Friend or Foe: Protein Inhibitors of DNA Gyrase
Abstract
Simple Summary
Abstract
1. Introduction
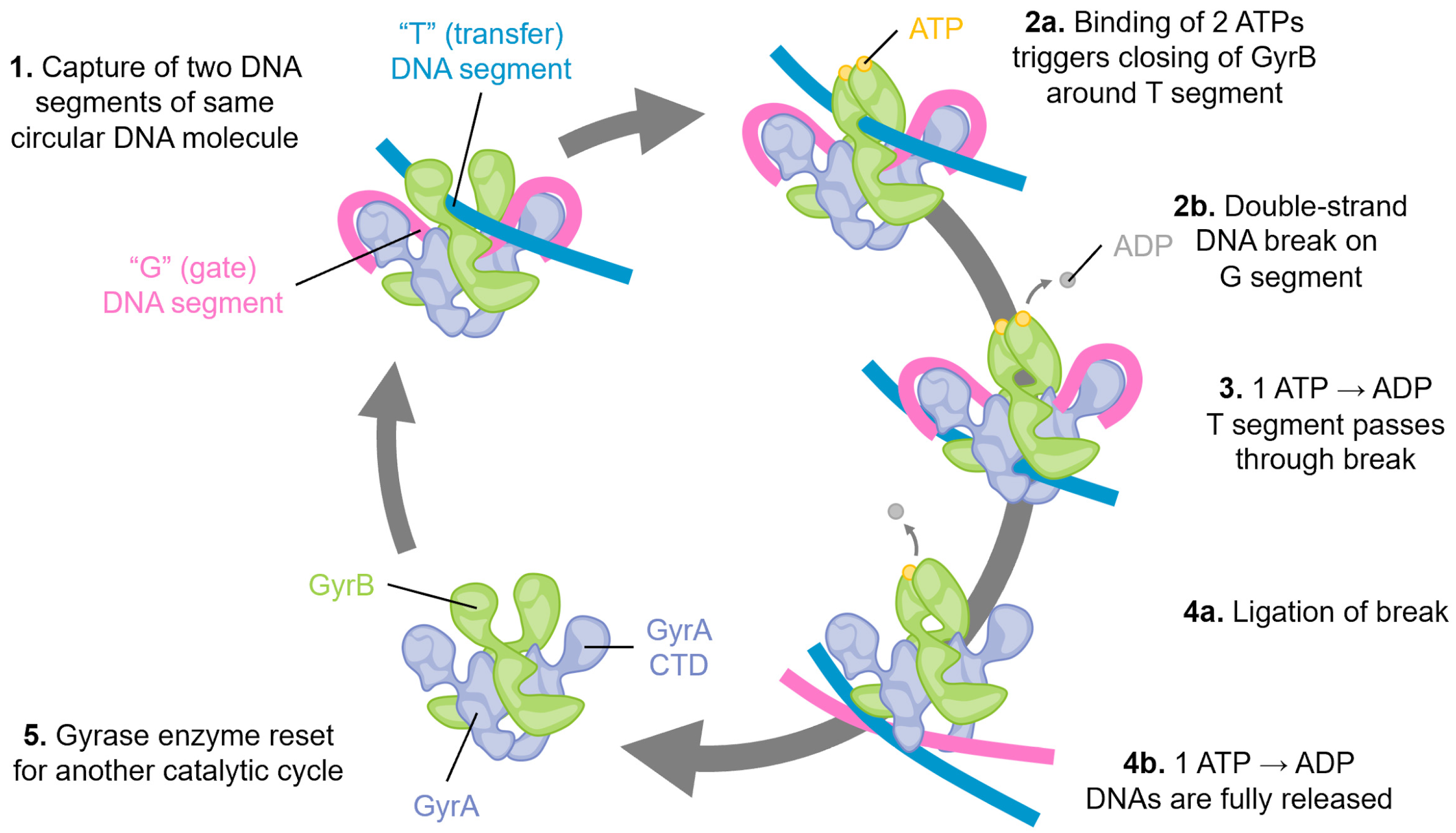
2. DNA Gyrase Functions as an Essential Type II Topoisomerase in Bacteria
3. Proteins Acting as Foes, Producing Damaging Gyrase Inhibition
3.1. ParE Toxin Proteins from ParDE Type II TA Systems

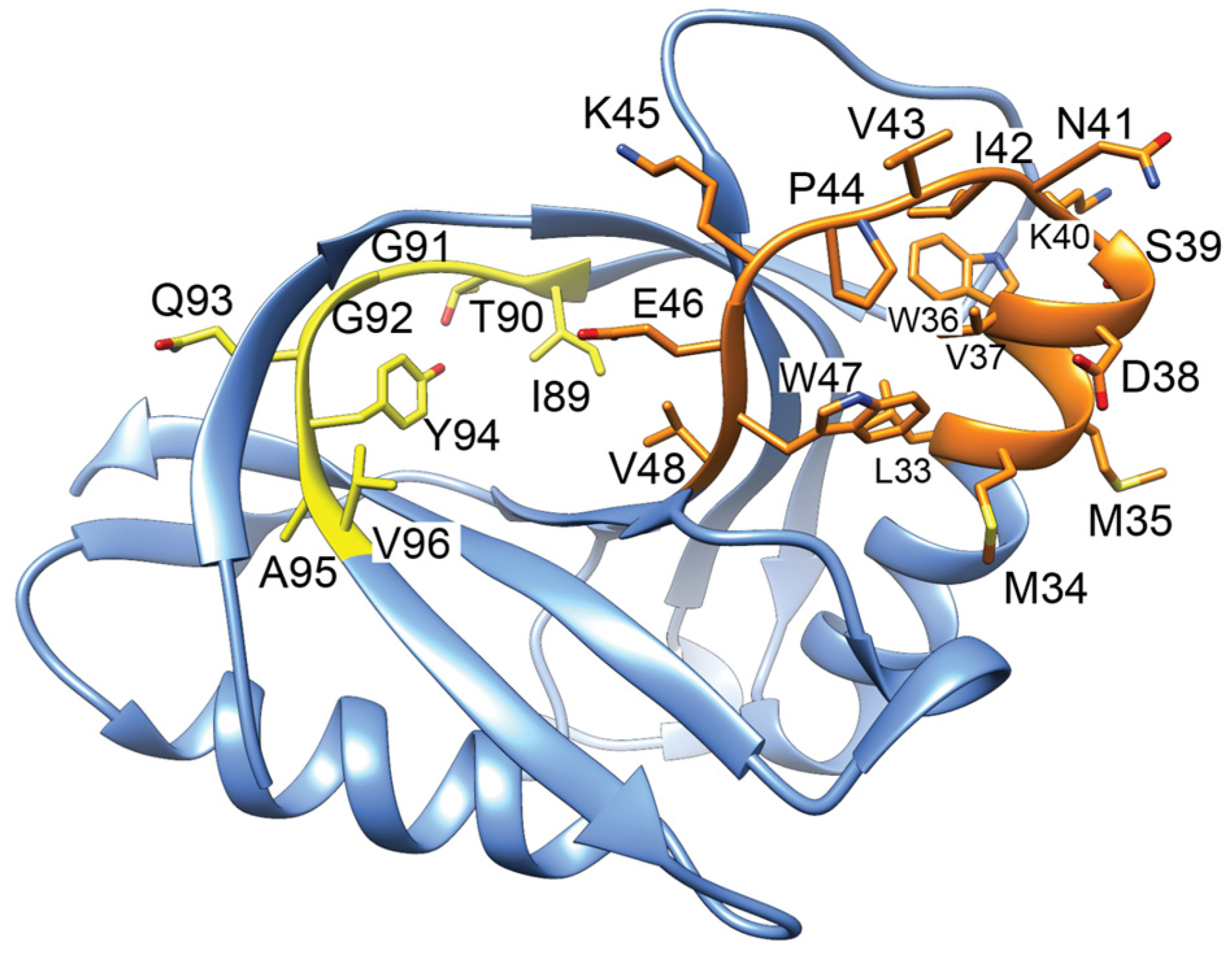
3.2. CcdB Toxin Proteins from CcdAB Type II TA Systems

3.3. TsbT Toxin Proteins from TsbAT Type II TA Systems
3.4. Microcin B17
4. Proteins Acting as Friends, Protecting Gyrase from Damaging Inhibition
4.1. Phage-Derived Peptides
4.2. FicT Toxins from FicTA Type II TA Systems
4.3. Pentapeptide Repeat Proteins
4.3.1. Qnr Proteins
4.3.2. MfpA Proteins
4.4. YacG
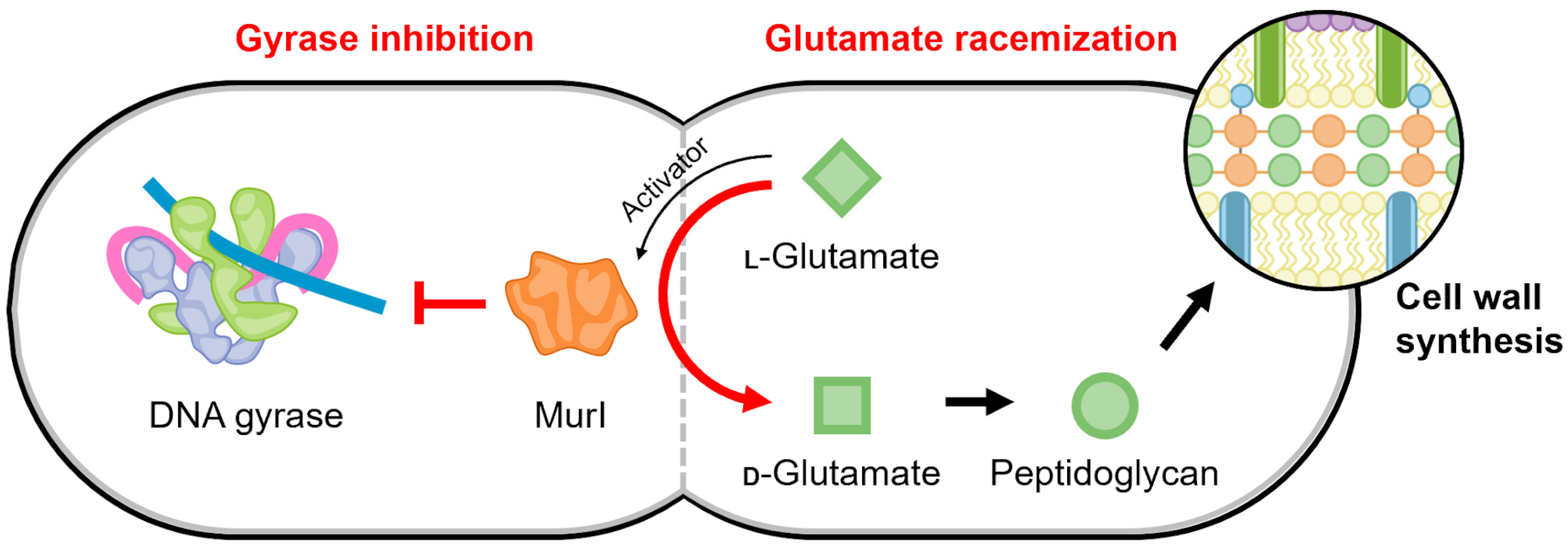
4.5. MurI
4.6. GyrI
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Ross, B.N.; Whiteley, M. Ignoring Social Distancing: Advances in Understanding Multi-Species Bacterial Interactions. Fac. Rev. 2020, 9, 23. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial Adaptation to Different Environmental Conditions: Molecular Perspective of Evolved Genetic and Cellular Systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef]
- Cuthbert, B.J.; Hayes, C.S.; Goulding, C.W. Functional and Structural Diversity of Bacterial Contact-Dependent Growth Inhibition Effectors. Front. Mol. Biosci. 2022, 9, 866854. [Google Scholar] [CrossRef]
- Harms, A.; Liesch, M.; Körner, J.; Québatte, M.; Engel, P.; Dehio, C. A Bacterial Toxin-Antitoxin Module is the Origin of Inter-Bacterial and Inter-Kingdom Effectors of Bartonella. PLoS Genet. 2017, 13, e1007077. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Wu, A.Y.; Iredell, J.R. Biological Functions of Type II Toxin-Antitoxin Systems in Bacteria. Microorganisms 2021, 9, 1276. [Google Scholar] [CrossRef]
- Pizzolato-Cezar, L.R.; Spira, B.; Machini, M.T. Bacterial Toxin-Antitoxin Systems: Novel Insights on Toxin Activation across Populations and Experimental Shortcomings. Curr. Res. Microb. Sci. 2023, 5, 100204. [Google Scholar] [CrossRef]
- Weisberg, A.J.; Chang, J.H. Mobile Genetic Element Flexibility as an Underlying Principle to Bacterial Evolution. Annu. Rev. Microbiol. 2023, 77, 603–624. [Google Scholar] [CrossRef]
- Mayo-Muñoz, D.; Pinilla-Redondo, R.; Birkholz, N.; Fineran, P.C. A Host of Armor: Prokaryotic Immune Strategies against Mobile Genetic Elements. Cell Rep. 2023, 42, 112672. [Google Scholar] [CrossRef]
- Al-Shayeb, B.; Schoelmerich, M.C.; West-Roberts, J.; Valentin-Alvarado, L.E.; Sachdeva, R.; Mullen, S.; Crits-Christoph, A.; Wilkins, M.J.; Williams, K.H.; Doudna, J.A.; et al. Borgs are Giant Genetic Elements with Potential to Expand Metabolic Capacity. Nature 2022, 610, 731–736. [Google Scholar] [CrossRef]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and Evolution of Bacterial Toxin–Antitoxin Systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef]
- Booth, S.C.; Smith, W.P.J.; Foster, K.R. The Evolution of Short- and Long-Range Weapons for Bacterial Competition. Nat. Ecol. Evol. 2023, 7, 2080–2091. [Google Scholar] [CrossRef]
- Ruggieri, F.; Compagne, N.; Antraygues, K.; Eveque, M.; Flipo, M.; Willand, N. Antibiotics with Novel Mode of Action as New Weapons to Fight Antimicrobial Resistance. Eur. J. Med. Chem. 2023, 256, 115413. [Google Scholar] [CrossRef]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting Bacterial DNA Gyrase as a Drug Target: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef]
- Klostermeier, D. Towards Conformation-Sensitive Inhibition of Gyrase: Implications of Mechanistic Insight for the Identification and Improvement of Inhibitors. Molecules 2021, 26, 1234. [Google Scholar] [CrossRef]
- Bax, B.D.; Murshudov, G.; Maxwell, A.; Germe, T. DNA Topoisomerase Inhibitors: Trapping a DNA-Cleaving Machine in Motion. J. Mol. Biol. 2019, 431, 3427–3449. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, L. The Antibacterial Activity of Fluoroquinolone Derivatives: An Update (2018–2021). Eur. J. Med. Chem. 2021, 224, 113741. [Google Scholar] [CrossRef]
- Hiasa, H. DNA Topoisomerases as Targets for Antibacterial Agents. In DNA Topoisomerases: Methods and Protocols; Drolet, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 47–62. ISBN 978-1-4939-7459-7. [Google Scholar]
- Sissi, C.; Palumbo, M. In Front of and behind the Replication Fork: Bacterial Type IIA Topoisomerases. Cell. Mol. Life Sci. 2010, 67, 2001–2024. [Google Scholar] [CrossRef]
