The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications
Simple Summary
Abstract
1. Introduction
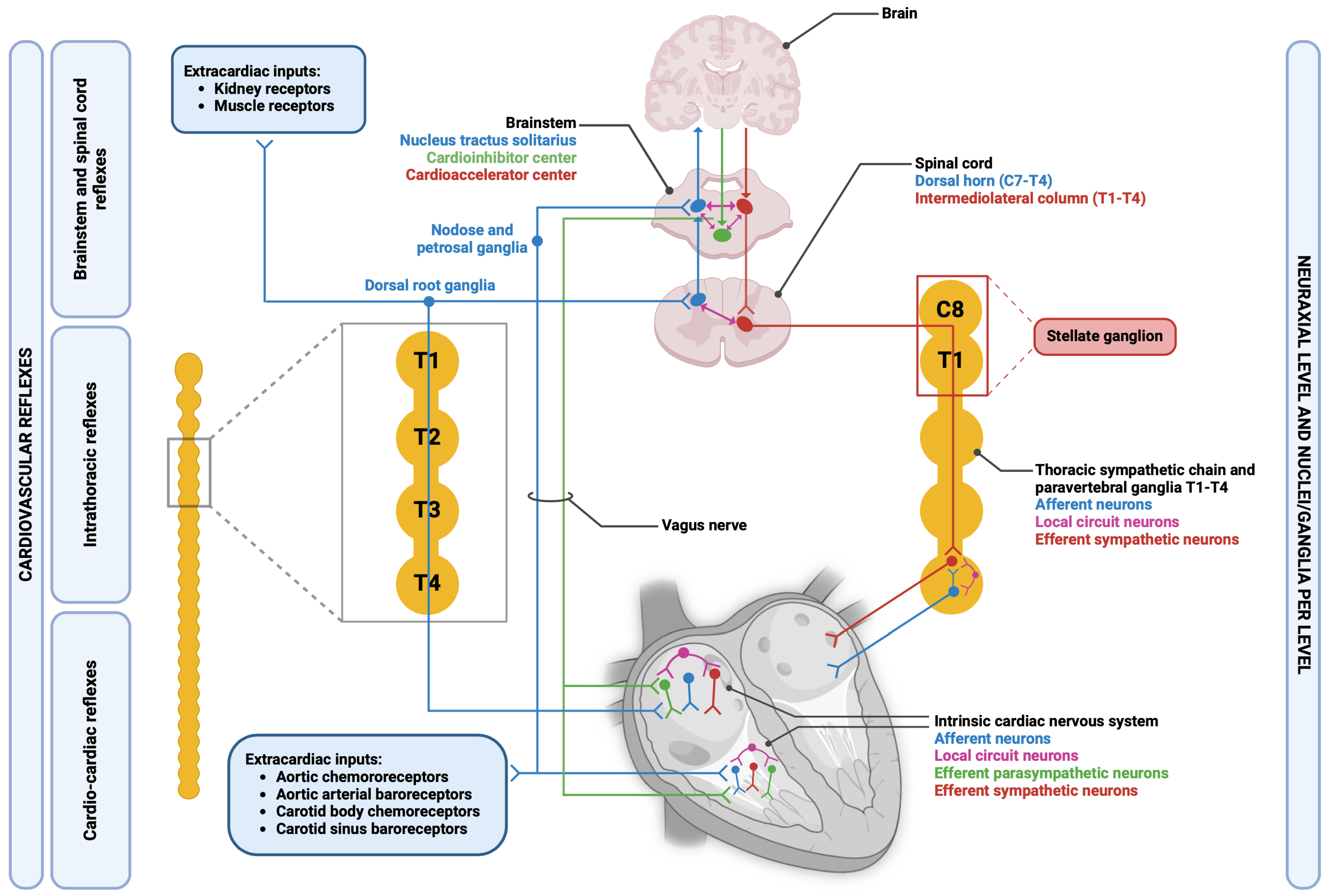
2. General Principles of Functioning of Cardiac Autonomic Nervous System
3. Embryological Origin of Cardiac Innervation
4. Anatomical and Functional Organization of CANS
4.1. Sympathetic Branch
4.2. Parasympathetic Branch
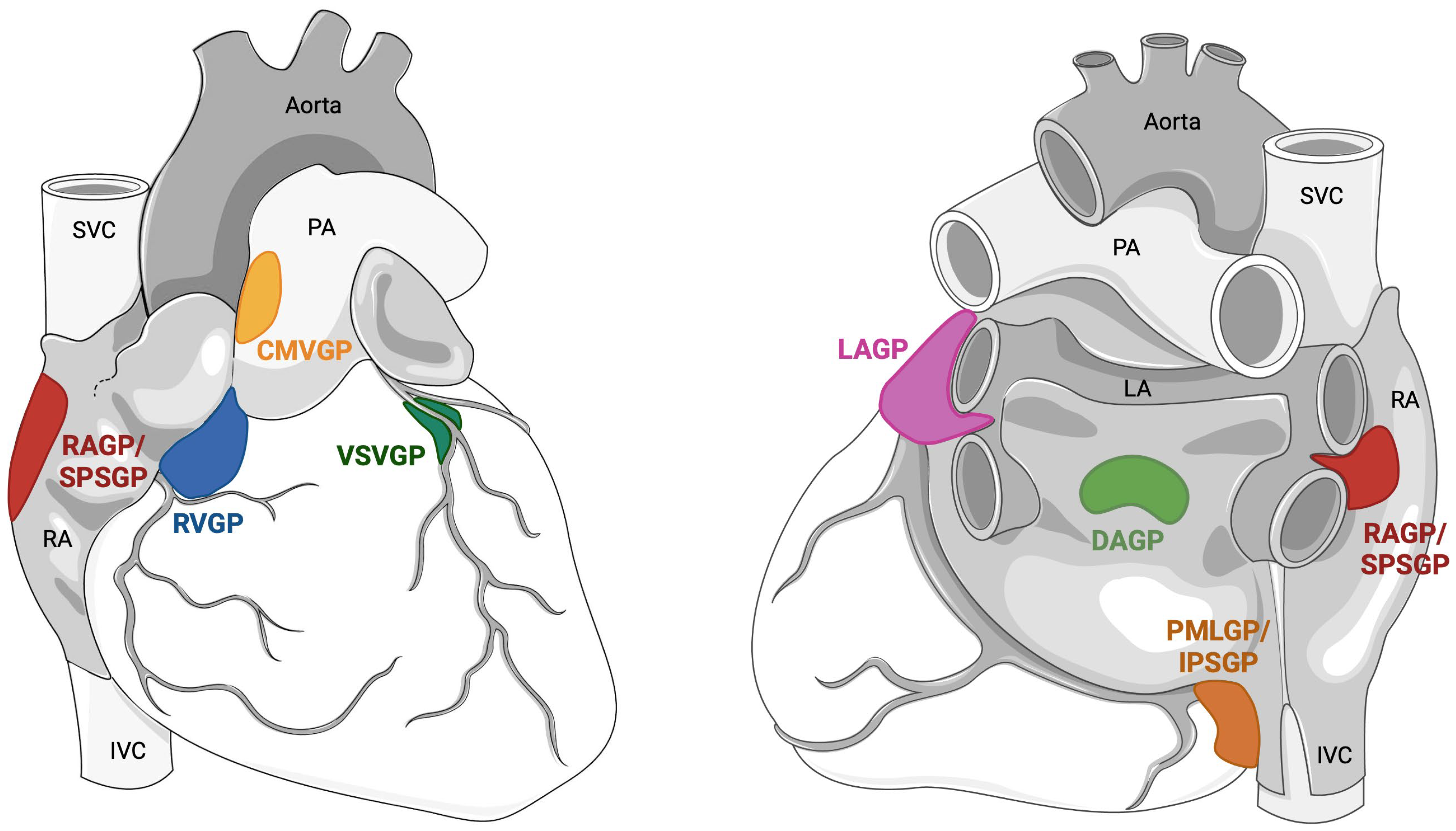
5. Anatomy of the Intrinsic Cardiac Nervous System
6. The Autonomic Neurocardiac Junction
6.1. The Sympathetic-Cardiac Junction
6.2. The Parasympathetic-Neurocardiac Junction
7. ICNS in Pathology: Pre-Clinical Data
7.1. Myocardial Infarction
7.2. Atrial Fibrillation
7.3. Ventricular Arrhythmias
7.4. Heart Failure
7.5. Heart Transplantation
8. ICNS as a Direct Therapeutic Target: Clinical Data
8.1. ICNS Modulation in Atrial Fibrillation
8.2. ICNS Modulation to Prevent Bradyarrhythmia
8.3. ICNS Modulation in Heart Failure
8.4. ICNS Preservation after OHT
9. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-Based Function. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: New York, NY, USA, 2016; pp. 1635–1653. Available online: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c150046 (accessed on 27 December 2023).
