Absence of the Klotho Function Causes Cornea Degeneration with Specific Features Resembling Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Klotho Null Mutant Mice
2.2. Genotyping of Klotho Mutant Mice
2.3. Cornea Surface Topography
2.4. Tissue Fixation, Micro-Section, and Hematoxylin–Eosin Staining
2.5. Immunohistochemical and Immunofluorescent Detections
2.6. In Situ TUNEL Staining
2.7. Statistics
3. Results
3.1. Cornea Surface Irregularity with Klotho Null Mutation
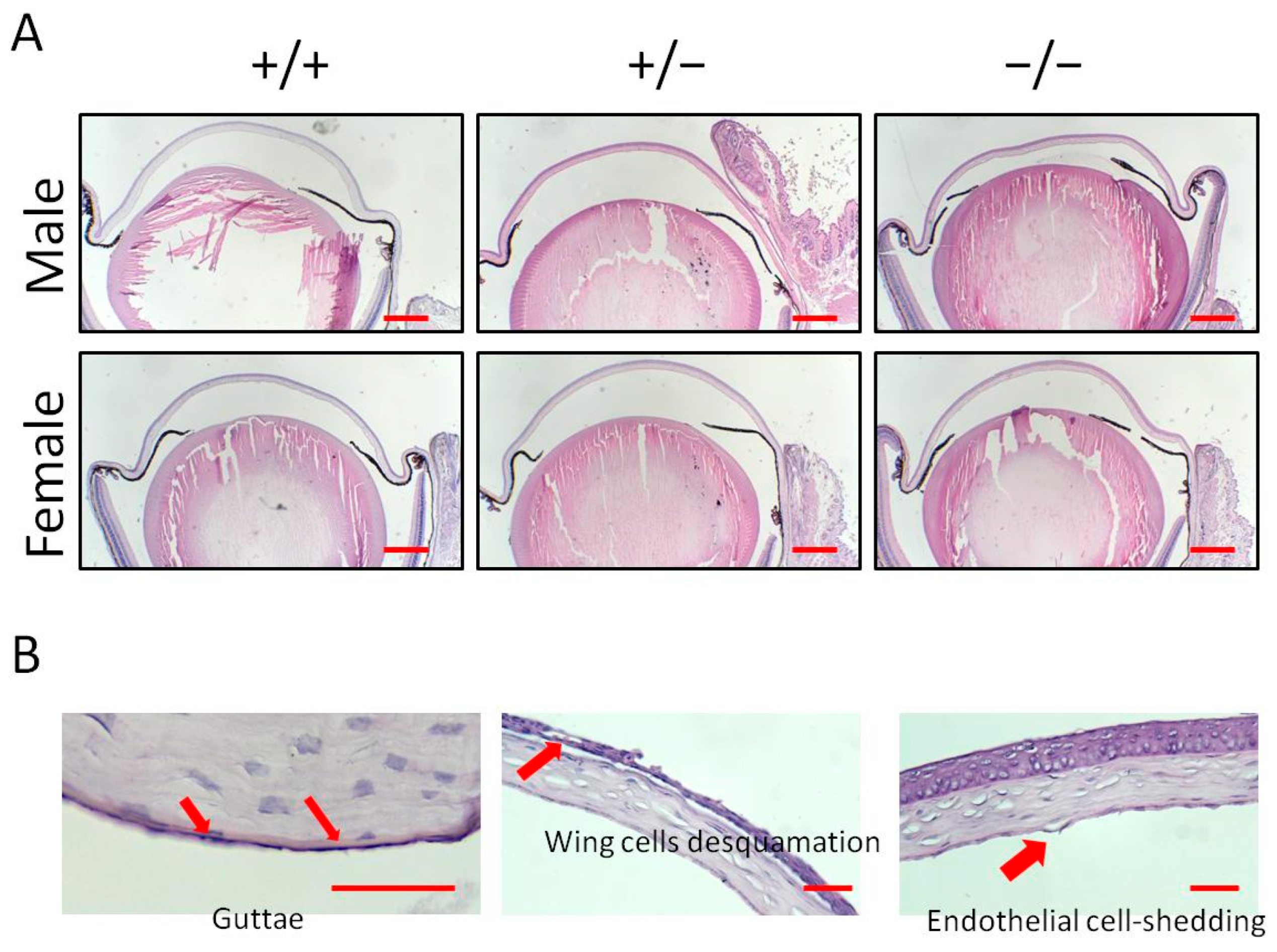
3.2. Histological Analysis Indicates Cornea Pathological Alterations with a Klotho Null Mutation
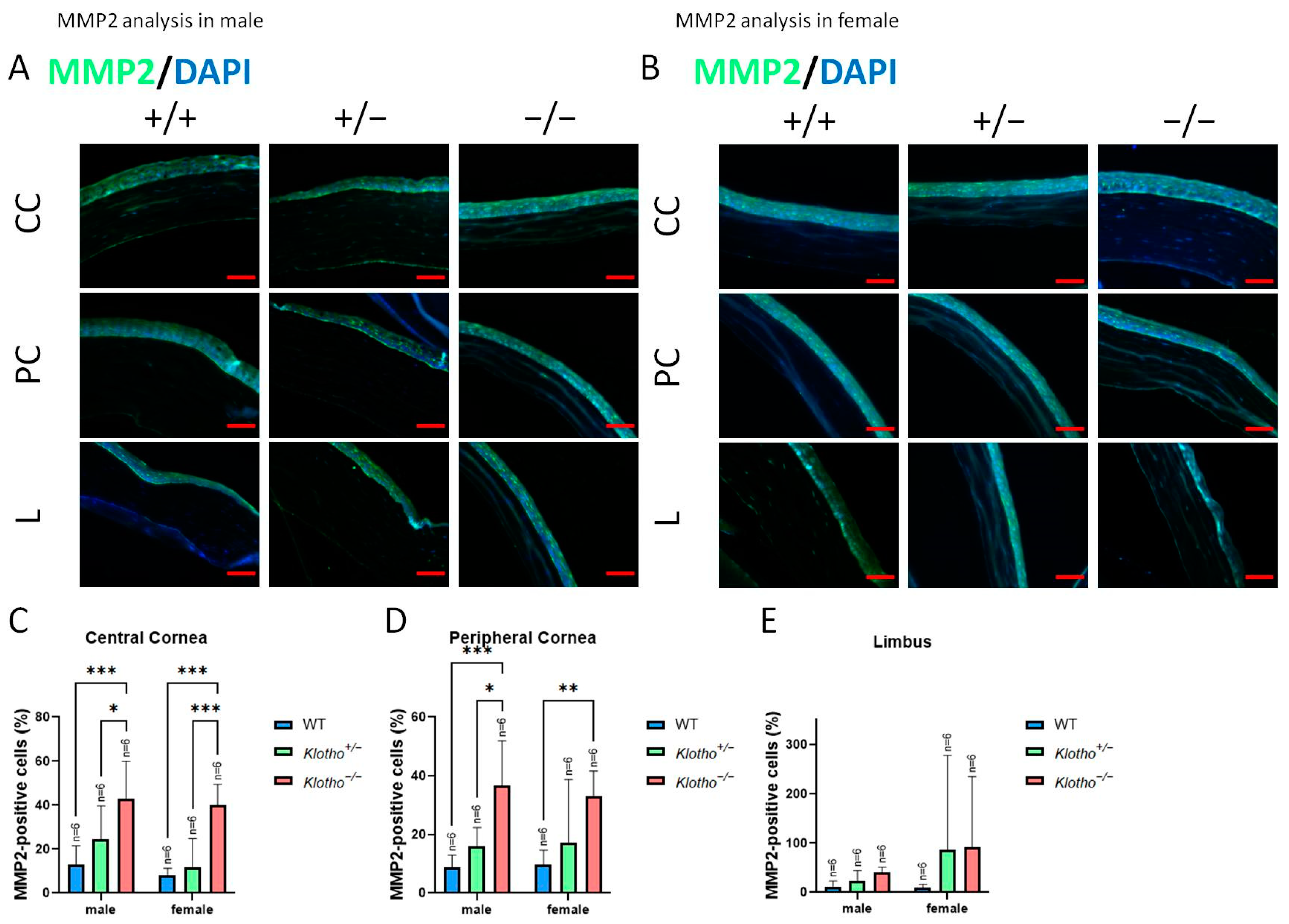
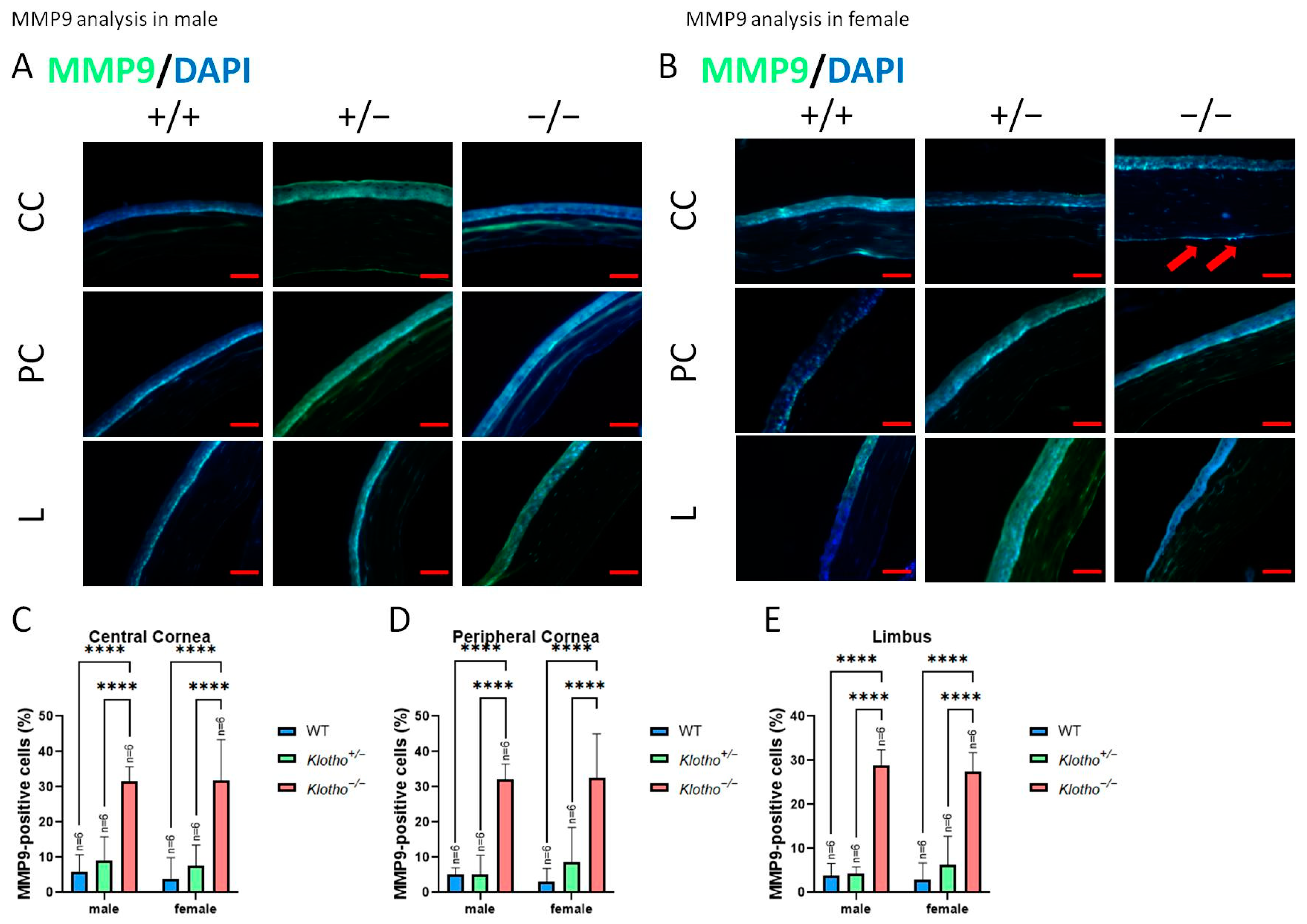
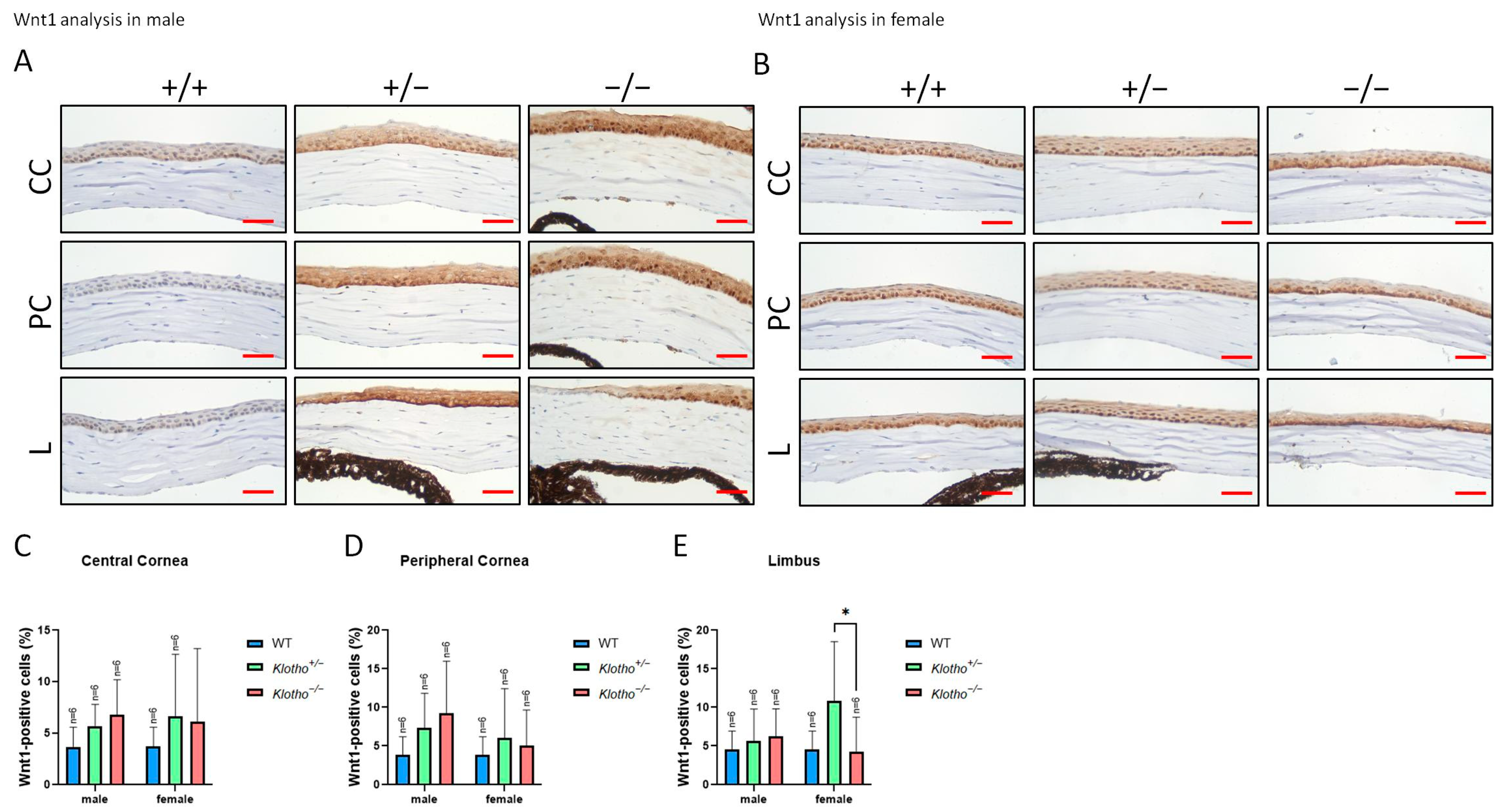
3.3. Significant Elevations of MMP2, MMP9, and Wnt-1 Expression with the Klotho Null Mutation
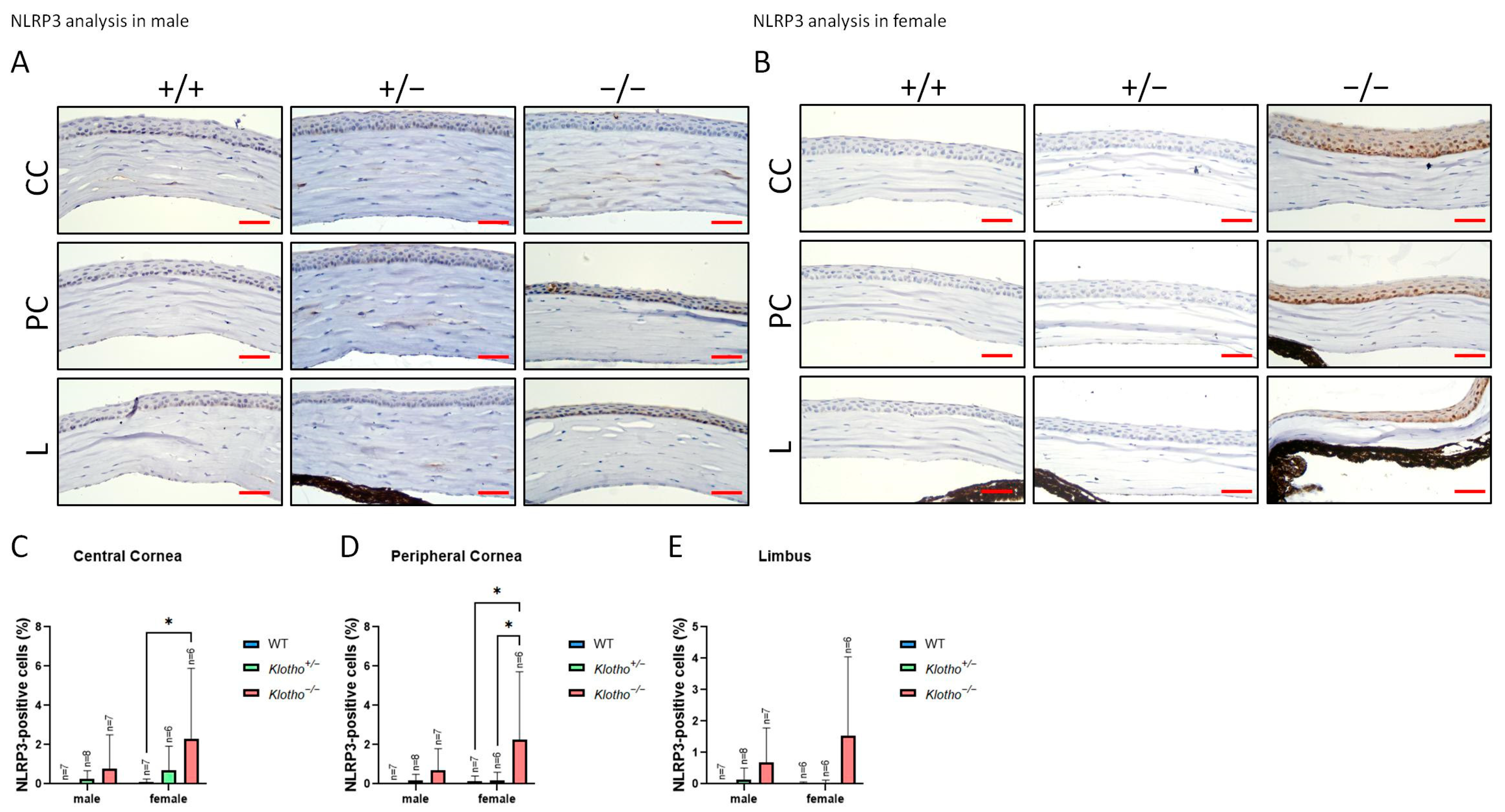
3.4. The Status of NLRP3 with the Klotho Null Mutation
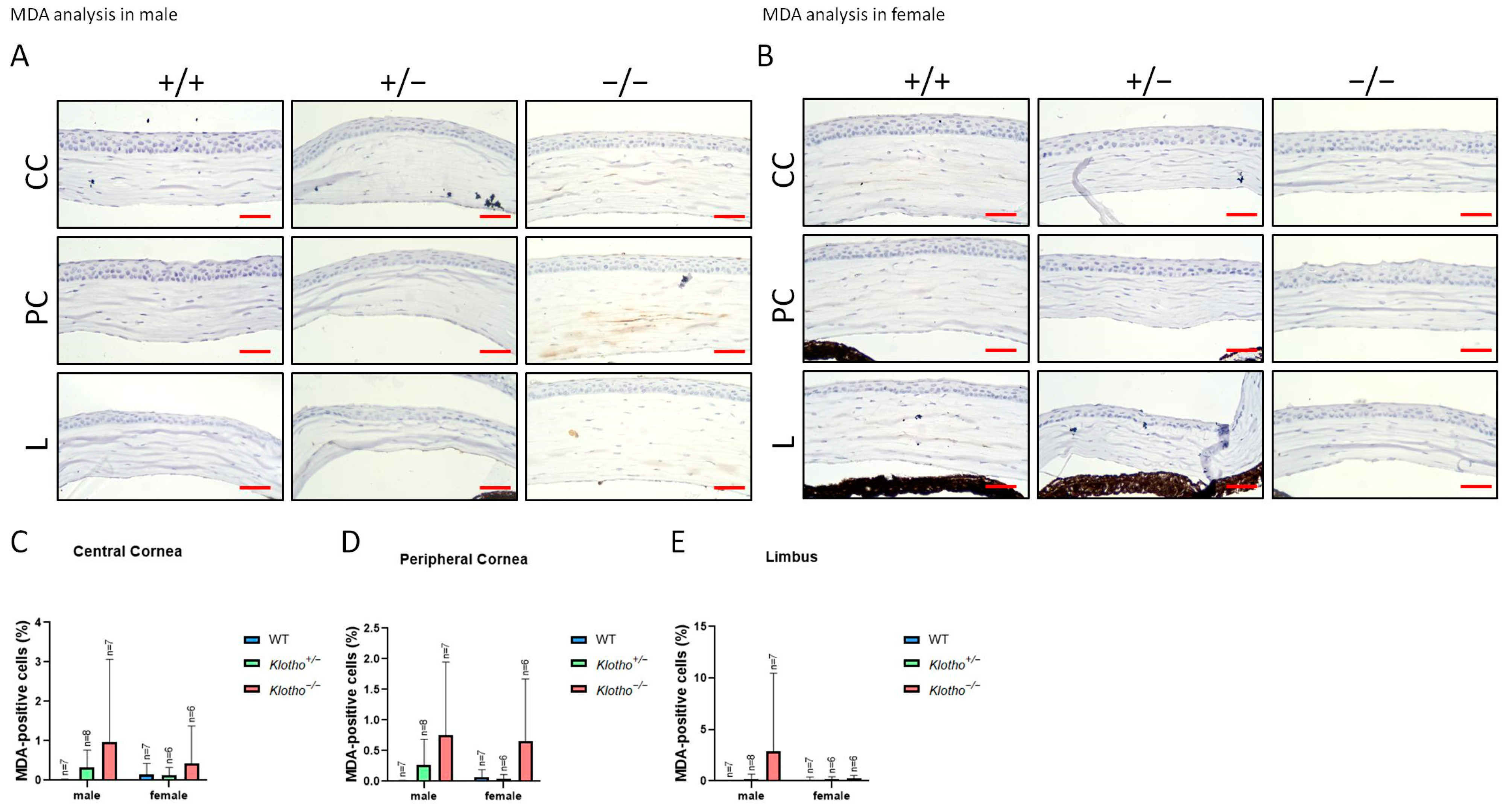
3.5. Increased Oxidative Stress as Indicated by 8-OHdG and MDA Markers
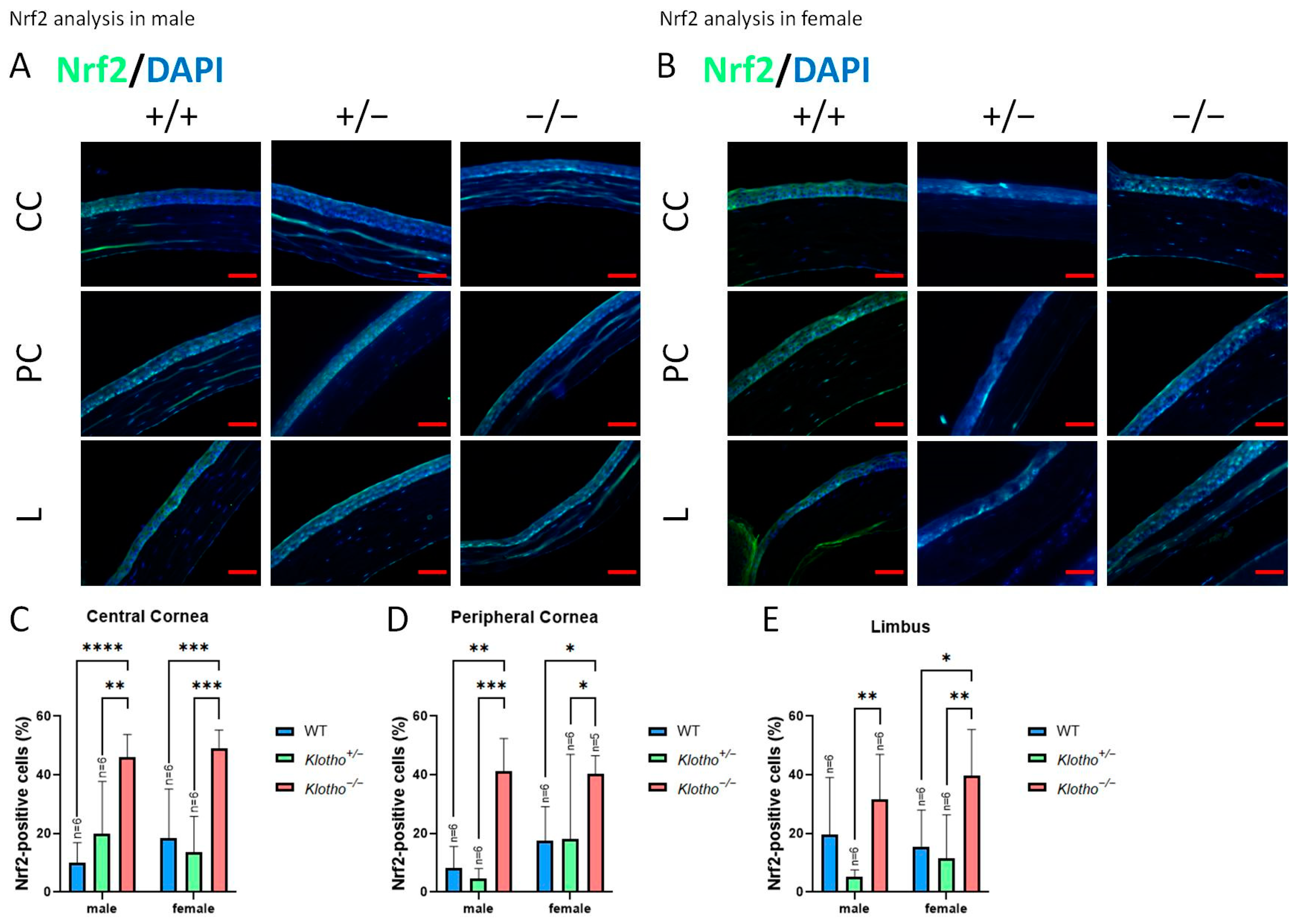
3.6. Klotho Null Mutation Leads to an Increase in Nrf2 Expression
3.7. Klotho Null Mutation Leads to a Comcimitant Increase in p53, Ki67, and Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W., Jr. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, B.J.; Jenkins, M.W.; Rollins, A.M.; Dupps, W.J. A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking. Front. Bioeng. Biotechnol. 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Faragher, R.G.; Mulholland, B.; Tuft, S.J.; Sandeman, S.; Khaw, P.T. Aging and the cornea. Br. J. Ophthalmol. 1997, 81, 814–817. [Google Scholar] [CrossRef]