- Forquet, R.; Pineau, M.; Nasser, W.; Reverchon, S.; Meyer, S. Role of the Discriminator Sequence in the Supercoiling Sensitivity of Bacterial Promoters. mSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Stracy, M.; Wollman, A.J.M.; Kaja, E.; Gapinski, J.; Lee, J.-E.; Leek, V.A.; McKie, S.J.; Mitchenall, L.A.; Maxwell, A.; Sherratt, D.J.; et al. Single-Molecule Imaging of DNA Gyrase Activity in Living Escherichia coli. Nucleic Acids Res. 2019, 47, 210–220. [Google Scholar] [CrossRef]
- Galvin, C.J.; Hobson, M.; Meng, J.X.; Ierokomos, A.; Ivanov, I.E.; Berger, J.M.; Bryant, Z. Single-Molecule Dynamics of DNA Gyrase in Evolutionarily Distant Bacteria Mycobacterium tuberculosis and Escherichia coli. J. Biol. Chem. 2023, 299, 103003. [Google Scholar] [CrossRef]
- Hobson, M.J.; Bryant, Z.; Berger, J.M. Modulated Control of DNA Supercoiling Balance by the DNA-Wrapping Domain of Bacterial Gyrase. Nucleic Acids Res. 2020, 48, 2035–2049. [Google Scholar] [CrossRef]
- Gubaev, A.; Klostermeier, D. The Mechanism of Negative DNA Supercoiling: A Cascade of DNA-Induced Conformational Changes Prepares Gyrase for Strand Passage. DNA Repair. 2014, 16, 23–34. [Google Scholar] [CrossRef]
- Ashley, R.E.; Dittmore, A.; McPherson, S.A.; Turnbough, C.L., Jr.; Neuman, K.C.; Osheroff, N. Activities of Gyrase and Topoisomerase IV on Positively Supercoiled DNA. Nucleic Acids Res. 2017, 45, 9611–9624. [Google Scholar] [CrossRef]
- Hirsch, J.; Klostermeier, D. What Makes a Type IIA Topoisomerase a Gyrase or a Topo IV? Nucleic Acids Res. 2021, 49, 6027–6042. [Google Scholar] [CrossRef]
- Basu, A.; Hobson, M.; Lebel, P.; Fernandes, L.E.; Tretter, E.M.; Berger, J.M.; Bryant, Z. Dynamic Coupling between Conformations and Nucleotide States in DNA Gyrase. Nat. Chem. Biol. 2018, 14, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Parente, A.C.; Bryant, Z. Structural Dynamics and Mechanochemical Coupling in DNA Gyrase. J. Mol. Biol. 2016, 428, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hauk, G.; Quigley, J.; Liang, L.; Son, S.; Ghiglieri, M.; Gates, M.F.; Morrissette, M.; Shahsavari, N.; Niles, S.; et al. Evybactin is a DNA Gyrase Inhibitor that Selectively Kills Mycobacterium tuberculosis. Nat. Chem. Biol. 2022, 18, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, E.; Hommernick, K.; Behroz, I.; Kulike, M.; Pakosz-Stępień, Z.; Mazurek, L.; Seidel, M.; Kunert, M.; Santos, K.; von Moeller, H.; et al. Molecular Mechanism of Topoisomerase Poisoning by the Peptide Antibiotic Albicidin. Nat. Catal. 2023, 6, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; Germe, T.; Bax, B.D.; Huang, J.; Thalji, R.K.; Bacqué, E.; Checchia, A.; Chen, D.; Cui, H.; Ding, X.; et al. Thiophene Antibacterials that Allosterically Stabilize DNA-Cleavage Complexes with DNA Gyrase. Proc. Natl. Acad. Sci. USA 2017, 114, E4492–E4500. [Google Scholar] [CrossRef]
- Schoeffler, A.J.; May, A.P.; Berger, J.M. A Domain Insertion in Escherichia coli GyrB Adopts a Novel Fold That Plays a Critical Role in Gyrase Function. Nucleic Acids Res. 2010, 38, 7830–7844. [Google Scholar] [CrossRef]
- Sada, M.; Kimura, H.; Nagasawa, N.; Akagawa, M.; Okayama, K.; Shirai, T.; Sunagawa, S.; Kimura, R.; Saraya, T.; Ishii, H.; et al. Molecular Evolution of the Pseudomonas Aeruginosa DNA Gyrase gyrA Gene. Microorganisms 2022, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, D.; Klostermeier, D. Functional Interactions between Gyrase Subunits are Optimized in a Species-Specific Manner. J. Biol. Chem. 2020, 295, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Duprey, A.; Groisman, E.A. The Regulation of DNA Supercoiling across Evolution. Protein Sci. 2021, 30, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Petrella, S.; Capton, E.; Raynal, B.; Giffard, C.; Thureau, A.; Bonneté, F.; Alzari, P.M.; Aubry, A.; Mayer, C. Overall Structures of Mycobacterium tuberculosis DNA Gyrase Reveal the Role of a Corynebacteriales GyrB-Specific Insert in ATPase Activity. Structure 2019, 27, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Mark Fisher, L.; Jarlier, V.; Cambau, E. First Functional Characterization of a Singly Expressed Bacterial Type II Topoisomerase: The Enzyme from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2006, 348, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Chen, Y.; Goh, Y.-X.; Wang, M.; Tai, C.; Deng, Z.; Song, J.; Ou, H.-Y. TADB 3.0: An Updated Database of Bacterial Toxin–Antitoxin Loci and Associated Mobile Genetic Elements. Nucleic Acids Res. 2023, 52, D784–D790. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Iredell, J. A ParDE-Family Toxin Antitoxin System in Major Resistance Plasmids of Enterobacteriaceae Confers Antibiotic and Heat Tolerance. Sci. Rep. 2019, 9, 9872. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; White, J.C.; Murphy, T.; Ames, J.R.; Bourne, C.R. The Toxin from a ParDE Toxin-antitoxin System Found in Pseudomonas Aeruginosa Offers Protection to Cells Challenged with Anti-gyrase Antibiotics. Mol. Microbiol. 2019, 111, 441–454. [Google Scholar] [CrossRef]