- Armour, J.A. Cardiac neuronal hierarchy in health and disease. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R262–R271. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Ghidoni, A.; Ravera, A.; De Ferrari, G.M.; Calvillo, L. Chemokines and Heart Disease: A Network Connecting Cardiovascular Biology to Immune and Autonomic Nervous Systems. Mediat. Inflamm. 2016, 2016, 5902947. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.N. Neural control of cardiac function. Baillieres Clin. Neurol. 1997, 6, 227–244. [Google Scholar] [PubMed]
- Végh, A.; Duim, S.; Smits, A.; Poelmann, R.; Ten Harkel, A.; DeRuiter, M.; Goumans, M.J.; Jongbloed, M.R.M. Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. J. Cardiovasc. Dev. Dis. 2016, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Durães Campos, I.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell. Cardiol. 2018, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W. Autonomic cardiac innervation: Development and adult plasticity. Organogenesis 2013, 9, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Kasemeier-Kulesa, J.C.; Bradley, R.; Pasquale, E.B.; Lefcort, F.; Kulesa, P.M. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development 2006, 133, 4839–4847. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, V.; Webb, S.; Bradshaw, L.; Brown, N.A.; Anderson, R.H.; Henderson, D.J. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J. Anat. 2008, 212, 1–11. [Google Scholar] [CrossRef]
- Morikawa, Y.; Zehir, A.; Maska, E.; Deng, C.; Schneider, M.D.; Mishina, Y.; Cserjesi, P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development 2009, 136, 3575–3584. [Google Scholar] [CrossRef]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 1999, 399, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, A.; Guillemot, F.; Brunet, J.F. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev. Biol. 2006, 295, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Quaife, C.J.; Palmiter, R.D. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 1995, 374, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.; Matsumoto, A.M.; Palmiter, R.D. Noradrenaline is essential for mouse fetal development. Nature 1995, 374, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Friend, D.S.; Sunday, M.E.; Singh, K.; Haley, K.; Austen, K.F.; Kelly, R.A.; Smith, T.W. An intrinsic adrenergic system in mammalian heart. J. Clin. Investig. 1996, 98, 1298–1303. [Google Scholar] [CrossRef]
- Ebert, S.N.; Rong, Q.; Boe, S.; Thompson, R.P.; Grinberg, A.; Pfeifer, K. Targeted insertion of the Cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: A new model for studying the developmental distribution of adrenergic cells. Dev. Dyn. 2004, 231, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Owji, A.; Varudkar, N.; Ebert, S.N. Therapeutic potential of Pnmt+ primer cells for neuro/myocardial regeneration. Am. J. Stem. Cells 2013, 2, 137–154. [Google Scholar]
- Tamura, Y.; Sano, M.; Nakamura, H.; Ito, K.; Sato, Y.; Shinmura, K.; Ieda, M.; Fujita, J.; Kurosawa, H.; Ogawa, S.; et al. Neural crest-derived resident cardiac cells contribute to the restoration of adrenergic function of transplanted heart in rodent. Cardiovasc. Res. 2016, 109, 350–357. [Google Scholar] [CrossRef]
- Huang, M.H.; Nguyen, V.; Wu, Y.; Rastogi, S.; Lui, C.Y.; Birnbaum, Y.; Wang, H.-Q.; Ware, D.L.; Chauhan, M.; Garg, N.; et al. Reducing ischaemia/reperfusion injury through -opioid-regulated intrinsic cardiac adrenergic cells: Adrenopeptidergic co-signalling. Cardiovasc. Res. 2009, 84, 452–460. [Google Scholar] [CrossRef]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 2005, 209, 425–438. [Google Scholar] [CrossRef]
- Geis, W.; Kaye, M.; Randall, W. Major autonomic pathways to the atria and S-A and A-V nodes of the canine heart. Am. J. Physiol. Content. 1973, 224, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Randall, W.C.; Rohse, W.G. The Augmentor Action of the Sympathetic Cardiac Nerves. Circ. Res. 1956, 4, 470–475. [Google Scholar] [CrossRef]
- Chauhan, R.A.; Coote, J.; Allen, E.; Pongpaopattanakul, P.; Brack, K.E.; Ng, G.A. Functional selectivity of cardiac preganglionic sympathetic neurones in the rabbit heart. Int. J. Cardiol. 2018, 264, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Stone, H.L. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ. Res. 1979, 44, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; De Ferrari, G.M.; Pugliese, L.; Schwartz, P.J. Cardiac Sympathetic Denervation in Channelopathies. Front. Cardiovasc. Med. 2019, 6, 27. [Google Scholar] [CrossRef]
- Dusi, V.; Sorg, J.M.; Gornbein, J.; Gima, J.; Yanagawa, J.; Lee, J.M.; Vecerek, N.; Vaseghi, M.; Bradfield, J.S.; De Ferrari, G.M.; et al. Prognostic impact of atrial rhythm and dimension in patients with structural heart disease undergoing cardiac sympathetic denervation for ventricular arrhythmias. Heart Rhythm 2020, 17, 714–720. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Challis, R.C.; Fowlkes, C.C.; Hanna, P.; Tompkins, J.D.; Jordan, M.C.; Hiyari, S.; Gabris-Weber, B.A.; Greenbaum, A.; Chan, K.Y.; et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat. Commun. 2019, 10, 1944. [Google Scholar] [CrossRef]
- Yanowitz, F.; Preston, J.B.; Abildskov, J.A. Functional Distribution of Right and Left Stellate Innervation to the Ventricles: Production of Neurogenic Electrocardiographs Changes by Unilateral Alteration of Sympathetic TONE. Circ. Res. 1966, 18, 416–428. [Google Scholar] [CrossRef]