- Yassa, H.; Hussein, G.; Kotb, S. Effect of Aging on Cornea of Male Albino Rat and the Possible Protective Role of Vitamin, C. Egypt. J. Histol. 2020, 45, 640–652. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Faragher, R.G.; Allen, M.C.; Liu, C.; Lloyd, A.W. Does MMP-2 expression and secretion change with increasing serial passage of keratocytes in culture? Mech. Ageing Dev. 2001, 122, 157–167. [Google Scholar] [CrossRef]
- Sie, N.M.; Yam, G.H.; Soh, Y.Q.; Lovatt, M.; Dhaliwal, D.; Kocaba, V.; Mehta, J.S. Regenerative capacity of the corneal transition zone for endothelial cell therapy. Stem Cell Res. Ther. 2020, 11, 523. [Google Scholar] [CrossRef]
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Ong Tone, S.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583. [Google Scholar] [CrossRef]
- Wang, Q.; Dou, S.; Zhang, B.; Jiang, H.; Qi, X.; Duan, H.; Wang, X.; Dong, C.; Zhang, B.N.; Xie, L.; et al. Heterogeneity of human corneal endothelium implicates lncRNA NEAT1 in Fuchs endothelial corneal dystrophy. Mol. Ther. Nucleic Acids 2022, 27, 880–893. [Google Scholar] [CrossRef]
- Stunf Pukl, S. MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy. Genes 2022, 13, 1711. [Google Scholar] [CrossRef] [PubMed]
- White, T.L.; Deshpande, N.; Kumar, V.; Gauthier, A.G.; Jurkunas, U.V. Cell cycle re-entry and arrest in G2/M phase induces senescence and fibrosis in Fuchs Endothelial Corneal Dystrophy. Free. Radic. Biol. Med. 2021, 164, 34–43. [Google Scholar] [CrossRef]
- Cui, Z.; Zeng, Q.; Guo, Y.; Liu, S.; Wang, P.; Xie, M.; Chen, J. Pathological molecular mechanism of symptomatic late-onset Fuchs endothelial corneal dystrophy by bioinformatic analysis. PLoS ONE 2018, 13, e0197750. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, B.J.; Gasymov, O.K.; Casey, R.C. Exfoliative epitheliopathy of bullous keratopathy with breaches in the MUC16 Glycocalyx. