.; Shi, X.N.; Zhu, Y.; Liu, X.; Wong, M.H.; et al. Econazole nitrate inhibits PI3K activity and promotes apoptosis in lung cancer cells. Sci. Rep. 2017, 7, 17987. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.; Silvestri, C.; Martella, A.; Vanoevelen, J.M.; Di Marzo, V.; Voets, T. Δ9-tetrahydrocannabivarin impairs epithelial calcium transport through inhibition of TRPV5 and TRPV6. Pharmacol. Res. 2018, 136, 83–89. [Google Scholar] [CrossRef]
- Landowski, C.P.; Bolanz, K.A.; Suzuki, Y.; Hediger, M.A. Chemical inhibitors of the calcium entry channel TRPV6. Pharm. Res. 2011, 28, 322–330. [Google Scholar] [CrossRef]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells—Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Cunha, M.R.; Bhardwaj, R.; Carrel, A.L.; Lindinger, S.; Romanin, C.; Parise-Filho, R.; Hediger, M.A.; Reymond, J.-L. Natural product inspired optimization of a selective TRPV6 calcium channel inhibitor. RSC Med. Chem. 2020, 11, 1032–1040. [Google Scholar] [CrossRef]
| Compound | Remarks |
|---|---|
| PCHDs | |
| Ruthenium Red (RR) | |
| 2-aminoethoxydiphenyl borate (2-APB) | |
| Econazole | |
| Phytocannabinoid tetrahydrocannabivarin (THCV) | |
| SOR-C13 (13 amino acid peptide) | |
| TH-1177 | |
| Capsaicin | Apoptosis of human gastric cancer cells is induced by TRPV6 pathway [109,110]. |
| Piperazine derivative Cis-22a | The first submicromolar, small molecule inhibitor, it decreased the cell viability of tumor cell lines overexpressing TRPV6 [111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deng, X.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Ali, D.W.; Michalak, M.; Zhou, C.; Chen, X.-Z.; et al. The TRPV6 Calcium Channel and Its Relationship with Cancer. Biology 2024, 13, 168. https://doi.org/10.3390/biology13030168
Wang Y, Deng X, Zhang R, Lyu H, Xiao S, Guo D, Ali DW, Michalak M, Zhou C, Chen X-Z, et al. The TRPV6 Calcium Channel and Its Relationship with Cancer. Biology. 2024; 13(3):168. https://doi.org/10.3390/biology13030168
Chicago/Turabian StyleWang, Yifang, Xiaoling Deng, Rui Zhang, Hao Lyu, Shuai Xiao, Dong Guo, Declan William Ali, Marek Michalak, Cefan Zhou, Xing-Zhen Chen, and et al. 2024. "The TRPV6 Calcium Channel and Its Relationship with Cancer" Biology 13, no. 3: 168. https://doi.org/10.3390/biology13030168
APA StyleWang, Y., Deng, X., Zhang, R., Lyu, H., Xiao, S., Guo, D., Ali, D. W., Michalak, M., Zhou, C., Chen, X.-Z., & Tang, J. (2024). The TRPV6 Calcium Channel and Its Relationship with Cancer. Biology, 13(3), 168. https://doi.org/10.3390/biology13030168