- Randall, W.C.; Armour, J.A.; Geis, W.P.; Lippincott, D.B. Regional cardiac distribution of the sympathetic nerves. Fed. Proc. 1972, 31, 1199–1208. [Google Scholar]
- Haws, C.W.; Burgess, M.J. Effects of bilateral and unilateral stellate stimulation on canine ventricular refractory periods at sites overlapping innervation. Circ. Res. 1978, 42, 195–198. [Google Scholar] [CrossRef]
- Janse, M.J.; Schwartz, P.J.; Wilms-Schopman, F.; Peters, R.J.; Durrer, D. Effects of unilateral stellate ganglion stimulation and ablation on electrophysiologic changes induced by acute myocardial ischemia in dogs. Circulation 1985, 72, 585–595. [Google Scholar] [CrossRef]
- Vaseghi, M.; Zhou, W.; Shi, J.; Ajijola, O.A.; Hadaya, J.; Shivkumar, K.; Mahajan, A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm 2012, 9, 1303–1309. [Google Scholar] [CrossRef]
- Holmgren, S.; Abrahamsson, T.; Almgren, O. Adrenergic innervation of coronary arteries and ventricular myocardium in the pig: Fluorescence microscopic appearance in the normal state and after ischemia. Basic Res. Cardiol. 1985, 80, 18–26. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K.; Mya-Tu, M.; Zetterström, B.E.M. Observations on adrenergic innervation of dog heart. Am. J. Physiol. Content 1965, 209, 689–692. [Google Scholar] [CrossRef]
- Jacobowitz, D.; Cooper, T.; Barner, H.B. Histochemical and Chemical Studies of the Localization of Adrenergic and Cholinergic Nerves in Normal and Denervated Cat Hearts. Circ. Res. 1967, 20, 289–298. [Google Scholar] [CrossRef]
- Kawano, H.; Okada, R.; Yano, K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessel. 2003, 18, 32–39. [Google Scholar] [CrossRef]
- Habecker, B.A.; Anderson, M.E.; Birren, S.J.; Fukuda, K.; Herring, N.; Hoover, D.B.; Kanazawa, H.; Paterson, D.J.; Ripplinger, C.M. Molecular and cellular neurocardiology: Development, and cellular and molecular adaptations to heart disease. J. Physiol. 2016, 594, 3853–3875. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Zipes, D.P. Efferent sympathetic and vagal innervation of the canine right ventricle. Circulation 1994, 90, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Randall, W.C. In vivo papillary muscle responses to cardiac nerve stimulation. Exp. Biol. Med. 1970, 133, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Gatti, P.J.; Johnson, T.A.; John Massari, V. Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction? J. Auton. Nerv. Syst. 1996, 57, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, L.W.; Rodak, D.J.; Fleming, T.J.; Gatti, P.J.; Massari, V.J.; McKenzie, J.C.; Gillis, R.A. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J. Auton. Nerv. Syst. 1998, 70, 129–141. [Google Scholar] [CrossRef]
- Gray, A.L.; Johnson, T.A.; Lauenstein, J.M.; Newton, S.S.; Ardell, J.L.; Massari, V.J. Parasympathetic control of the heart. III. Neuropeptide Y-immunoreactive nerve terminals synapse on three populations of negative chronotropic vagal preganglionic neurons. J. Appl. Physiol. 2004, 96, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Machhada, A.; Trapp, S.; Spyer, K.M. Cardiac vagal preganglionic neurones: An update. Auton. Neurosci. 2016, 199, 24–28. [Google Scholar] [CrossRef]
- Machhada, A.; Hosford, P.S.; Dyson, A.; Ackland, G.L.; Mastitskaya, S.; Gourine, A.V. Optogenetic Stimulation of Vagal Efferent Activity Preserves Left Ventricular Function in Experimental Heart Failure. JACC Basic Transl. Sci. 2020, 5, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; De Ferrari, G.M.; Mann, D.L. Cardiac Sympathetic-Parasympathetic Interaction. JACC Basic Transl. Sci. 2020, 5, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Butler, C.K.; Smith, F.M.; Hopkins, D.A.; Armour, J.A. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am. J. Physiol.-Heart Circ. Physiol. 1991, 260, H713–H721. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.L.; Johnson, T.A.; Ardell, J.L.; Massari, V.J. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J. Appl. Physiol. 2004, 96, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- McAllen, R.M.; Salo, L.M.; Paton, J.F.R.; Pickering, A.E. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: An intracellular analysis. J. Physiol. 2011, 589, 5801–5818. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, E.; Salavatian, S.; Southerland, E.M.; Vinet, A.; Jacquemet, V.; Armour, J.A.; Ardell, J.L. Network interactions within the canine intrinsic cardiac nervous system: Implications for reflex control of regional cardiac function. J. Physiol. 2013, 591, 4515–4533. [Google Scholar] [CrossRef]
- Yuan, B.X.; Ardell, J.L.; Hopkins, D.A.; Armour, J.A. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc. Res. 1993, 27, 760–769. [Google Scholar] [CrossRef]
- Petraitiene, V.; Pauza, D.H.; Benetis, R. Distribution of adrenergic and cholinergic nerve fibres within intrinsic nerves at the level of the human heart hilum. Eur. J. Cardio-Thorac Surg. 2014, 45, 1097–1105. [Google Scholar] [CrossRef]
- “Über Herzreizung und ihr Verhältniss zum Blutdruck: (Aus dem XXXVIII. Bde, S. 345, des Jahrg. 1859 der Sitzungsberichte der Math.-Naturn. Cl. der K. A. d. W. Besonders Abgedruckt.)”—Details|MDZ. Available online: https://www.digitale-sammlungen.de/en/details/bsb10368227 (accessed on 31 January 2024).