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Leonard, B.C.; Raghunathan, V.K.; Kim, S.; Li, J.Y.; Mannis, M.J.; Murphy, C.J.; Thomasy, S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Transl. Med. 2021, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Cheikhi, A.; Barchowsky, A.; Sahu, A.; Shinde, S.N.; Pius, A.; Clemens, Z.J.; Li, H.; Kennedy, C.A.; Hoeck, J.D.; Franti, M.; et al. Klotho: An Elephant in Aging Research. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Moos, W.H.; Faller, D.V.; Glavas, I.P.; Harpp, D.N.; Kanara, I.; Mavrakis, A.N.; Pernokas, J.; Pernokas, M.; Pinkert, C.A.; Powers, W.R.; et al. Klotho Pathways, Myelination Disorders, Neurodegenerative Diseases, and Epigenetic Drugs. Biores. Open Access 2020, 9, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Wu, Z.; Yu, X.; Du, X.; Li, X. The Expressions of Klotho Family Genes in Human Ocular Tissues and in Anterior Lens Capsules of Age-Related Cataract. Curr. Eye Res. 2017, 42, 871–875. [Google Scholar] [CrossRef]
- Jin, S.L.; Zhang, Y.; Chen, Z.H.; Qian, D.W.; Qine, Y.J.; Yongjie, Q.; He, S.K.; Guo, H.K. Epigenetic changes of the Klotho gene in age-related cataracts. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2544–2553. [Google Scholar]
- Ghemame, M.; Charpentier, P.; Mouriaux, F. Corneal topography in clinical practice. J. Fr. Ophtalmol. 2019, 42, e439–e451. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, D.P.; Wu, C.Y.; Teng, M.C.; Sun, C.Y.; Tsai, Y.T.; Su, K.C.; Wang, S.R.; Chang, H.H. Dietary zerumbone prevents mouse cornea from UVB-induced photokeratitis through inhibition of NF-κB, iNOS, and TNF-α expression and reduction of MDA accumulation. Mol. Vis. 2011, 17, 854–863. [Google Scholar]
- De Paiva, C.S.; Pangelinan, S.B.; Chang, E.; Yoon, K.C.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch. Ophthalmol. 2009, 127, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2021, 37, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013, 102, 14–15. