DNA Damage, Cell Death, and Alteration of Cell Proliferation Insights Caused by Copper Oxide Nanoparticles Using a Plant-Based Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
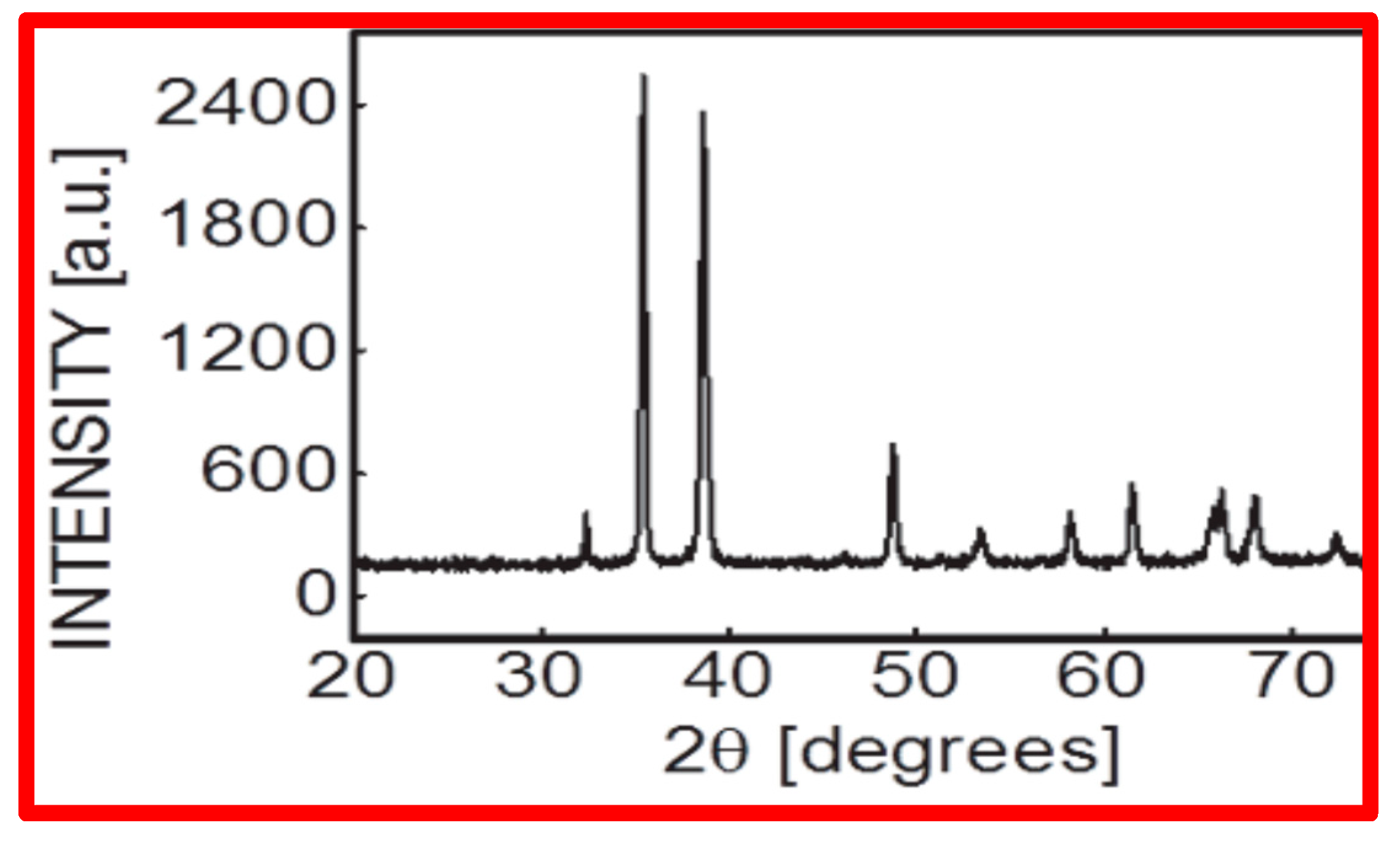
2.1. Nanoparticle (NP) Characterization
2.2. Procurement of Seeds and Chemical
2.3. Preparation of CuO NP Solution
2.4. Treatment of Seeds with CuO NPs
2.5. Determining Seed Germination and Radicle Length
2.6. Cytotoxicity Evaluation in Root Tip Cells of Pisum Sativum