- Cooper, T.F.; Paixão, T.; Heinemann, J.A. Within-Host Competition Selects for Plasmid-Encoded Toxin–Antitoxin Systems. Proc. R. Soc. B Biol. Sci. 2010, 277, 3149–3155. [Google Scholar] [CrossRef]
- Guérout, A.-M.; Iqbal, N.; Mine, N.; Ducos-Galand, M.; Van Melderen, L.; Mazel, D. Characterization of the Phd-Doc and Ccd Toxin-Antitoxin Cassettes from Vibrio Superintegrons. J. Bacteriol. 2013, 195, 2270–2283. [Google Scholar] [CrossRef]
- Roberts, R.C.; Burioni, R.; Helinski, D.R. Genetic Characterization of the Stabilizing Functions of a Region of Broad-Host-Range Plasmid RK2. J. Bacteriol. 1990, 172, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Roberts, R.C.; Helinski, D.R. A Region of the Broad-Host-Range Plasmid RK2 Causes Stable in Planta Inheritance of Plasmids in Rhizobium Meliloti Cells Isolated from Alfalfa Root Nodules. J. Bacteriol. 1992, 174, 7486–7489. [Google Scholar] [CrossRef]
- Anantharaman, V.; Aravind, L. New Connections in the Prokaryotic Toxin-Antitoxin Network: Relationship with the Eukaryotic Nonsense-Mediated RNA Decay System. Genome Biol. 2003, 4, R81. [Google Scholar] [CrossRef]
- Pandey, D.P.; Gerdes, K. Toxin–Antitoxin Loci Are Highly Abundant in Free-Living but Lost from Host-Associated Prokaryotes. Nucleic Acids Res. 2005, 33, 966–976. [Google Scholar] [CrossRef]
- Yuan, J.; Yamaichi, Y.; Waldor, M.K. The Three Vibrio Cholerae Chromosome II-Encoded ParE Toxins Degrade Chromosome I Following Loss of Chromosome II. J. Bacteriol. 2011, 193, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Hallez, R.; Geeraerts, D.; Sterckx, Y.; Mine, N.; Loris, R.; Van Melderen, L. New Toxins Homologous to ParE Belonging to Three-Component Toxin–Antitoxin Systems in Escherichia coli O157:H7. Mol. Microbiol. 2010, 76, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Guo, Y.; Wang, P.; Zeng, Z.; Li, B.; Tang, K.; Liu, X.; Wang, X. Type II Toxin/Antitoxin System ParESO/CopASO Stabilizes Prophage CP4So in Shewanella Oneidensis. Environ. Microbiol. 2018, 20, 1224–1239. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Tang, K.; Wang, W.; Guo, Y.; Wang, X. Prophage Encoding Toxin/Antitoxin System PfiT/PfiA Inhibits Pf4 Production in Pseudomonas Aeruginosa. Microb. Biotechnol. 2020, 13, 1132–1144. [Google Scholar] [CrossRef]
- Sterckx, Y.G.J.; Garcia-Pino, A.; Haesaerts, S.; Jové, T.; Geerts, L.; Sakellaris, V.; Van Melderen, L.; Loris, R. The ParE2–PaaA2 Toxin–Antitoxin Complex from Escherichia coli O157 Forms a Heterodocecamer in Solution and in the Crystal. Acta Cryst. F 2012, 68, 724–729. [Google Scholar] [CrossRef]
- Zander, I.; Shmidov, E.; Roth, S.; Ben-David, Y.; Shoval, I.; Shoshani, S.; Danielli, A.; Banin, E. Characterization of PfiT/PfiA Toxin–Antitoxin System of Pseudomonas Aeruginosa that Affects Cell Elongation and Prophage Induction. Environ. Microbiol. 2020, 22, 5048–5057. [Google Scholar] [CrossRef]
- Roberts, R.C.; Ström, A.R.; Helinski, D.R. The parDE Operon of the Broad-Host-Range Plasmid RK2 Specifies Growth Inhibition Associated with Plasmid Loss. J. Mol. Biol. 1994, 237, 35–51. [Google Scholar] [CrossRef]
- Jiang, Y.; Pogliano, J.; Helinski, D.R.; Konieczny, I. ParE Toxin Encoded by the Broad-Host-Range Plasmid RK2 is an Inhibitor of Escherichia coli Gyrase. Mol. Microbiol. 2002, 44, 971–979. [Google Scholar] [CrossRef]
- Yuan, J.; Sterckx, Y.; Mitchenall, L.A.; Maxwell, A.; Loris, R.; Waldor, M.K. Vibrio Cholerae ParE2 Poisons DNA Gyrase via a Mechanism Distinct from Other Gyrase Inhibitors. J. Biol. Chem. 2010, 285, 40397–40408. [Google Scholar] [CrossRef]
- Fiebig, A.; Castro Rojas, C.M.; Siegal-Gaskins, D.; Crosson, S. Interaction Specificity, Toxicity and Regulation of a Paralogous Set of ParE/RelE-Family Toxin–Antitoxin Systems. Mol. Microbiol. 2010, 77, 236–251. [Google Scholar] [CrossRef]
- Gupta, M.; Nayyar, N.; Chawla, M.; Sitaraman, R.; Bhatnagar, R.; Banerjee, N. The Chromosomal parDE2 Toxin–Antitoxin System of Mycobacterium tuberculosis H37Rv: Genetic and Functional Characterization. Front. Microbiol. 2016, 7, 886. [Google Scholar] [CrossRef]