- Tcheng, K.T. Innervation of the dog’s heart. Am. Heart J. 1951, 41, 512–524. [Google Scholar] [CrossRef]
- Davies, F.; Francis, E.T.B.; King, T.S. Neurological studies of the cardiac ventricles of mammals. J Anat. 1952, 86 Pt 2, 130–143. [Google Scholar] [PubMed]
- Coote, J.H. Myths and realities of the cardiac vagus. J. Physiol. 2013, 591, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.Z.; Roeske, W.R.; Morkin, E.; Yamamura, H.I. Cardiac muscarinic cholinergic receptors. Biochemical identification and characterization. J. Biol. Chem. 1978, 253, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Ulphani, J.S.; Cain, J.H.; Inderyas, F.; Gordon, D.; Gikas, P.V.; Shade, G.; Mayor, D.; Arora, R.; Kadish, A.H.; Goldberger, J.J. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm 2010, 7, 1113–1119. [Google Scholar] [CrossRef]
- Armour, J.A.; Murphy, D.A.; Yuan, B.X.; MacDonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Kimura, K.; Ieda, M.; Fukuda, K. Development, Maturation, and Transdifferentiation of Cardiac Sympathetic Nerves. Circ. Res. 2012, 110, 325–336. [Google Scholar] [CrossRef]
- Pauza, D.H.; Pauziene, N.; Pakeltyte, G.; Stropus, R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann. Anat. Anat. Anz. 2002, 184, 125–136. [Google Scholar] [CrossRef]
- Wake, E.; Brack, K. Characterization of the intrinsic cardiac nervous system. Auton. Neurosci. Basic Clin. 2016, 199, 3–16. [Google Scholar] [CrossRef]
- Fedele, L.; Brand, T. The Intrinsic Cardiac Nervous System and Its Role in Cardiac Pacemaking and Conduction. J. Cardiovasc. Dev. Dis. 2020, 7, 54. [Google Scholar] [CrossRef]
- Brignole, M.; Aksu, T.; Calò, L.; Debruyne, P.; Deharo, J.C.; Fanciulli, A.; Fedorowski, A.; Kulakowski, P.; Morillo, C.; Moya, A.; et al. Clinical controversy: Methodology and indications of cardioneuroablation for reflex syncope. Europace 2023, 25, euad033. [Google Scholar] [CrossRef]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-Based Function. Compr. Physiol. 2016, 6, 1635–1653. [Google Scholar] [PubMed]
- Leiria, T.L.L.; Glavinovic, T.; Armour, J.A.; Cardinal, R.; de Lima, G.G.; Kus, T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton. Neurosci. Basic Clin. 2011, 161, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Autonomic Neurotransmission: 60 Years Since Sir Henry Dale. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 1–30. [Google Scholar] [CrossRef]
- Wei, W.; Smrcka, A.V. Subcellular β-Adrenergic Receptor Signaling in Cardiac Physiology and Disease. J. Cardiovasc. Pharmacol. 2022, 80, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kokoz, Y.M.; Evdokimovskii, E.V.; Maltsev, A.V.; Nenov, M.N.; Nakipova, O.V.; Averin, A.S.; Pimenov, O.Y.; Teplov, I.Y.; Berezhnov, A.V.; Reyes, S.; et al. Sarcolemmal α2-adrenoceptors control protective cardiomyocyte-delimited sympathoadrenal response. J. Mol. Cell Cardiol. 2016, 100, 9–20. [Google Scholar] [CrossRef]
- Evdokimovskii, E.V.; Jeon, R.; Park, S.; Pimenov, O.Y.; Alekseev, A.E. Role of α2-Adrenoceptor Subtypes in Suppression of L-Type Ca2+ Current in Mouse Cardiac Myocytes. Int. J. Mol. Sci. 2021, 22, 4135. [Google Scholar] [CrossRef]
- Schobesberger, S.; Wright, P.T.; Poulet, C.; Sanchez Alonso Mardones, J.L.; Mansfield, C.; Friebe, A.; Harding, S.E.; Balligand, J.-L.; Nikolaev, V.O.; Gorelik, J. β3-Adrenoceptor redistribution impairs NO/cGMP/PDE2 signalling in failing cardiomyocytes. eLife 2020, 9, e52221. [Google Scholar] [CrossRef]
- Choate, J.K.; Edwards, F.R.; Hirst, G.D.; O’Shea, J.E. Effects of sympathetic nerve stimulation on the sino-atrial node of the guinea-pig. J. Physiol. 1993, 471, 707–727. [Google Scholar] [CrossRef]
- Bramich, N.J.; Brock, J.A.; Edwards, F.R.; Hirst, G.D. Responses to sympathetic nerve stimulation of the sinus venosus of the toad. J. Physiol. 1993, 461, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Ajijola, O.A.; Vaseghi, M.; Zhou, W.; Yamakawa, K.; Benharash, P.; Hadaya, J.; Lux, R.L.; Mahajan, A.; Shivkumar, K.; Howard-Quijano, K.; et al. Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T. Sympathetic innervation improves the contractile performance of neonatal cardiac ventricular myocytes in culture. J. Mol. Cell Cardiol. 1990, 22, 333–342. [Google Scholar] [CrossRef]
- van Weperen, V.Y.H.; Ripplinger, C.M.; Vaseghi, M. Autonomic control of ventricular function in health and disease: Current state of the art. Clin. Auton. Res. 2023, 33, 491–517. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Do some nerve cells release more than one transmitter? Neuroscience 1976, 1, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Herring, N. Autonomic control of the heart: Going beyond the classical neurotransmitters. Exp. Physiol. 2015, 100, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Cranley, J.; Lokale, M.N.; Li, D.; Shanks, J.; Alston, E.N.; Girard, B.M.; Carter, E.; Parsons, R.L.; Habecker, B.A.; et al. The cardiac sympathetic co-transmitter galanin reduces acetylcholine release and vagal bradycardia: Implications for neural control of cardiac excitability. J. Mol. Cell Cardiol. 2012, 52, 667–676. [Google Scholar] [CrossRef]
- Beaulieu, P. Peptidic regulation of heart rate and interactions with the autonomic nervous system. Cardiovasc. Res. 1998, 37, 578–585. [Google Scholar] [CrossRef]
- Potter, E.K. Prolonged non-adrenergic inhibition of cardiac vagal action following sympathetic stimulation: Neuromodulation by neuropeptide Y? Neurosci. Lett. 1985, 54, 117–121. [Google Scholar] [CrossRef]
- Dusi, V.; De Ferrari, G.M.; Schwartz, P.J. There are 100 ways by which the sympathetic nervous system can trigger life-threatening arrhythmias. Eur. Heart J. 2020, 41, 2180–2182. [Google Scholar] [CrossRef]
- Kalla, M.; Hao, G.; Tapoulal, N.; Tomek, J.; Liu, K.; Woodward, L.; Dall’Armellina, E.; Banning, A.P.; Choudhury, R.P.; Neubauer, S.; et al. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur. Heart J. 2020, 41, 2168–2179. [Google Scholar] [CrossRef]
- Edvinsson, L.; Copeland, J.R.; Emson, P.C.; McCulloch, J.; Uddman, R. Nerve fibers containing neuropeptide Y in the cerebrovascular bed: Immunocytochemistry, radioimmunoassay, and vasomotor effects. J. Cereb. Blood Flow Metab. 1987, 7, 45–57. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral Control of Sinoatrial Node Activity and Heart Rate: Insight from Experimental Models and Findings from Humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Introduction and Perspective, Historical Note. Front. Cell Neurosci. 2013, 21, 227. Available online: http://journal.frontiersin.org/article/10.3389/fncel.2013.00227/abstract (accessed on 27 December 2023). [CrossRef] [PubMed]
- Burnstock, G.; Pelleg, A. Cardiac purinergic signalling in health and disease. Purinergic. Signal. 2015, 11, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.; Harmer, S.C.; Tinker, A. The control of cardiac ventricular excitability by autonomic pathways. Pharmacol. Ther. 2017, 174, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bywater, R.A.; Campbell, G.; Edwards, F.R.; Hirst, G.D.; O’Shea, J.E. The effects of vagal stimulation and applied acetylcholine on the sinus venosus of the toad. J. Physiol. 1989, 415, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.D.; Edwards, F.R.; Hirst, G.D.; O’Shea, J.E. Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea-pig heart. J. Physiol. 1989, 415, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D. Nitric oxide regulation of autonomic function in heart failure. Curr. Heart Fail. Rep. 2009, 6, 71–80. [Google Scholar] [CrossRef]
- Henning, R. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc. Res. 2001, 49, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Ardell, J.L. Brain-Heart Afferent-Efferent Traffic. In Brain and Heart Dynamics; Govoni, S., Politi, P., Vanoli, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–24. Available online: http://link.springer.com/10.1007/978-3-030-28008-6_2 (accessed on 3 January 2024).