Fixing of Roots and Analysis of Cell Proliferation Kinetics (CPK) and Mitotic Index (MI)
2.7. Detection of Cell Death (CD) in Root Tips (RTs)
2.8. Bud Collection and Fixation
2.9. Genotoxicity Evaluation
2.9.1. Chromosomal Aberration Frequency (CAF) Analysis in Pollen Mother Cells (PMCs)
2.9.2. Micronucleus Frequency (MNF) Analysis in Pollen Mother Cells (PMCs)
2.10. Data Analysis
3. Results
3.1. Nanoparticle (NP) Size Determination
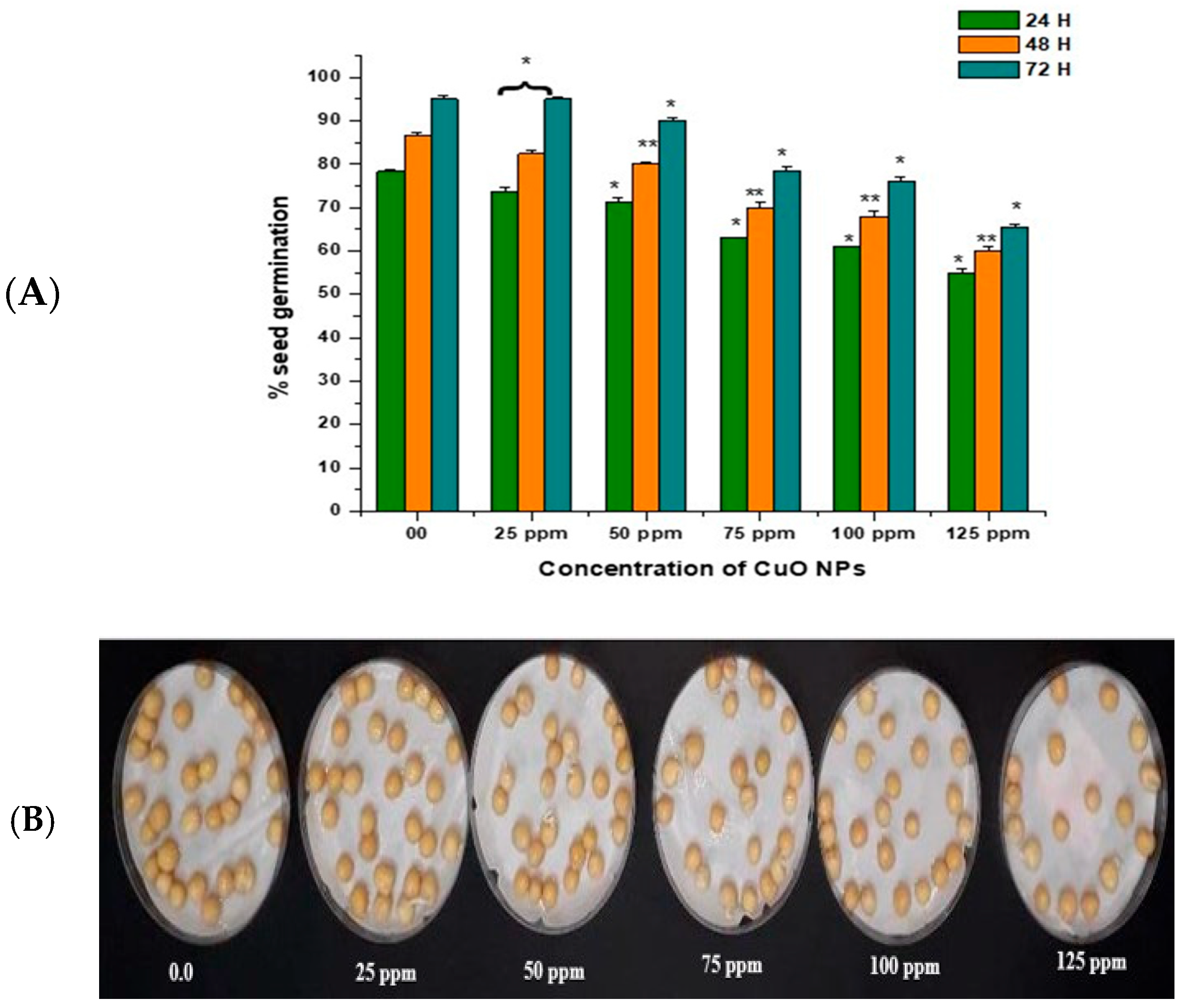
3.2. Effect of CuO NPs on Seed Germination (SG) of Pea
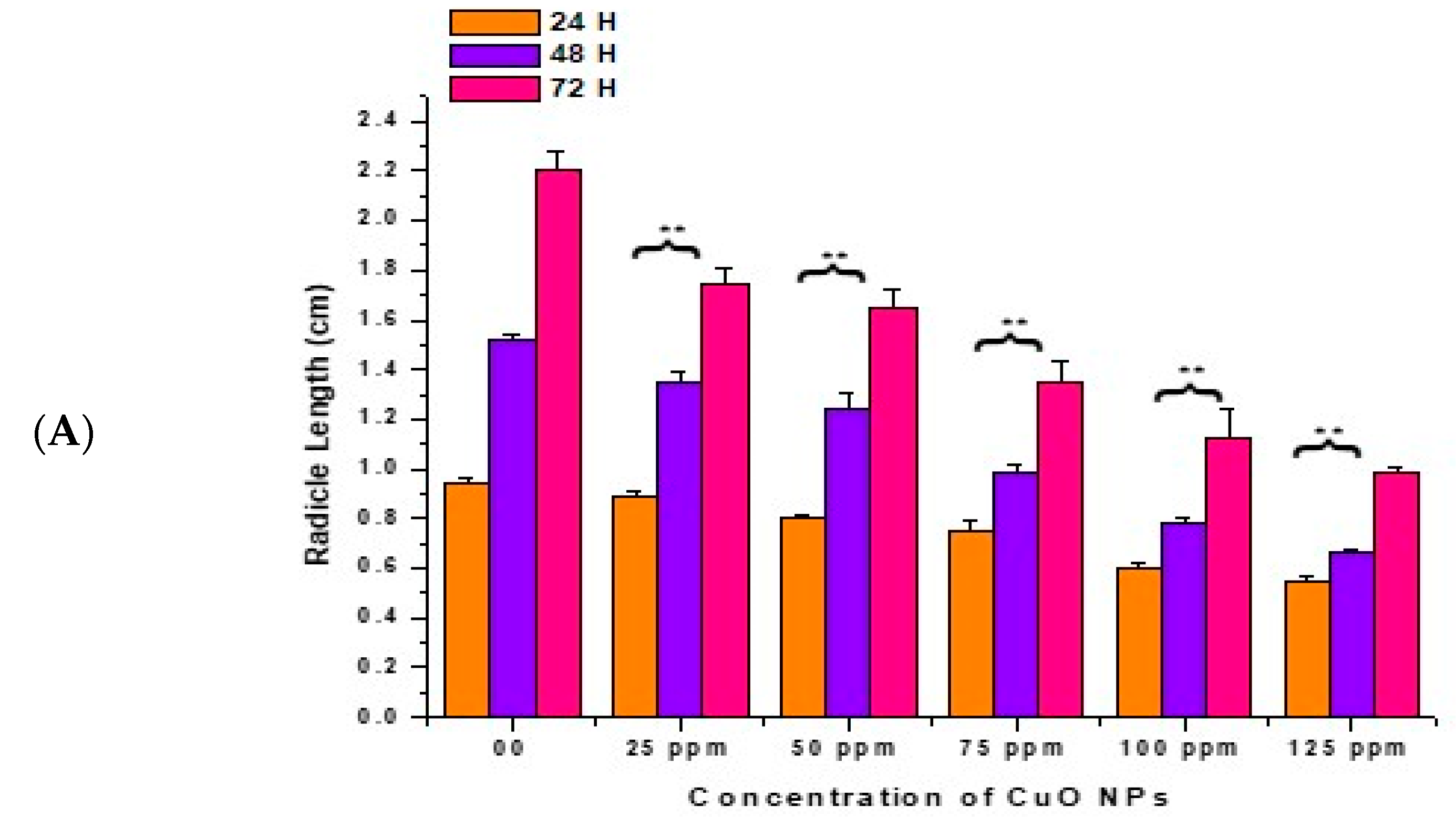
3.3. Effect of CuO NPs on Radicle Length (RL) of Pea
3.4. Effect of CuO NPs on Cell Proliferation Kinetics (CPK) of Pea
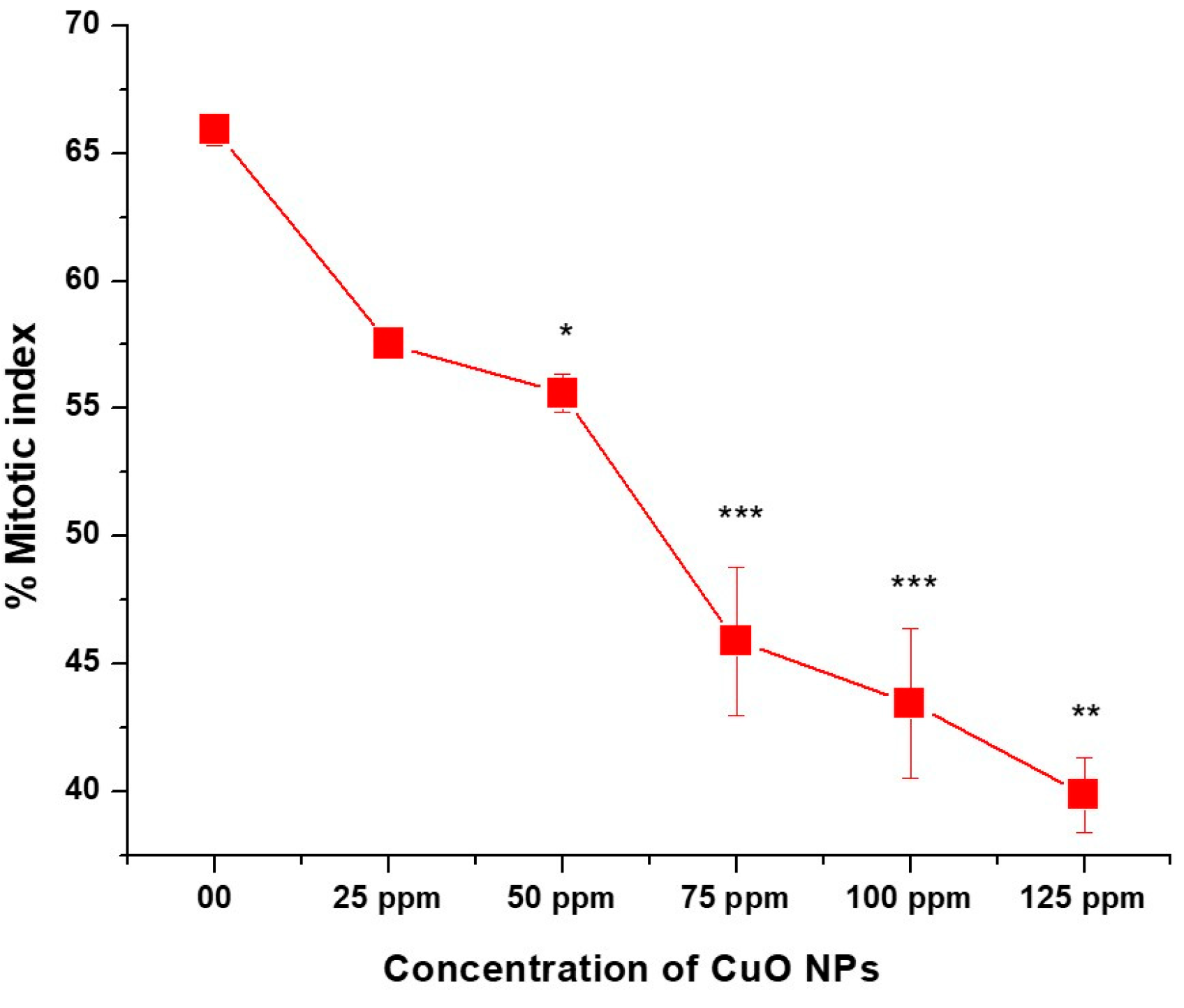
3.5. Effect of CuO NPs on Mitotic Index (MI) of Pea
3.6. Effect of CuO NPs on Cell Death (CD) in Root Tip Cells (RTCs) of Pea
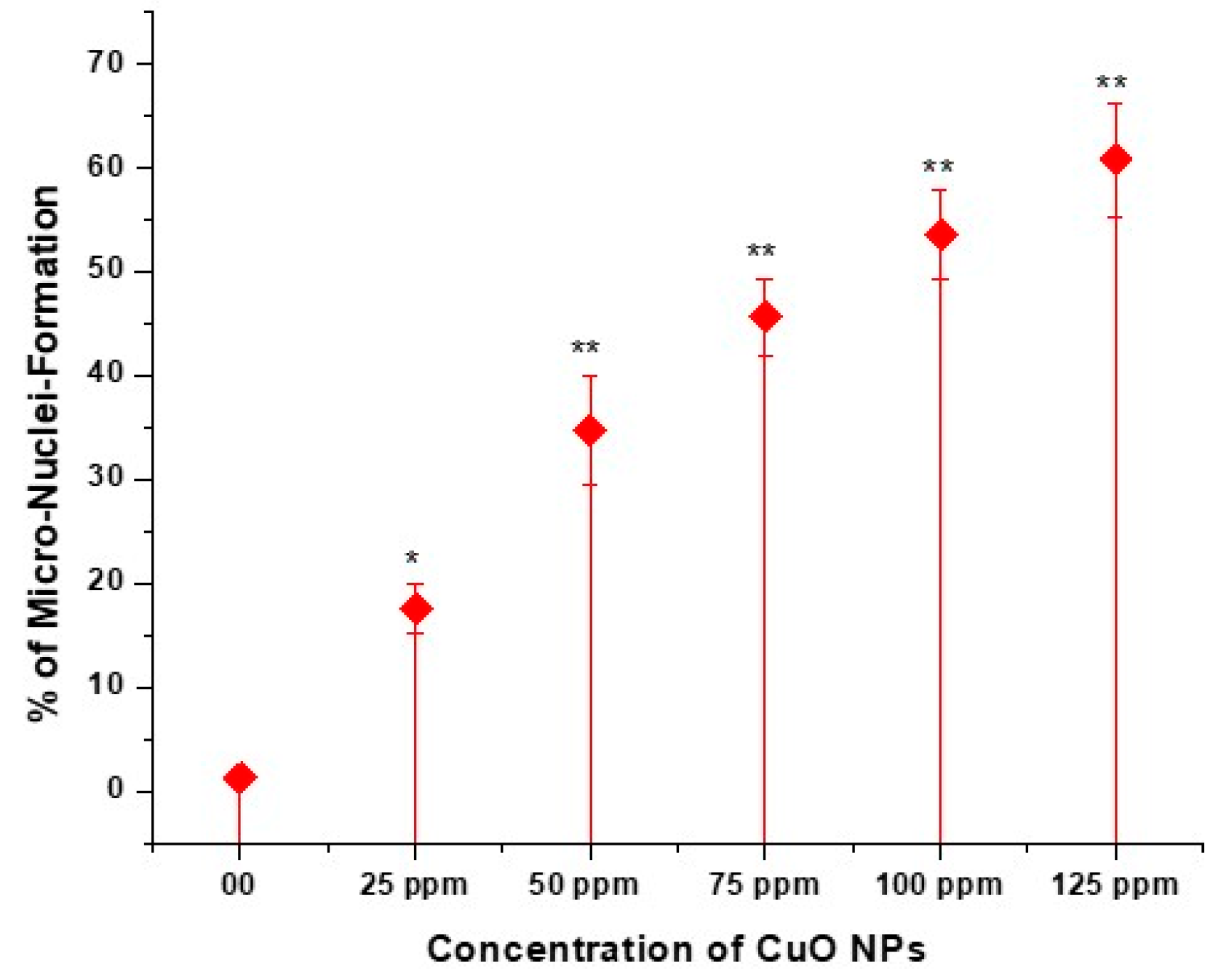