- Aakre, C.D.; Herrou, J.; Phung, T.N.; Perchuk, B.S.; Crosson, S.; Laub, M.T. Evolving New Protein-Protein Interaction Specificity through Promiscuous Intermediates. Cell 2015, 163, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Schott, S.; Scheuer, R.; Ermoli, F.; Glatter, T.; Evguenieva-Hackenberg, E.; Diepold, A. A ParDE Toxin–Antitoxin System is Responsible for the Maintenance of the Yersinia Virulence Plasmid but not for Type III Secretion-Associated Growth Inhibition. Front. Cell. Infect. Microbiol. 2023, 13, 1166077. [Google Scholar] [CrossRef] [PubMed]
- Gubaev, A.; Weidlich, D.; Klostermeier, D. DNA Gyrase with a Single Catalytic Tyrosine Can Catalyze DNA Supercoiling by a Nicking-Closing Mechanism. Nucleic Acids Res. 2016, 44, 10354–10366. [Google Scholar] [CrossRef] [PubMed]
- Soczek, K.M.; Grant, T.; Rosenthal, P.B.; Mondragón, A. CryoEM Structures of Open Dimers of Gyrase A in Complex with DNA Illuminate Mechanism of Strand Passage. eLife 2018, 7, e41215. [Google Scholar] [CrossRef]
- Sterckx, Y.G.-J.; Jové, T.; Shkumatov, A.V.; Garcia-Pino, A.; Geerts, L.; De Kerpel, M.; Lah, J.; De Greve, H.; Van Melderen, L.; Loris, R. A Unique Hetero-Hexadecameric Architecture Displayed by the Escherichia coli O157 PaaA2–ParE2 Antitoxin–Toxin Complex. J. Mol. Biol. 2016, 428, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Tripathi, A.; Sahu, A.; Varadarajan, R. Contribution of the Chromosomal ccdAB Operon to Bacterial Drug Tolerance. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef] [PubMed]
- Klemenčič, M.; Halužan Vasle, A.; Dolinar, M. The Cysteine Protease MaOC1, a Prokaryotic Caspase Homolog, Cleaves the Antitoxin of a Type II Toxin-Antitoxin System. Front. Microbiol. 2021, 12, 635684. [Google Scholar] [CrossRef]
- Snead, K.J.; Moore, L.L.; Bourne, C.R. ParD Antitoxin Hotspot Alters a Disorder-to-Order Transition upon Binding to Its Cognate ParE Toxin, Lessening Its Interaction Affinity and Increasing Its Protease Degradation Kinetics. Biochemistry 2022, 61, 34–45. [Google Scholar] [CrossRef]
- Miallau, L.; Jain, P.; Arbing, M.A.; Cascio, D.; Phan, T.; Ahn, C.J.; Chan, S.; Chernishof, I.; Maxson, M.; Chiang, J.; et al. Comparative Proteomics Identifies the Cell-Associated Lethality of M. Tuberculosis RelBE-like Toxin-Antitoxin Complexes. Structure 2013, 21, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.M.; Crosson, S. A Conserved Mode of Protein Recognition and Binding in a ParD-ParE Toxin-Antitoxin Complex. Biochemistry 2010, 49, 2205–2215. [Google Scholar] [CrossRef]
- Bernard, P.; Couturier, M. Cell Killing by the F Plasmid CcdB Protein Involves Poisoning of DNA-Topoisomerase II Complexes. J. Mol. Biol. 1992, 226, 735–745. [Google Scholar] [CrossRef]
- Ogura, T.; Hiraga, S. Mini-F Plasmid Genes That Couple Host Cell Division to Plasmid Proliferation. Proc. Natl. Acad. Sci. USA 1983, 80, 4784–4788. [Google Scholar] [CrossRef]
- Mine, N.; Guglielmini, J.; Wilbaux, M.; Van Melderen, L. The Decay of the Chromosomally Encoded ccdO157 Toxin–Antitoxin System in the Escherichia coli Species. Genetics 2009, 181, 1557–1566. [Google Scholar] [CrossRef]
- Saavedra De Bast, M.; Mine, N.; Van Melderen, L. Chromosomal Toxin-Antitoxin Systems May Act as Antiaddiction Modules. J. Bacteriol. 2008, 190, 4603–4609. [Google Scholar] [CrossRef]
- Figueroa-Bossi, N.; Balbontín, R.; Bossi, L. Scarless DNA Recombineering. Cold Spring Harb. Protoc. 2023, 2023. [Google Scholar] [CrossRef]
- Murakami, M.; Murakami, A.M.; Yonekura, M.; Miyoshi, I.; Itagaki, S.; Niwa, Y. A Simple, Dual Direct Expression Plasmid System in Prokaryotic and Mammalian Cells. PNAS Nexus 2023, 2, pgad139. [Google Scholar] [CrossRef]
- Menestreau, M.; Rachedi, R.; Risoul, V.; Foglino, M.; Latifi, A. The CcdB Toxin is an Efficient Selective Marker for CRISPR-Plasmids Developed for Genome Editing in Cyanobacteria. MicroPubl. Biol. 2022, 2022. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H. A Highly Sensitive Selection Method for Directed Evolution of Homing Endonucleases. Nucleic Acids Res. 2005, 33, e154. [Google Scholar] [CrossRef]
- López-Igual, R.; Dorado-Morales, P.; Mazel, D. Increasing the Scalability of Toxin–Intein Orthogonal Combinations. ACS Synth. Biol. 2023, 12, 618–623. [Google Scholar] [CrossRef]
- Bhowmick, J.; Nag, M.; Ghosh, P.; Rajmani, R.S.; Chatterjee, R.; Karmakar, K.; Chandra, K.; Chatterjee, J.; Chakravortty, D.; Varadarajan, R. A CcdB Toxin-Derived Peptide Acts as a Broad-Spectrum Antibacterial Therapeutic in Infected Mice. EMBO Rep. 2023, 24, e55338. [Google Scholar] [CrossRef]
- De Jonge, N.; Simic, M.; Buts, L.; Haesaerts, S.; Roelants, K.; Garcia-Pino, A.; Sterckx, Y.; De Greve, H.; Lah, J.; Loris, R. Alternative Interactions Define Gyrase Specificity in the CcdB Family. Mol. Microbiol. 2012, 84, 965–978. [Google Scholar] [CrossRef]