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and aging. Biochim. Biophys. Acta 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, P.; Arango-Gonzalez, B.; Völkl, J.; Alesutan, I.; Lang, F.; Zrenner, E.; Paquet-Durand, F.; Ekström, P.A. Retinitis Pigmentosa: Over-expression of anti-ageing protein Klotho in degenerating photoreceptors. J. Neurochem. 2013, 127, 868–879. [Google Scholar] [CrossRef]
- Reish, N.J.; Maltare, A.; McKeown, A.S.; Laszczyk, A.M.; Kraft, T.W.; Gross, A.K.; King, G.D. The age-regulating protein klotho is vital to sustain retinal function. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6675–6685. [Google Scholar] [CrossRef]
- Jang, H.Y.; Kim, S.J.; Park, K.S.; Kim, J.H. Klotho prevents transforming growth factor-beta2-induced senescent-like morphological changes in the retinal pigment epithelium. Cell Death Dis. 2023, 14, 334. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, K.; Yukita, M.; Yasuda, M.; Omodaka, K.; Ryu, M.; Fujita, K.; Nishiguchi, K.M.; Machida, S.; Nakazawa, T. The neuroprotective effect of latanoprost acts via klotho-mediated suppression of calpain activation after optic nerve transection. J. Neurochem. 2017, 140, 495–508. [Google Scholar] [CrossRef]
- Wu, C.Y.; Song, D.F.; Lu, T.H.; Chen, Z.J.; Tsai, S.M.; Liu, Y.J.; Chang, H.H.; Lin, D.P. Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice. Biology 2023, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Inomata, T.; Shih, K.; Hughes, J.B.; Bozza, N.; Tomioka, Y.; Numa, K.; Yokoi, N.; Campisi, J.; Dana, R.; et al. Impact of aging on the pathophysiology of dry eye disease: A systematic review and meta-analysis. Ocul. Surf. 2022, 25, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Pal-Ghosh, S.; Tadvalkar, G.; Williams, A.; Pflugfelder, S.C.; de Paiva, C.S. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp. Eye Res. 2018, 169, 91–98. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am. J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.E.H.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. The Effects of Aging on Corneal and Ocular Surface Homeostasis in Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2705–2715. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.Q.; Fini, M.E. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef]
- Ihanamaki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef]
- Pouw, A.E.; Greiner, M.A.; Coussa, R.G.; Jiao, C.; Han, I.C.; Skeie, J.M.; Fingert, J.H.; Mullins, R.F.; Sohn, E.H. Cell-Matrix Interactions in the Eye: From Cornea to Choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef]
- Takegahara, K.; Usuda, J.; Inoue, T.; Sonokawa, T.; Matsui, T.; Matsumoto, M. Antiaging gene Klotho regulates epithelial-mesenchymal transition and increases sensitivity to pemetrexed by inducing lipocalin-2 expression. Oncol. Lett. 2021, 21, 418. [Google Scholar] [CrossRef]
- Chu, H.; Sun, X.; Wang, J.; Lei, K.; Shan, Z.; Zhao, C.; Ning, Y.; Gong, R.; Ren, H.; Cui, Z. Synergistic effects of sodium butyrate and cisplatin against cervical carcinoma in vitro and in vivo. Front. Oncol. 2022, 12, 999667. [Google Scholar] [CrossRef]
- Tian, S.; Peng, P.; Li, J.; Deng, H.; Zhan, N.; Zeng, Z.; Dong, W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging 2020, 12, 3574–3593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, M.; Li, J.; Danielson, P.; Yang, L.; Zhou, Q. Induction of Fibroblast Senescence During Mouse Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Inoue, H.; Kawakita, T.; Ogishima, T.; Tsubota, K. Selenium compound protects corneal epithelium against oxidative stress. PLoS ONE 2012, 7, e45612. [Google Scholar] [CrossRef] [PubMed]