3.7. Effect of CuO NPs on Micronucleus Frequency (MNF) of Pea in Pollen Mother Cells (PMCs)
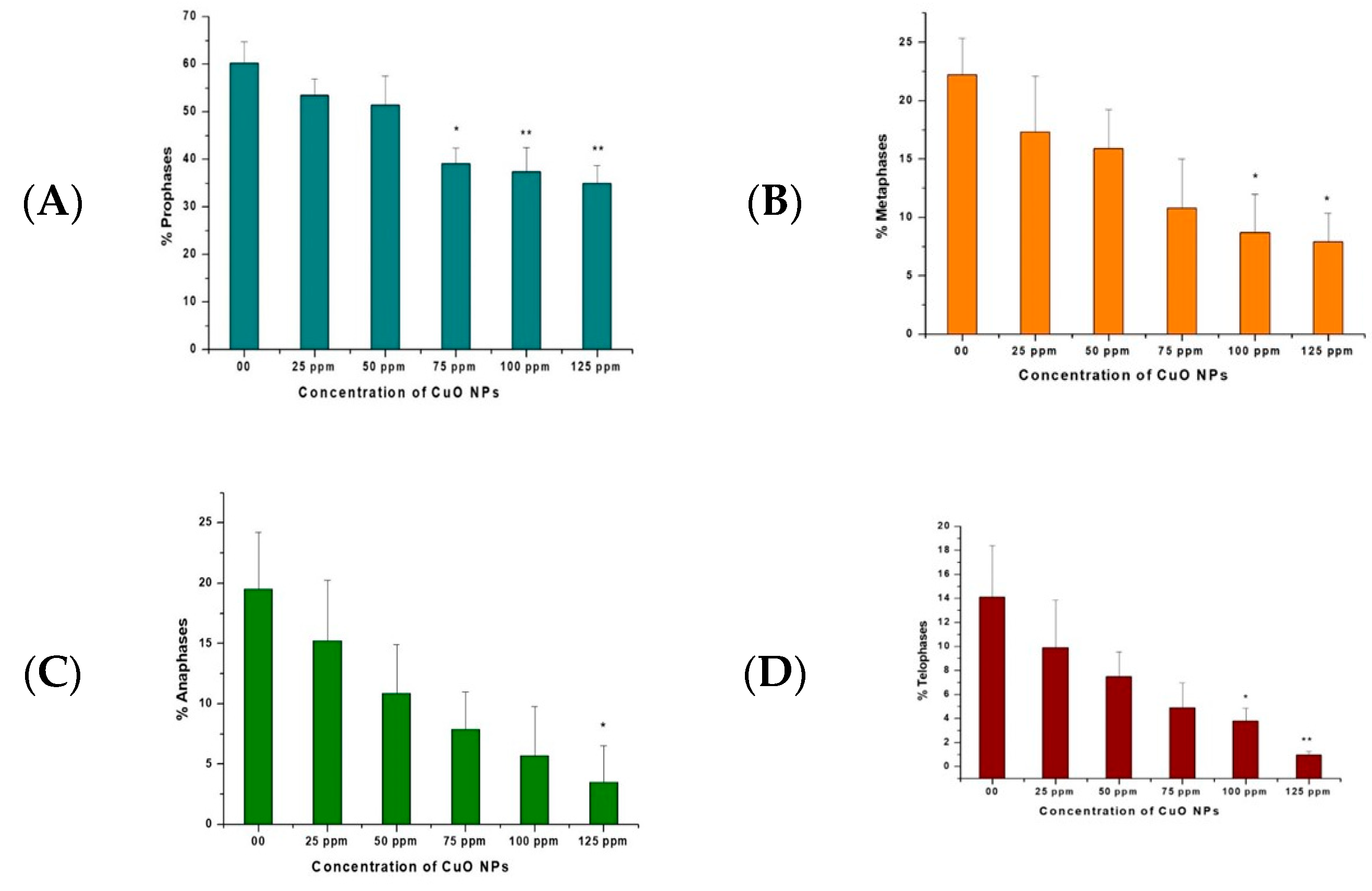
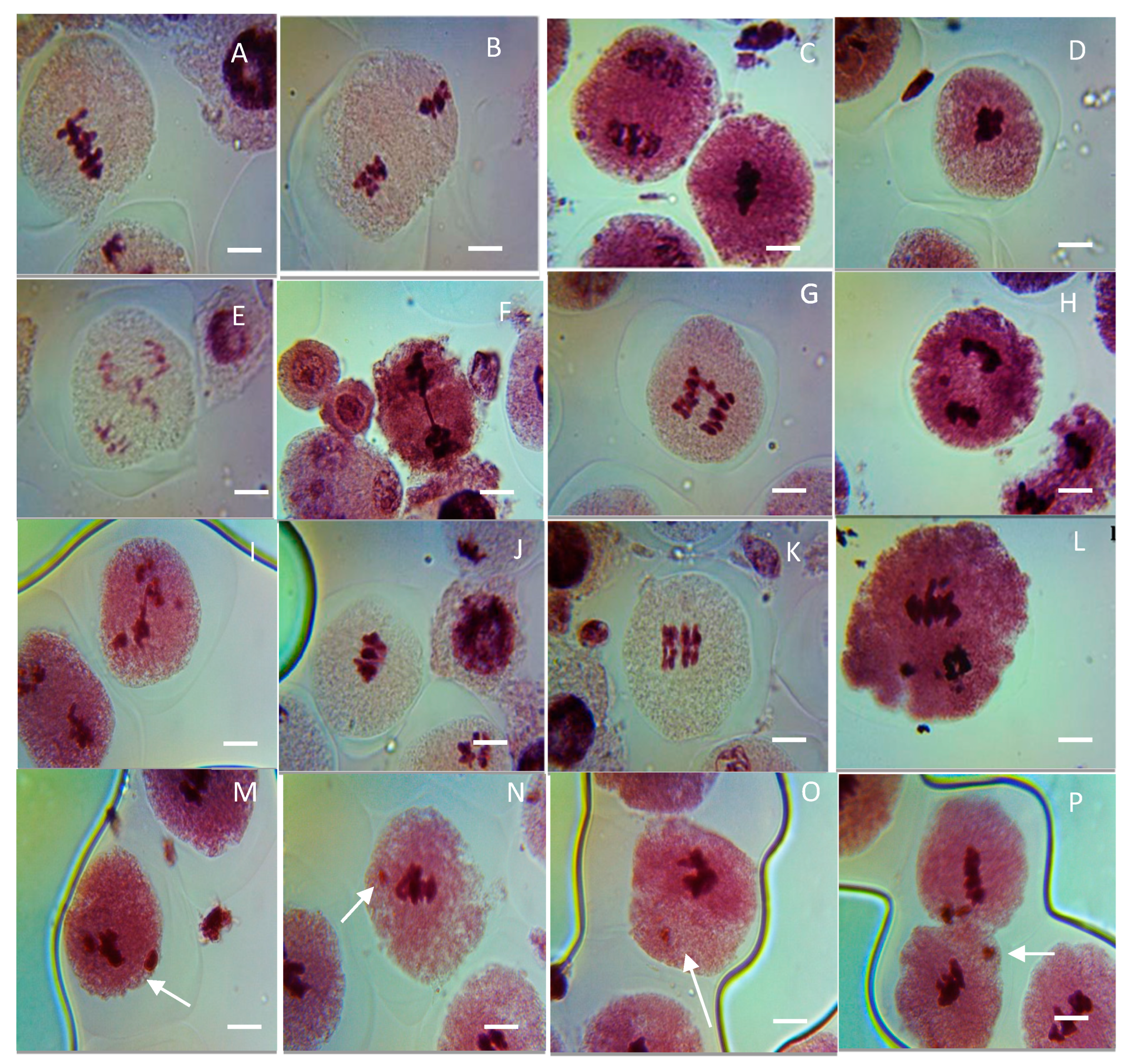
3.8. Effect of CuO NPs on Chromosomal Aberration Frequency (CAF) of Pea in Pollen Mother Cells (PMCs)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kushwah, K.S.; Patel, S.; Verma, D.K. Synthesis and effect of TiO2 nanoparticles on phytotoxicity and genotoxicity in Pisum sativum L. Vegetos 2022, 35, 204–211. [Google Scholar] [CrossRef]