- Wilbaux, M.; Mine, N.; Guérout, A.-M.; Mazel, D.; Van Melderen, L. Functional Interactions between Coexisting Toxin-Antitoxin Systems of the Ccd Family in Escherichia coli O157:H7. J. Bacteriol. 2007, 189, 2712–2719. [Google Scholar] [CrossRef]
- Bahassi, E.M.; O’Dea, M.H.; Allali, N.; Messens, J.; Gellert, M.; Couturier, M. Interactions of CcdB with DNA Gyrase: Inactivation of GyrA, Poisoning of The Gyrase-DNA Complex, and the Antidote Action of CcdA. J. Biol. Chem. 1999, 274, 10936–10944. [Google Scholar] [CrossRef]
- Smith, A.B.; Maxwell, A. A Strand-Passage Conformation of DNA Gyrase is Required to Allow the Bacterial Toxin, CcdB, to Access Its Binding Site. Nucleic Acids Res. 2006, 34, 4667–4676. [Google Scholar] [CrossRef]
- Dao-Thi, M.-H.; Van Melderen, L.; De Genst, E.; Afif, H.; Buts, L.; Wyns, L.; Loris, R. Molecular Basis of Gyrase Poisoning by the Addiction Toxin CcdB. J. Mol. Biol. 2005, 348, 1091–1102. [Google Scholar] [CrossRef]
- Jonge, N.D.; Hohlweg, W.; Garcia-Pino, A.; Respondek, M.; Buts, L.; Haesaerts, S.; Lah, J.; Zangger, K.; Loris, R. Structural and Thermodynamic Characterization of Vibrio Fischeri CcdB. J. Biol. Chem. 2010, 285, 5606–5613. [Google Scholar] [CrossRef]
- Trovatti, E.; Cotrim, C.A.; Garrido, S.S.; Barros, R.S.; Marchetto, R. Peptides Based on CcdB Protein as Novel Inhibitors of Bacterial Topoisomerases. Bioorganic Med. Chem. Lett. 2008, 18, 6161–6164. [Google Scholar] [CrossRef]
- De Jonge, N.; Garcia-Pino, A.; Buts, L.; Haesaerts, S.; Charlier, D.; Zangger, K.; Wyns, L.; De Greve, H.; Loris, R. Rejuvenation of CcdB-Poisoned Gyrase by an Intrinsically Disordered Protein Domain. Mol. Cell 2009, 35, 154–163. [Google Scholar] [CrossRef]
- Simic, M.; De Jonge, N.; Loris, R.; Vesnaver, G.; Lah, J. Driving Forces of Gyrase Recognition by the Addiction Toxin CcdB. J. Biol. Chem. 2009, 284, 20002–20010. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Lotz, C.; Ortiz, J.; Lamour, V. Cryo-EM Structure of the Complete E. coli DNA Gyrase Nucleoprotein Complex. Nat. Commun. 2019, 10, 4935. [Google Scholar] [CrossRef]
- Kato, F.; Yoshizumi, S.; Yamaguchi, Y.; Inouye, M. Genome-Wide Screening for Identification of Novel Toxin-Antitoxin Systems in Staphylococcus Aureus. Appl. Environ. Microbiol. 2019, 85, e00915-19. [Google Scholar] [CrossRef]
- Kato, F.; Yamaguchi, Y.; Inouye, K.; Matsuo, K.; Ishida, Y.; Inouye, M. A Novel Gyrase Inhibitor from Toxin-Antitoxin System Expressed by Staphylococcus Aureus. FEBS J. 2022, 290, 1502–1518. [Google Scholar] [CrossRef]
- Severinov, K.; Semenova, E.; Kazakov, A.; Kazakov, T.; Gelfand, M.S. Low-Molecular-Weight Post-Translationally Modified Microcins. Mol. Microbiol. 2007, 65, 1380–1394. [Google Scholar] [CrossRef]
- Davagnino, J.; Herrero, M.; Furlong, D.; Moreno, F.; Kolter, R. The DNA Replication Inhibitor Microcin B17 is a Forty-Three-Amino-Acid Protein Containing Sixty Percent Glycine. Proteins Struct. Funct. Bioinform. 1986, 1, 230–238. [Google Scholar] [CrossRef]
- Collin, F.; Maxwell, A. The Microbial Toxin Microcin B17: Prospects for the Development of New Antibacterial Agents. J. Mol. Biol. 2019, 431, 3400–3426. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Baquero, M.-R.; del Campo, R.; Bravo-Vázquez, D.A. Microcins in Enterobacteriaceae: Peptide Antimicrobials in the Eco-Active Intestinal Chemosphere. Front. Microbiol. 2019, 10, 2261. [Google Scholar] [CrossRef]
- Genilloud, O.; Moreno, F.; Kolter, R. DNA Sequence, Products, and Transcriptional Pattern of the Genes Involved in Production of the DNA Replication Inhibitor Microcin B17. J. Bacteriol. 1989, 171, 1126–1135. [Google Scholar] [CrossRef]
- Montero, C.; Mateu, G.; Rodriguez, R.; Takiff, H. Intrinsic Resistance of Mycobacterium smegmatis to Fluoroquinolones May Be Influenced by New Pentapeptide Protein MfpA. Antimicrob. Agents Chemother. 2001, 45, 3387–3392. [Google Scholar] [CrossRef]
- San Millán, J.L.; Kolter, R.; Moreno, F. Evidence that Colicin X is Microcin B17. J. Bacteriol. 1987, 169, 2899–2901. [Google Scholar] [CrossRef]
- Ghilarov, D.; Stevenson, C.E.M.; Travin, D.Y.; Piskunova, J.; Serebryakova, M.; Maxwell, A.; Lawson, D.M.; Severinov, K. Architecture of Microcin B17 Synthetase: An Octameric Protein Complex Converting a Ribosomally Synthesized Peptide into a DNA Gyrase Poison. Mol. Cell 2019, 73, 749–762. [Google Scholar] [CrossRef]
- Yorgey, P.; Lee, J.; Kördel, J.; Vivas, E.; Warner, P.; Jebaratnam, D.; Kolter, R. Posttranslational Modifications in Microcin B17 Define an Additional Class of DNA Gyrase Inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 4519–4523. [Google Scholar] [CrossRef]