- Reeves, T.J.; Hefner, L.L. The effect of vagal stimulation on ventricular contractility. Trans. Assoc. Am. Physicians 1961, 74, 260–270. [Google Scholar] [PubMed]
- Du, X.Y.; Schoemaker, R.G.; Bos, E.; Saxena, P.R. Characterization of the positive and negative inotropic effects of acetylcholine in the human myocardium. Eur. J. Pharmacol. 1995, 284, 119–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilmour, R.F.; Zipes, D.P. Positive inotropic effect of acetylcholine in canine cardiac Purkinje fibers. Am. J. Physiol. 1985, 249, H735–H740. [Google Scholar] [CrossRef]
- Yang, J.M.; Chung, K.T.; Yang, S.N. Muscarinic activation causes biphasic inotropic response and decreases cellular Na+ activity in canine cardiac Purkinje fibers. J. Biomed. Sci. 1999, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Mizukawa, Y.; Urushidani, T.; Tachibana, S.; Naito, Y. An inotropic action caused by muscarinic receptor subtype 3 in canine cardiac purkinje fibers. ISRN Pharmacol. 2013, 2013, 207671. [Google Scholar] [CrossRef] [PubMed]
- Buccino, R.A.; Sonnenblick, E.H.; Cooper, T.; Braunwald, E. Direct Positive Inotropic Effect of Acetylcholine on Myocardium. Circ. Res. 1966, 19, 1097–1108. [Google Scholar] [CrossRef]
- Hopkins, D.A.; Macdonald, S.E.; Murphy, D.A.; Armour, J.A. Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat. Rec. 2000, 259, 424–436. [Google Scholar] [CrossRef]
- Hardwick, J.C.; Southerland, E.M.; Ardell, J.L. Chronic myocardial infarction induces phenotypic and functional remodeling in the guinea pig cardiac plexus. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1926–R1933. [Google Scholar] [CrossRef]
- Hardwick, J.C.; Ryan, S.E.; Beaumont, E.; Ardell, J.L.; Southerland, E.M. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton. Neurosci. Basic Clin. 2014, 181, 4–12. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Nakamura, K.; Ajijola, O.A.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Southerland, E.M. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J. Physiol. 2016, 594, 321–341. [Google Scholar] [CrossRef]
- Vaseghi, M.; Salavatian, S.; Rajendran, P.S.; Yagishita, D.; Woodward, W.R.; Hamon, D.; Yamakawa, K.; Irie, T.; Habecker, B.A.; Shivkumar, K. Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight 2017, 2, e86715. [Google Scholar] [CrossRef]
- Dusi, V.; De Ferrari, G.M. Vagal stimulation in heart failure. Herz 2021, 46, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Angelini, F.; Zile, M.R.; De Ferrari, G.M. Neuromodulation devices for heart failure. Eur. Hear. J. Suppl. 2022, 24 (Suppl. E), E12–E27. [Google Scholar] [CrossRef] [PubMed]
- Magyar, T.; Árpádffy-Lovas, T.; Pászti, B.; Tóth, N.; Szlovák, J.; Gazdag, P.; Kohajda, Z.; Gyökeres, A.; Györe, B.; Gurabi, Z.; et al. Muscarinic agonists inhibit the ATP-dependent potassium current and suppress the ventricle-Purkinje action potential dispersion. Can. J. Physiol. Pharmacol. 2021, 99, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, J.O.; Laurikainen, A.; Airaksinen, M.S.; Saarma, M. GDNF family receptors in the embryonic and postnatal rat heart and reduced cholinergic innervation in mice hearts lacking ret or GFRalpha2. Dev. Dyn. 2000, 219, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Ieda, M.; Kimura, K.; Arai, T.; Kawaguchi-Manabe, H.; Matsuhashi, T.; Endo, J.; Sano, M.; Kawakami, T.; Kimura, T.; et al. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J. Clin. Investig. 2010, 120, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Scherlag, B.J.; Po, S. The intrinsic cardiac nervous system and atrial fibrillation. Curr. Opin. Cardiol. 2006, 21, 51–54. [Google Scholar] [CrossRef]
- Alexander, S.; Mathie, A.; Peters, J. Guide to Receptors and Channels (GRAC), 5th Edition. Br. J. Pharmacol. 2011, 164, S1–S2. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2011.01649_1.x (accessed on 19 December 2023). [CrossRef]
- Jamali, H.K.; Waqar, F.; Gerson, M.C. Cardiac autonomic innervation. J. Nucl. Cardiol. 2017, 24, 1558–1570. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Tang, Q.; Jiang, H.; Huang, C.; He, B.; Hu, X.; Huang, J.; Zhu, X.; Wang, H. The increase in sympathetic nerve density in the atrium facilitates atrial fibrillation in patients with rheumatic heart disease. Int. J. Cardiol. 2013, 165, 174–178. [Google Scholar] [CrossRef]
- Patterson, E.; Po, S.S.; Scherlag, B.J.; Lazzara, R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005, 2, 624–631. [Google Scholar] [CrossRef]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Lu, Z.; Scherlag, B.J.; Lin, J.; Niu, G.; Fung, K.M.; Zhao, L.; Ghias, M.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Atrial fibrillation begets atrial fibrillation: Autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ. Arrhythm. Electrophysiol. 2008, 1, 184–192. [Google Scholar] [CrossRef]
- Yu, L.; Scherlag, B.J.; Sha, Y.; Li, S.; Sharma, T.; Nakagawa, H.; Jackman, W.M.; Lazzara, R.; Jiang, H.; Po, S.S. Interactions between atrial electrical remodeling and autonomic remodeling: How to break the vicious cycle. Heart Rhythm 2012, 9, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.; Chang, H.; Scherlag, B.J.; Lin, Y.; Chou, Y.; Lin, W.; Chen, S.; Po, S.S. Temporary Suppression of Cardiac Ganglionated Plexi Leads to Long-Term Suppression of Atrial Fibrillation: Evidence of Early Autonomic Intervention to Break the Vicious Cycle of “AF Begets AF”. J. Am. Heart Assoc. 2016, 5, e003309. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Scherlag, B.J.; Lin, J.; Zhang, Y.; Lu, Z.; Truong, K.; Patterson, E.; Lazzara, R.; Jackman, W.M.; Po, S.S. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J. Am. Coll. Cardiol. 2007, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Zhang, Y.; Xie, X.; Wang, W.; Li, Z.; Gao, M.; Wang, Z.; Hou, Y. Long-Term Effects of Ganglionated Plexi Ablation on Electrophysiological Characteristics and Neuron Remodeling in Target Atrial Tissues in a Canine Model. Circ. Arrhythm. Electrophysiol. 2015, 8, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Scherlag, B.J.; Yu, L.; Sheng, X.; Zhang, Y.; Ali, R.; Dong, Y.; Ghias, M.; Po, S.S. Low-level vagosympathetic stimulation: A paradox and potential new modality for the treatment of focal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2009, 2, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Scherlag, B.J.; Li, S.; Fan, Y.; Dyer, J.; Male, S.; Varma, V.; Sha, Y.; Stavrakis, S.; Po, S.S. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: A noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm 2013, 10, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, X.; Liu, Q.; Sheng, X.; Yu, L.; Wang, Z.; Wang, S.; Zhou, S. Left-sided Noninvasive Vagus Nerve Stimulation Suppresses Atrial Fibrillation by Upregulating Atrial Gap Junctions in Canines. J. Cardiovasc. Pharmacol. 2015, 66, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, T.; Wan, J.; Yuan, Y.; Chen, P.S. Neural Mechanisms and Therapeutic Opportunities for Atrial Fibrillation. Methodist DeBakey Cardiovasc. J. 2021, 17, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Pugliese, L.; De Ferrari, G.M.; Odero, A.; Crotti, L.; Dagradi, F.; Castelletti, S.; Vicentini, A.; Rordorf, R.; Li, C.; et al. Left Cardiac Sympathetic Denervation for Long QT Syndrome: 50 Years’ Experience Provides Guidance for Management. JACC Clin. Electrophysiol. 2022, 8, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Angelini, F.; Gravinese, C.; Frea, S.; De Ferrari, G.M. Electrical storm management in structural heart disease. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C242–C248. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Baldi, E.; Compagnoni, S.; Rordorf, R.; Sanzo, A.; Gentile, F.R.; Dusi, V.; Frea, S.; Gravinese, C.; Cauti, F.M.; et al. Electrical storm treatment by percutaneous stellate ganglion block: The STAR study. Eur. Heart J. 2024, 30, ehae021. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, R.; Pagé, P.; Vermeulen, M.; Ardell, J.L.; Armour, J.A. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton. Neurosci. Basic Clin. 2009, 145, 55–62. [Google Scholar] [CrossRef]
- He, B.; Lu, Z.; He, W.; Wu, L.; Cui, B.; Hu, X.; Yu, L.; Huang, C.; Jiang, H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int. J. Cardiol. 2013, 168, 86–93. [Google Scholar] [CrossRef]
- Wu, B.; Xu, S.; Dai, R.; Hong, M.; Wu, H.; Lin, R. Epicardial ganglionated plexi ablation increases the inducibility of ventricular tachyarrhythmias in a canine postmyocardial infarction model. J. Cardiovasc. Electrophysiol. 2019, 30, 741–746. [Google Scholar] [CrossRef]
- Jungen, C.; Scherschel, K.; Eickholt, C.; Kuklik, P.; Klatt, N.; Bork, N.; Salzbrunn, T.; Alken, F.; Angendohr, S.; Klene, C.; et al. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat. Commun. 2017, 8, 14155. [Google Scholar] [CrossRef]
- Münkler, P.; Wutzler, A.; Attanasio, P.; Huemer, M.; Parwani, A.S.; Haverkamp, W.; Meyer, C.; Boldt, L.-H. Ventricular Tachycardia (VT) Storm After Cryoballoon-Based Pulmonary Vein Isolation. Am. J. Case Rep. 2018, 19, 1078–1082. [Google Scholar] [CrossRef]
- Boles, U.; Refila, B.; Gul, E.E.; Szeplaki, G.; Keaney, J.; Galvin, J.; Keelan, E. Ventricular Tachycardia Storm After Standard Radiofrequency Pulmonary Vein Isolation. Am. J. Case Rep. 2019, 20, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Li, X.; Ma, C. Takotsubo syndrome following radiofrequency ablation of atrial fibrillation in a patient with coronary artery anomaly: A case report. Eur. Heart J. Case Rep. 2022, 6, ytac147. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Ahlemeyer, L.; Freas, M.; Cooper, J.M.; Marchlinski, F.E.; Callans, D.J.; Hutchinson, M.D. Outflow tract premature ventricular depolarizations after atrial fibrillation ablation may reflect autonomic influences. J. Interv. Card. Electrophysiol. 2014, 41, 187–192. [Google Scholar] [CrossRef]
- Chung, W.H.; Masuyama, K.; Challita, R.; Hayase, J.; Mori, S.; Cha, S.; Bradfield, J.S.; Ardell, J.L.; Shivkumar, K.; Ajijola, O.A. Ischemia-induced ventricular proarrhythmia and cardiovascular autonomic dysreflexia after cardioneuroablation. Heart Rhythm 2023, 20, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, Z.; He, W.; Wu, L.; Huang, B.; Yu, L.; Cui, B.; Hu, X.; Jiang, H. Effects of low-intensity atrial ganglionated plexi stimulation on ventricular electrophysiology and arrhythmogenesis. Auton. Neurosci. 2013, 174, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gronda, E.; Dusi, V.; D’Elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2022, 24 (Suppl. E), E4–E11. [Google Scholar] [CrossRef]
- Arora, R.C.; Cardinal, R.; Smith, F.M.; Ardell, J.L.; Dell’Italia, L.J.; Armour, J.A. Intrinsic cardiac nervous system in tachycardia induced heart failure. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R1212–R1223. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.E.; Durston, M.; Zherebitskaya, E.; Smith, D.R.; Freed, D.; Glazner, G.W.; Tian, G.; Fernyhough, P.; Arora, R.C. Temporal dystrophic remodeling within the intrinsic cardiac nervous system of the streptozotocin-induced diabetic rat model. Acta Neuropathol. Commun. 2014, 2, 60. [Google Scholar] [CrossRef]
- Bassil, G.; Chang, M.; Pauza, A.; Diaz Vera, J.; Tsalatsanis, A.; Lindsey, B.G.; Noujaim, S.F. Pulmonary Vein Ganglia Are Remodeled in the Diabetic Heart. J. Am. Heart Assoc. 2018, 7, e008919. [Google Scholar] [CrossRef]
- Dyavanapalli, J.; Hora, A.J.; Escobar, J.B.; Schloen, J.; Dwyer, M.K.; Rodriguez, J.; Spurney, C.F.; Kay, M.W.; Mendelowitz, D. Chemogenetic activation of intracardiac cholinergic neurons improves cardiac function in pressure overload-induced heart failure. Am. J. Physiol. Circ. Physiol. 2020, 319, H3–H12. [Google Scholar] [CrossRef]
- Luo, D.; Hu, H.; Qin, Z.; Liu, S.; Yu, X.; Ma, R.; He, W.; Xie, J.; Lu, Z.; He, B.; et al. Stimulation of ganglionated plexus attenuates cardiac neural remodeling and heart failure progression in a canine model of acute heart failure post-myocardial infarction. Auton. Neurosci. 2017, 208, 73–79. [Google Scholar] [CrossRef]
- He, B.; Lu, Z.; Jiang, H. Atrial ganglionated plexi stimulation may be an effective therapeutic tool for the treatment of heart failure. Med. Hypotheses 2013, 81, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; O’Blenes, S.; Hanna, B.D.; Armour, J.A. Capacity of intrinsic cardiac neurons to modify the acutely autotransplanted mammalian heart. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1994, 13, 847–856. [Google Scholar]