- Kvanta, A.; Sarman, S.; Fagerholm, P.; Seregard, S.; Steen, B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp. Eye Res. 2000, 70, 419–428. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, S.H.; Jin, H.; Kim, S.J.; Ryu, Y.; Lee, K.J.; Kim, B.; Shin, H.J.; Won, K.J. Bioluminescence Imaging of Matrix Metalloproteinases-2 and -9 Activities in Ethanol-injured Cornea of Mice. In Vivo 2021, 35, 1521–1528. [Google Scholar] [CrossRef]
- Kocaba, V.; Katikireddy, K.R.; Gipson, I.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Association of the Gutta-Induced Microenvironment With Corneal Endothelial Cell Behavior and Demise in Fuchs Endothelial Corneal Dystrophy. JAMA Ophthalmol. 2018, 136, 886–892. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Huang, Y.H.; Cunningham, C.M.; Han, K.Y.; Chang, M.; Seiki, M.; Zhou, Z.; Azar, D.T. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv. Ophthalmol. 2016, 61, 478–497. [Google Scholar] [CrossRef]
- Diwanji, N.; Bergmann, A. Basement membrane damage by ROS- and JNK-mediated Mmp2 activation drives macrophage recruitment to overgrown tissue. Nat. Commun. 2020, 11, 3631. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Xiang, J.; Li, Y.; Wu, D.; Xu, J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019, 21, 101093. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gomez-Ambrosi, J.; Ramirez, B.; Rodriguez, A.; Becerril, S.; Valenti, V.; Moncada, R.; Silva, C.; Salvador, J.; Fruhbeck, G.; et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol. Immunol. 2021, 18, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Petznick, A.; Madigan, M.C.; Garrett, Q.; Sweeney, D.F.; Evans, M.D. Contributions of ocular surface components to matrix-metalloproteinases (MMP)-2 and MMP-9 in feline tears following corneal epithelial wounding. PLoS ONE 2013, 8, e71948. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.G.; Inanc-Surer, S.; Cakan-Akdogan, G.; Oktay, G.; Utine, C.A.; Kalyoncu, S. Roles of matrix metalloproteinases in the cornea: A special focus on macular corneal dystrophy. Med. Drug Discov. 