- Bayer, A.; Freund, S.; Nicholson, G.; Jung, G. Posttranslational Backbone Modifications in the Ribosomal Biosynthesis of the Glycine-Rich Antibiotic Microcin B17. Angew. Chem. Int. Ed. Engl. 1993, 32, 1336–1339. [Google Scholar] [CrossRef]
- Metelev, M.; Serebryakova, M.; Ghilarov, D.; Zhao, Y.; Severinov, K. Structure of Microcin B-Like Compounds Produced by Pseudomonas Syringae and Species Specificity of Their Antibacterial Action. J. Bacteriol. 2013, 195, 4129–4137. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.-J.; Yang, J.Y.; Jung, Y.-J.; Hong, S.G.; Kim, O.-S. Complete Genome Sequence of Pseudomonas Antarctica PAMC 27494, a Bacteriocin-Producing Psychrophile Isolated from Antarctica. J. Biotechnol. 2017, 259, 15–18. [Google Scholar] [CrossRef]
- Vizán, J.L.; Hernández-Chico, C.; del Castillo, I.; Moreno, F. The Peptide Antibiotic Microcin B17 Induces Double-Strand Cleavage of DNA Mediated by E. coli DNA Gyrase. EMBO J. 1991, 10, 467–476. [Google Scholar] [CrossRef]
- Pierrat, O.A.; Maxwell, A. The Action of the Bacterial Toxin Microcin B17: Insight into the Cleavage-Religation Reaction of DNA Gyrase. J. Biol. Chem. 2003, 278, 35016–35023. [Google Scholar] [CrossRef]
- Parks, W.M.; Bottrill, A.R.; Pierrat, O.A.; Durrant, M.C.; Maxwell, A. The Action of the Bacterial Toxin, Microcin B17, on DNA Gyrase. Biochimie 2007, 89, 500–507. [Google Scholar] [CrossRef]
- Pierrat, O.A.; Maxwell, A. Evidence for the Role of DNA Strand Passage in the Mechanism of Action of Microcin B17 on DNA Gyrase. Biochemistry 2005, 44, 4204–4215. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, Gene-Encoded Antibacterial Peptides from Enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef]
- De Smet, J.; Wagemans, J.; Boon, M.; Ceyssens, P.-J.; Voet, M.; Noben, J.-P.; Andreeva, J.; Ghilarov, D.; Severinov, K.; Lavigne, R. The Bacteriophage LUZ24 “Igy” Peptide Inhibits the Pseudomonas DNA Gyrase. Cell Rep. 2021, 36, 109567. [Google Scholar] [CrossRef]
- Kever, L.; Hünnefeld, M.; Brehm, J.; Heermann, R.; Frunzke, J. Identification of Gip as a Novel Phage-Encoded Gyrase Inhibitor Protein of Corynebacterium Glutamicum. Mol. Microbiol. 2021, 116, 1268–1280. [Google Scholar] [CrossRef]
- Ofir, G.; Sorek, R. Contemporary Phage Biology: From Classic Models to New Insights. Cell 2018, 172, 1260–1270. [Google Scholar] [CrossRef]
- Utsumi, R.; Nakamoto, Y.; Kawamukai, M.; Himeno, M.; Komano, T. Involvement of Cyclic AMP and Its Receptor Protein in Filamentation of an Escherichia coli Fic Mutant. J. Bacteriol. 1982, 151, 807–812. [Google Scholar] [CrossRef]
- Kawamukai, M.; Matsuda, H.; Fujii, W.; Nishida, T.; Izumoto, Y.; Himeno, M.; Utsumi, R.; Komano, T. Cloning of the Fic-1 Gene Involved in Cell Filamentation Induced by Cyclic AMP and Construction of a Delta Fic Escherichia coli Strain. J. Bacteriol. 1988, 170, 3864–3869. [Google Scholar] [CrossRef]
- Harms, A.; Stanger, F.V.; Scheu, P.D.; de Jong, I.G.; Goepfert, A.; Glatter, T.; Gerdes, K.; Schirmer, T.; Dehio, C. Adenylylation of Gyrase and Topo IV by FicT Toxins Disrupts Bacterial DNA Topology. Cell Rep. 2015, 12, 1497–1507. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, L.; Zhen, X.; Yang, D.; Li, C.; Chen, Y.; Wang, H.; Qu, Y.; Liu, X.; Yin, Y.; et al. A Secreted Effector with a Dual Role as a Toxin and as a Transcriptional Factor. Nat. Commun. 2022, 13, 7779. [Google Scholar] [CrossRef]
- Veyron, S.; Peyroche, G.; Cherfils, J. FIC Proteins: From Bacteria to Humans and Back Again. Pathog. Dis. 2018, 76, fty012. [Google Scholar] [CrossRef]
- Engel, P.; Goepfert, A.; Stanger, F.V.; Harms, A.; Schmidt, A.; Schirmer, T.; Dehio, C. Adenylylation Control by Intra- or Intermolecular Active-Site Obstruction in Fic Proteins. Nature 2012, 482, 107–110. [Google Scholar] [CrossRef]
- Lu, C.; Nakayasu, E.S.; Zhang, L.-Q.; Luo, Z.-Q. Identification of Fic-1 as an Enzyme That Inhibits Bacterial DNA Replication by AMPylating GyrB, Promoting Filament Formation. Sci. Signal. 2016, 9, ra11. [Google Scholar] [CrossRef]
- Lu, C.-H.; McCloskey, A.; Chen, F.-R.; Nakayasu, E.S.; Zhang, L.-Q.; Luo, Z.-Q. Fic Proteins Inhibit the Activity of Topoisomerase IV by AMPylation in Diverse Bacteria. Front. Microbiol. 2020, 11, 2084. [Google Scholar] [CrossRef]
- Castro-Roa, D.; Garcia-Pino, A.; De Gieter, S.; van Nuland, N.A.J.; Loris, R.; Zenkin, N. The Fic Protein Doc Uses an Inverted Substrate to Phosphorylate and Inactivate EF-Tu. Nat. Chem. Biol. 2013, 9, 811–817. [Google Scholar] [CrossRef]