- Murphy, D.A.; Thompson, G.W.; Ardell, J.L.; McCraty, R.; Stevenson, R.S.; Sangalang, V.E.; Cardinal, R.; Wilkinson, M.; Craig, S.; Smith, F.M.; et al. The heart reinnervates after transplantation. Ann. Thorac. Surg. 2000, 69, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Te, A.L.D.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chung, F.P.; Tuan, T.-C.; Chao, T.-F.; Liao, J.-N.; Chang, Y.-T.; et al. Vasovagal responses during cryoballoon pulmonary vein isolation in paroxysmal atrial fibrillation predict favorable mid-term outcomes. Int. J. Cardiol. 2018, 258, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Santinelli, V.; Manguso, F.; Vicedomini, G.; Gugliotta, F.; Augello, G.; Mazzone, P.; Tortoriello, V.; Landoni, G.; Zangrillo, A.; et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004, 109, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kampaktsis, P.N.; Oikonomou, E.K.; Choi, D.Y.; Cheung, J.W. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: A meta-analysis of randomized controlled clinical trials. J. Interv. Card. Electrophysiol. 2017, 50, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, S.; Wu, W.; Xie, Z.; Guo, Q. Different effects of additional ganglion plexus ablation on catheter and surgical ablation for atrial fibrillation: A systemic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2019, 30, 3039–3049. [Google Scholar] [CrossRef]
- Rackley, J.; Nudy, M.; Gonzalez, M.D.; Naccarelli, G.; Maheshwari, A. Pulmonary vein isolation with adjunctive left atrial ganglionic plexus ablation for treatment of atrial fibrillation: A meta-analysis of randomized controlled trials. J. Interv. Card. Electrophysiol. 2022, 66, 333–342. [Google Scholar] [CrossRef]
- Kim, M.Y.; Coyle, C.; Tomlinson, D.R.; Sikkel, M.B.; Sohaib, A.; Luther, V.; Leong, K.M.; Malcolme-Lawes, L.; Low, B.; Sandler, B.; et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm 2022, 19, 516–524. [Google Scholar] [CrossRef]
- Mikhaylov, E.; Kanidieva, A.; Sviridova, N.; Abramov, M.; Gureev, S.; Szili-Torok, T.; Lebedev, D. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: A 3-year follow-up study. Europace 2011, 13, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Krul, S.P.J.; Driessen, A.H.G.; Zwinderman, A.H.; van Boven, W.J.; Wilde, A.A.M.; de Bakker, J.M.T.; de Groot, J.R. Navigating the mini-maze: Systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int. J. Cardiol. 2013, 166, 132–140. [Google Scholar] [CrossRef]
- Krul, S.P.J.; Driessen, A.H.G.; Van Boven, W.J.; Linnenbank, A.C.; Geuzebroek, G.S.C.; Jackman, W.M.; Wilde, A.A.; de Bakker, J.M.; de Groot, J.R. Thoracoscopic Video-Assisted Pulmonary Vein Antrum Isolation, Ganglionated Plexus Ablation, and Periprocedural Confirmation of Ablation Lesions: First Results of a Hybrid Surgical-Electrophysiological Approach for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Driessen, A.H.G.; Berger, W.R.; Krul, S.P.J.; Van Den Berg, N.W.E.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.R.; de Jong, J.S.; van Boven, W.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.C.M.; Po, S.S.; Camm, A.J.; Ioannidis, J.P. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Neuzil, P.; Petru, J.; Koruth, J.S.; Kralovec, S.; Miller, M.A.; Funasako, M.; Chovanec, M.; Turagam, M.K.; Whang, W.; et al. Pulsed Field Ablation to Treat Atrial Fibrillation: Autonomic Nervous System Effects. JACC Clin. Electrophysiol. 2023, 9, 481–493. [Google Scholar] [CrossRef]
- Avazzadeh, S.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Keane, D.; Quinlan, L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension In Vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021, 10, 5443. [Google Scholar] [CrossRef] [PubMed]
- Avazzadeh, S.; Dehkordi, M.H.; Owens, P.; Jalali, A.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Fernhead, H.O.; Keane, D.; Quinlan, L.R. Establishing electroporation thresholds for targeted cell specific cardiac ablation in a 2D culture model. J. Cardiovasc. Electrophysiol. 2022, 33, 2050–2061. [Google Scholar] [CrossRef]
- Madhavan, M.; Venkatachalam, K.L.; Swale, M.J.; Desimone, C.V.; Gard, J.J.; Johnson, S.B.; Suddendorf, S.H.; Mikell, S.B.; Ladewig, D.J.; Nosbush, T.G.; et al. Novel Percutaneous Epicardial Autonomic Modulation in the Canine for Atrial Fibrillation: Results of an Efficacy and Safety Study. Pacing Clin. Electrophysiol. PACE 2016, 39, 407–417. [Google Scholar] [CrossRef]
- Saljic, A.; Hansen, M.E.H.; Dobrev, D. Botulinum toxin for prevention of post-operative atrial fibrillation. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 385–388. [Google Scholar] [CrossRef]
- Pokushalov, E.; Kozlov, B.; Romanov, A.; Strelnikov, A.; Bayramova, S.; Sergeevichev, D.; Bogachev-Prokophiev, A.; Zheleznev, S.; Shipulin, V.; Salakhutdinov, N.; et al. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: Results of a randomized pilot study. J. Am. Coll. Cardiol. 2014, 64, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.; Pokushalov, E.; Ponomarev, D.; Bayramova, S.; Shabanov, V.; Losik, D.; Stenin, I.; Elesin, D.; Mikheenko, I.; Strelnikov, A.; et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: Three-year follow-up of a randomized study. Heart Rhythm 2019, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Singh, J.P.; Parks, K.A.; Katritsis, D.G.; Stavrakis, S.; Armoundas, A.A. Low-Level Tragus Stimulation Modulates Atrial Alternans and Fibrillation Burden in Patients with Paroxysmal Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020865. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.; Iftikhar, O.; Parwani, P.; Abbas, M.; Filiberti, A.; Fleming, C.; Hu, Y.; Garabelli, P.; et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation. JACC Clin. Electrophysiol. 2017, 3, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Pachon, J.C.; Pachon, E.I.; Pachon, J.C.; Lobo, T.J.; Pachon, M.Z.; Vargas, R.N.A.; Jatene, A.D. “Cardioneuroablation”—New treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. EP Eur. 2005, 7, 1–13. [Google Scholar] [CrossRef]
- Pachon, J.C.; Pachon, E.I.; Aksu, T.; Gopinathannair, R.; Kautzner, J.; Yao, Y.; Kusumoto, F. Cardioneuroablation: Where are we at? Heart Rhythm O2 2023, 4, 401–413. [Google Scholar] [CrossRef]
- Kulakowski, P.; Baran, J.; Sikorska, A.; Krynski, T.; Niedzwiedz, M.; Soszynska, M.; Piotrowski, R. Cardioneuroablation for Reflex Asystolic Syncope—Mid-Term Safety, Efficacy and Patient’s Acceptance. Heart Rhythm 2023, 9, 85–95. Available online: https://pubmed.ncbi.nlm.nih.gov/38036236/ (accessed on 7 January 2024).