2021, 11, 100095. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, Y.; Deng, M.; Liu, Y.; Wang, S.; He, X.; Allaire-Leung, M.; Wan, J.; Zou, Y.; Yang, C.; et al. Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae. Chemosphere 2019, 226, 696–703. [Google Scholar] [CrossRef]
- Chen, K.; Wang, S.; Sun, Q.W.; Zhang, B.; Ullah, M.; Sun, Z. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ. Res. 2021, 128, 492–507. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Chen, J.; Wang, Y.; Wang, L.; Yao, X.; et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem. Biophys. Res. Commun. 2021, 534, 450–456. [Google Scholar] [CrossRef]
- Zhao, M.; Murakami, S.; Matsumaru, D.; Kawauchi, T.; Nabeshima, Y.I.; Motohashi, H. NRF2 pathway activation attenuates ageing-related renal phenotypes due to α-klotho deficiency. J. Biochem. 2022, 171, 579–589. [Google Scholar] [CrossRef]
- Hao, X.D.; Liu, Y.N.; Hu, S.H.; Pan, X.J.; Chen, P. Association of macular corneal dystrophy with excessive cell senescence and apoptosis induced by the novel mutant CHST6. Exp. Eye Res. 2022, 214, 108862. [Google Scholar] [CrossRef]
- Joyce, N.C.; Navon, S.E.; Roy, S.; Zieske, J.D. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1566–1575. [Google Scholar]
- Liu, M.; Wang, W.; Wang, J.; Fang, C.; Liu, T. Z-Guggulsterone alleviates renal fibrosis by mitigating G2/M cycle arrest through Klotho/p53 signaling. Chem. Biol. Interact. 2022, 354, 109846. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Campbell, C.; Venkitaraman, A.R.; Esposito, A. Pulsatile MAPK Signaling Modulates p53 Activity to Control Cell Fate Decisions at the G2 Checkpoint for DNA Damage. Cell. Rep. 2020, 30, 2083–2093. [Google Scholar] [CrossRef]