- Martínez-Martínez, L.; Pascual, A.; Jacoby, G.A. Quinolone Resistance from a Transferable Plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-Mediated Quinolone Resistance. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Jacoby, G.; Cattoir, V.; Hooper, D.; Martínez-Martínez, L.; Nordmann, P.; Pascual, A.; Poirel, L.; Wang, M. Qnr Gene Nomenclature. Antimicrob. Agents Chemother. 2008, 52, 2297–2299. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Corcoran, M.A.; Hooper, D.C. Protective Effect of Qnr on Agents Other than Quinolones that Target DNA Gyrase. Antimicrob. Agents Chemother. 2015, 59, 6689–6695. [Google Scholar] [CrossRef]
- Tran, J.H.; Jacoby, G.A.; Hooper, D.C. Interaction of the Plasmid-Encoded Quinolone Resistance Protein Qnr with Escherichia coli DNA Gyrase. Antimicrob. Agents Chemother. 2005, 49, 118–125. [Google Scholar] [CrossRef]
- Kim, E.S.; Chen, C.; Braun, M.; Kim, H.Y.; Okumura, R.; Wang, Y.; Jacoby, G.A.; Hooper, D.C. Interactions between QnrB, QnrB Mutants, and DNA Gyrase. Antimicrob. Agents Chemother. 2015, 59, 5413–5419. [Google Scholar] [CrossRef]
- Hegde, S.S.; Vetting, M.W.; Mitchenall, L.A.; Maxwell, A.; Blanchard, J.S. Structural and Biochemical Analysis of the Pentapeptide Repeat Protein EfsQnr, a Potent DNA Gyrase Inhibitor. Antimicrob. Agents Chemother. 2011, 55, 110–117. [Google Scholar] [CrossRef]
- Saga, T.; Sabtcheva, S.; Mitsutake, K.; Ishii, Y.; Tateda, K.; Yamaguchi, K.; Kaku, M. Characterization of qnrB-Like Genes in Citrobacter Species of the American Type Culture Collection. Antimicrob. Agents Chemother. 2013, 57, 2863–2866. [Google Scholar] [CrossRef]
- Da Re, S.; Garnier, F.; Guérin, E.; Campoy, S.; Denis, F.; Ploy, M.-C. The SOS Response Promotes qnrB Quinolone-Resistance Determinant Expression. EMBO Rep. 2009, 10, 929–933. [Google Scholar] [CrossRef]
- Kim, H.B.; Park, C.H.; Gavin, M.; Jacoby, G.A.; Hooper, D.C. Cold Shock Induces qnrA Expression in Shewanella Algae. Antimicrob. Agents Chemother. 2011, 55, 414–416. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Hooper, D.C. Phylogenetic Analysis of Chromosomally Determined Qnr and Related Proteins. Antimicrob. Agents Chemother. 2013, 57, 1930–1934. [Google Scholar] [CrossRef]
- Mérens, A.; Matrat, S.; Aubry, A.; Lascols, C.; Jarlier, V.; Soussy, C.-J.; Cavallo, J.-D.; Cambau, E. The Pentapeptide Repeat Proteins MfpAMt and QnrB4 Exhibit Opposite Effects on DNA Gyrase Catalytic Reactions and on the Ternary Gyrase-DNA-Quinolone Complex. J. Bacteriol. 2009, 191, 1587–1594. [Google Scholar] [CrossRef]
- Hegde, S.S.; Vetting, M.W.; Roderick, S.L.; Mitchenall, L.A.; Maxwell, A.; Takiff, H.E.; Blanchard, J.S. A Fluoroquinolone Resistance Protein from Mycobacterium tuberculosis That Mimics DNA. Science 2005, 308, 1480–1483. [Google Scholar] [CrossRef]
- Feng, L.; Mundy, J.E.A.; Stevenson, C.E.M.; Mitchenall, L.A.; Lawson, D.M.; Mi, K.; Maxwell, A. The Pentapeptide-Repeat Protein, MfpA, Interacts with Mycobacterial DNA Gyrase as a DNA T-Segment Mimic. Proc. Natl. Acad. Sci. USA 2021, 118, e2016705118. [Google Scholar] [CrossRef]
- Tao, J.; Han, J.; Wu, H.; Hu, X.; Deng, J.; Fleming, J.; Maxwell, A.; Bi, L.; Mi, K. Mycobacterium Fluoroquinolone Resistance Protein B, a Novel Small GTPase, is Involved in the Regulation of DNA Gyrase and Drug Resistance. Nucleic Acids Res. 2013, 41, 2370–2381. [Google Scholar] [CrossRef]
- Biswas, P.; Sengupta, S.; Nagaraja, V. Evolution of YacG to Safeguard DNA Gyrase from External Perturbation. Res. Microbiol. 2023, 174, 104093. [Google Scholar] [CrossRef]
- Sengupta, S.; Nagaraja, V. YacG from Escherichia coli is a Specific Endogenous Inhibitor of DNA Gyrase. Nucleic Acids Res. 2008, 36, 4310–4316. [Google Scholar] [CrossRef]
- Vos, S.M.; Lyubimov, A.Y.; Hershey, D.M.; Schoeffler, A.J.; Sengupta, S.; Nagaraja, V.; Berger, J.M. Direct Control of Type IIA Topoisomerase Activity by a Chromosomally Encoded Regulatory Protein. Genes Dev. 2014, 28, 1485–1497. [Google Scholar] [CrossRef]
- Ramelot, T.A.; Cort, J.R.; Yee, A.A.; Semesi, A.; Edwards, A.M.; Arrowsmith, C.H.; Kennedy, M.A. NMR Structure of the Escherichia coli Protein YacG: A Novel Sequence Motif in the Zinc-Finger Family of Proteins. Proteins Struct. Funct. Bioinform. 2002, 49, 289–293. [Google Scholar] [CrossRef]
- Walker, S.S.; Labroli, M.; Painter, R.E.; Wiltsie, J.; Sherborne, B.; Murgolo, N.; Sher, X.; Mann, P.; Zuck, P.; Garlisi, C.G.; et al. Antibacterial Small Molecules Targeting the Conserved TOPRIM Domain of DNA Gyrase. PLoS ONE 2017, 12, e0180965. [Google Scholar] [CrossRef]