- Francia, P.; Viveros, D.; Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Martí-Almor, J.; Alderete, J.; Saglietto, A.; Bellido, A.F.; Franco-Ocaña, P.; et al. Clinical impact of aging on outcomes of cardioneuroablation for reflex syncope or functional bradycardia: Results from the cardionEuroabLation: PatiEnt selection, imaGe integrAtioN and outComEs—The ELEGANCE multicenter study. Heart Rhythm 2023, 20, 1279–1286. [Google Scholar] [CrossRef]
- Scanavacca, M.; Hachul, D.; Pisani, C.; Sosa, E. Selective Vagal Denervation of the Sinus and Atrioventricular Nodes, Guided by Vagal Reflexes Induced by High Frequency Stimulation, to Treat Refractory Neurally Mediated Syncope. J. Cardiovasc. Electrophysiol. 2009, 20, 558–563. [Google Scholar] [CrossRef]
- Aksu, T.; Yalin, K.; Gopinathannair, R. Fractionation mapping software to map ganglionated plexus sites during sinus rhythm. J. Cardiovasc. Electrophysiol. 2020, 31, 3326–3329. [Google Scholar] [CrossRef]
- Francia, P.; Viveros, D.; Falasconi, G.; Soto-Iglesias, D.; Fernández-Armenta, J.; Penela, D.; Berruezo, A. Computed tomography-based identification of ganglionated plexi to guide cardioneuroablation for vasovagal syncope. EP Eur. 2023, 25, euad170. [Google Scholar] [CrossRef]
- Stirrup, J.; Gregg, S.; Baavour, R.; Roth, N.; Breault, C.; Agostini, D.; Ernst, S.; Underwood, S.R. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: Technique and validation in patients with atrial fibrillation. J. Nucl. Cardiol. 2020, 27, 1939–1950. [Google Scholar] [CrossRef]
- Vandenberk, B.; Lei, L.Y.; Ballantyne, B.; Vickers, D.; Liang, Z.; Sheldon, R.S.; Chew, D.S.; Aksu, T.; Raj, S.R.; Morillo, C.A. Cardioneuroablation for vasovagal syncope: A systematic review and meta-analysis. Heart Rhythm 2022, 19, 1804–1812. [Google Scholar] [CrossRef]
- Calo, L.; Rebecchi, M.; Sette, A.; Sciarra, L.; Borrelli, A.; Scara, A.; Grieco, D.; Politano, A.; Sgueglia, M.; De Luca, L.; et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: A long-term follow-up prospective study. J. Interv. Card. Electrophysiol. 2021, 61, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, L.; Qiao, Y.; Shi, R.; Hou, B.; Wu, L.; Guo, J.; Zhang, S.; Yao, Y. Catheter Ablation as a Treatment for Vasovagal Syncope: Long-Term Outcome of Endocardial Autonomic Modification of the Left Atrium. J. Am. Heart Assoc. 2016, 5, e003471. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, R.; Baran, J.; Sikorska, A.; Krynski, T.; Kulakowski, P. Cardioneuroablation for Reflex Syncope: Efficacy and Effects on Autonomic Cardiac Regulation-A Prospective Randomized Trial. JACC Clin. Electrophysiol. 2023, 9, 85–95. [Google Scholar] [CrossRef]
- Pachon, M.J.C.; Pachon, M.E.I.; Pachon, C.T.C.; Santillana, P.T.G.; Lobo, T.J.; Pachon, M.J.C.; Zerpa, A.J.C.; Cunha, P.M.Z.; Higuti, C.; Ortencio, F.A.; et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythm. Electrophysiol. 2020, 13, e008703. [Google Scholar] [CrossRef]
- Heart Rate as a Predictor of Cardiovascular Risk—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29355923/ (accessed on 7 January 2024).
- Wang, Y.; Yin, L.; Hu, B.; Tse, L.A.; Liu, Y.; Ma, H.; Li, W. Association of heart rate with cardiovascular events and mortality in hypertensive and normotensive population: A nationwide prospective cohort study. Ann. Transl. Med. 2021, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, P.; Rossenbacker, T.; Collienne, C.; Roosen, J.; Ector, B.; Janssens, L.; Charlier, F.; Vankelecom, B.; Dewilde, W.; Wijns, W. Unifocal Right-Sided Ablation Treatment for Neurally Mediated Syncope and Functional Sinus Node Dysfunction Under Computed Tomographic Guidance. Circ. Arrhythm. Electrophysiol. 2018, 11, e006604. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Raj, S.R. Cardioneuroablation for Vasovagal Syncope: Sober Second Thoughts. Heart Rhythm 2023. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, S.M.; Sant’Anna, L.B.; Sant’Anna, M.B.; Menezes, R.S.M.; Mesquita, E.T.; Sant’Anna, F.M. Auricular Vagal Neuromodulation and its Application in Patients with Heart Failure and Reduced Ejection Fraction. Arq. Bras. Cardiol. 2023, 120, e20220581. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Nagai, M.; Förster, C.Y. Exploration of transcutaneous vagus nerve stimulation as a treatment option for adjuvant cancer and heart failure therapy. Explor. Neuroprotective Ther. 2023, 31, 363–397. [Google Scholar] [CrossRef]
- Bernardi, L.; Valenti, C.; Wdowczyck-Szulc, J.; Frey, A.W.; Rinaldi, M.; Spadacini, G.; Passino, C.; Martinelli, L.; Viganò, M.; Finardi, G. Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation 1998, 97, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Kinoshita, O.; Nawata, K.; Kyo, S.; Ono, M. Recipients with shorter cardiopulmonary bypass time achieve improvement of parasympathetic reinnervation within 6 months after heart transplantation. Int. Heart J. 2014, 55, 440–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grupper, A.; Gewirtz, H.; Kushwaha, S. Reinnervation post-heart transplantation. Eur. Heart J. 2018, 39, 1799–1806. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, D.Y.; Cho, Y.; Cho, H.J.; Lee, H.Y.; Choi, E.K.; Oh, S. Early Parasympathetic Reinnervation Is Not Related to Reconnection of Major Branches of the Vagus Nerve after Heart Transplantation. Korean Circ. J. 2016, 46, 197–206. [Google Scholar] [CrossRef][Green Version]
- Bengel, F.M.; Ueberfuhr, P.; Schiepel, N.; Nekolla, S.G.; Reichart, B.; Schwaiger, M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N. Engl. J. Med. 2001, 345, 731–738. [Google Scholar] [CrossRef]
- Imamura, T.; Kinugawa, K.; Okada, I.; Kato, N.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Kinoshita, O.; Nawata, K.; et al. Parasympathetic reinnervation accompanied by improved post-exercise heart rate recovery and quality of life in heart transplant recipients. Int. Heart J. 2015, 56, 180–185. [Google Scholar] [CrossRef]
- Zijderhand, C.F.; Veen, K.M.; Caliskan, K.; Schoonen, T.; Mokhles, M.M.; Bekkers, J.A.; Manintveld, O.C.; Constantinescu, A.A.; Brugts, J.J.; Bogers, A.J.; et al. Biatrial Versus Bicaval Orthotopic Heart Transplantation: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2020, 110, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Raj, S.R. Treating syncope without drugs: Standing still, exercising hard, or simply the “expert’s touch”? J. Cardiovasc. Electrophysiol. 2022, 33, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannino, G.; Braia, V.; Griffith Brookles, C.; Giacobbe, F.; D’Ascenzo, F.; Angelini, F.; Saglietto, A.; De Ferrari, G.M.; Dusi, V. The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications. Biology 2024, 13, 105. https://doi.org/10.3390/biology13020105
Giannino G, Braia V, Griffith Brookles C, Giacobbe F, D’Ascenzo F, Angelini F, Saglietto A, De Ferrari GM, Dusi V. The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications. Biology. 2024; 13(2):105. https://doi.org/10.3390/biology13020105
Chicago/Turabian StyleGiannino, Giuseppe, Valentina Braia, Carola Griffith Brookles, Federico Giacobbe, Fabrizio D’Ascenzo, Filippo Angelini, Andrea Saglietto, Gaetano Maria De Ferrari, and Veronica Dusi. 2024. "The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications" Biology 13, no. 2: 105. https://doi.org/10.3390/biology13020105
APA StyleGiannino, G., Braia, V., Griffith Brookles, C., Giacobbe, F., D’Ascenzo, F., Angelini, F., Saglietto, A., De Ferrari, G. M., & Dusi, V. (2024). The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications. Biology, 13(2), 105. https://doi.org/10.3390/biology13020105