- Ştefănescu-Dima, A.; Mănescu, M.R.; Bălăşoiu, A.T.; Bălăşoiu, M.; Mocanu, C.L.; Stanca, H.T.; Mercuţ Nicolcescu, M.F. Behavior pattern of early-stage ocular surface squamous cell carcinoma in non-HIV patients. Rom. J. Morphol. Embryol. Rev. Roum. De Morphol. Et Embryol. 2019, 60, 455–461. [Google Scholar]






| +/+ | +/− | −/− | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Wing cell desquamation | 1/7 (14.3%) | 1/7 (14.3%) | 2/7 (28.6%) | 0/6 (0%) | 2/4 (50%) | 3/5 (60%) |
| Endothelial cell-shedding | 0/7 (0%) | 0/7 (0%) | 0/7 (0%) | 0/6 (0%) | 2/4 (50%) | 3/5 (60%) |
| Guttae | 0/7 (0%) | 0/7 (0%) | 1/7 (14.3%) | 1/6 (16.7%) | 3/4 (75%) | 5/5 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Song, D.-F.; Chen, Z.-J.; Hu, C.-S.; Lin, D.P.-C.; Chang, H.-H. Absence of the Klotho Function Causes Cornea Degeneration with Specific Features Resembling Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy. Biology 2024, 13, 133. https://doi.org/10.3390/biology13030133
Wu C-Y, Song D-F, Chen Z-J, Hu C-S, Lin DP-C, Chang H-H. Absence of the Klotho Function Causes Cornea Degeneration with Specific Features Resembling Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy. Biology. 2024; 13(3):133. https://doi.org/10.3390/biology13030133
Chicago/Turabian StyleWu, Chun-Yen, Da-Fong Song, Zhi-Jia Chen, Chao-Sheng Hu, David Pei-Cheng Lin, and Han-Hsin Chang. 2024. "Absence of the Klotho Function Causes Cornea Degeneration with Specific Features Resembling Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy" Biology 13, no. 3: 133. https://doi.org/10.3390/biology13030133
APA StyleWu, C.-Y., Song, D.-F., Chen, Z.-J., Hu, C.-S., Lin, D. P.-C., & Chang, H.-H. (2024). Absence of the Klotho Function Causes Cornea Degeneration with Specific Features Resembling Fuchs Endothelial Corneal Dystrophy and Bullous Keratopathy. Biology, 13(3), 133. https://doi.org/10.3390/biology13030133