- Fisher, S.L. Glutamate Racemase as a Target for Drug Discovery. Microb. Biotechnol. 2008, 1, 345–360. [Google Scholar] [CrossRef]
- Ashiuchi, M.; Kuwana, E.; Yamamoto, T.; Komatsu, K.; Soda, K.; Misono, H. Glutamate Racemase is an Endogenous DNA Gyrase Inhibitor. J. Biol. Chem. 2002, 277, 39070–39073. [Google Scholar] [CrossRef] [PubMed]
- Ashiuchi, M.; Kuwana, E.; Komatsu, K.; Soda, K.; Misono, H. Differences in Effects on DNA Gyrase Activity between Two Glutamate Racemases of Bacillus subtilis, the Poly-γ-Glutamate Synthesis-Linking Glr Enzyme and the YrpC (MurI) Isozyme. FEMS Microbiol. Lett. 2003, 223, 221–225. [Google Scholar] [CrossRef]
- Sengupta, S.; Shah, M.; Nagaraja, V. Glutamate Racemase from Mycobacterium tuberculosis Inhibits DNA Gyrase by Affecting Its DNA-Binding. Nucleic Acids Res. 2006, 34, 5567–5576. [Google Scholar] [CrossRef]
- Sengupta, S.; Nagaraja, V. Inhibition of DNA Gyrase Activity by Mycobacterium smegmatis MurI. FEMS Microbiol. Lett. 2008, 279, 40–47. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Cho, C.-S.; Kim, S.S.; Sung, H.-C.; Yu, Y.G.; Cho, Y. Structure and Mechanism of Glutamate Racemase from Aquifex Pyrophilus. Nat. Struct. Mol. Biol. 1999, 6, 422–426. [Google Scholar] [CrossRef]
- Kim, K.-H.; Bong, Y.-J.; Park, J.K.; Shin, K.-J.; Hwang, K.Y.; Kim, E.E. Structural Basis for Glutamate Racemase Inhibition. J. Mol. Biol. 2007, 372, 434–443. [Google Scholar] [CrossRef]
- Lundqvist, T.; Fisher, S.L.; Kern, G.; Folmer, R.H.A.; Xue, Y.; Newton, D.T.; Keating, T.A.; Alm, R.A.; de Jonge, B.L.M. Exploitation of Structural and Regulatory Diversity in Glutamate Racemases. Nature 2007, 447, 817–822. [Google Scholar] [CrossRef]
- May, M.; Mehboob, S.; Mulhearn, D.C.; Wang, Z.; Yu, H.; Thatcher, G.R.J.; Santarsiero, B.D.; Johnson, M.E.; Mesecar, A.D. Structural and Functional Analysis of Two Glutamate Racemase Isozymes from Bacillus anthracis and Implications for Inhibitor Design. J. Mol. Biol. 2007, 371, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Poen, S. Biochemical, Biophysical, and Structural Characterisation of Glutamate Racemase from Mycobacterium tuberculosis and Mycobacterium smegmatis. Doctoral Dissertation, University of Otago, Dunedin, New Zealand, 2016. [Google Scholar]
- Ruzheinikov, S.N.; Taal, M.A.; Sedelnikova, S.E.; Baker, P.J.; Rice, D.W. Substrate-Induced Conformational Changes in Bacillus subtilis Glutamate Racemase and Their Implications for Drug Discovery. Structure 2005, 13, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Imajoh-Ohmi, S.; Hanaoka, F. Characterization of the Interaction between DNA Gyrase Inhibitor and DNA Gyrase of Escherichia coli. J. Biol. Chem. 2002, 277, 8949–8954. [Google Scholar] [CrossRef]
- Chatterji, M.; Nagaraja, V. GyrI: A Counter-Defensive Strategy against Proteinaceous Inhibitors of DNA Gyrase. EMBO Rep. 2002, 3, 261–267. [Google Scholar] [CrossRef]
- Baquero, M.R.; Bouzon, M.; Varea, J.; Moreno, F. sbmC, a Stationary-Phase Induced SOS Escherichia coli Gene, Whose Product Protects Cells from the DNA Replication Inhibitor Microcin B17. Mol. Microbiol. 1995, 18, 301–311. [Google Scholar] [CrossRef]
- Chatterji, M.; Sengupta, S.; Nagaraja, V. Chromosomally Encoded Gyrase Inhibitor GyrI Protects Escherichia coli against DNA-Damaging Agents. Arch. Microbiol. 2003, 180, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Vollmer, A.C.; LaRossa, R.A. In Vivo Titration of Mitomycin C Action by Four Escherichia coli Genomic Regions on Multicopy Plasmids. J. Bacteriol. 2001, 183, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Fielden, J.; Popović, M.; Ramadan, K. TEX264 at the Intersection of Autophagy and DNA Repair. Autophagy 2022, 18, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, M.J.; Gibney, S.A.; Burley, S.K. Crystal Structure of the Escherichia coli SbmC Protein that Protects Cells from the DNA Replication Inhibitor Microcin B17. Proteins Struct. Funct. Bioinform. 2002, 47, 403–407. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, S.; Tu, C.-H.; Bourne, C.R. Friend or Foe: Protein Inhibitors of DNA Gyrase. Biology 2024, 13, 84. https://doi.org/10.3390/biology13020084
Ruan S, Tu C-H, Bourne CR. Friend or Foe: Protein Inhibitors of DNA Gyrase. Biology. 2024; 13(2):84. https://doi.org/10.3390/biology13020084
Chicago/Turabian StyleRuan, Shengfeng, Chih-Han Tu, and Christina R. Bourne. 2024. "Friend or Foe: Protein Inhibitors of DNA Gyrase" Biology 13, no. 2: 84. https://doi.org/10.3390/biology13020084
APA StyleRuan, S., Tu, C.-H., & Bourne, C. R. (2024). Friend or Foe: Protein Inhibitors of DNA Gyrase. Biology, 13(2), 84. https://doi.org/10.3390/biology13020084