- Kumari, A.; Chokheli, V.A.; Lysenko, V.S.; Mandzhieva, S.S.; Minkina, T.M.; Mazarji, M.; Rajput, V.D.; Shuvaeva, V.A.; Sushkova, S.S.; Barakhov, A. Genotoxic and morpho-physiological responses of ZnO macro-and nano-forms in plants. Environ. Geochem. Health 2022, 45, 9345–9357. [Google Scholar] [CrossRef]
- Labeeb, M.; Haroun, S.; Badr, A.; Matter, M.; El Kholy, A. Impact of ecofriendly synthesized silver nanoparticles on yield parameters and molecular traits of pea (Pisum sativum L.). Catrina Int. J. Environ. Sci. 2023, 27, 1–11. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Varduny, T.V.; Lysenko, V.S.; Kapralova, O.A.; Chokheli, V.A.; Sereda, M.M.; Dmitriev, P.A.; Varduny, V.M. Effect of nano-and crystalline metal oxides on growth, gene-and cytotoxicity of plants in vitro and ex vitro. Turczaninowia 2018, 21, 207–214. [Google Scholar]
- Ghouri, F.; Shahid, M.J.; Zhong, M.; Zia, M.A.; Alomrani, S.O.; Liu, J.; Sun, L.; Ali, S.; Liu, X.; Shahid, M.Q. Alleviated lead toxicity in rice plants by co-augmented action of genome doubling and TiO2 nanoparticles on gene expression, cytological and physiological changes. Sci. Total Environ. 2024, 911, 168709. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. Development of hydrophilic microporous PES ultra ltration membrane containing CuO nanoparticles with improved antifouling and separation performance. Mater. Chem. Phys. 2019, 222, 338–350. [Google Scholar] [CrossRef]
- Kopytina, N.I.; Andreeva, N.A.; Sizova, O.S.; Mosunov, A.A.; Evstigneev, V.P.; Bocharova, E.A. Communities of fungi on plates coated with antifouling paint modified by nanoparticles. Inland Water Biol. 2023, 16, 656–663. [Google Scholar] [CrossRef]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of copper oxide (CuO) nanoparticles on human blood lymphocytes. Biol. Trace Elem. Res. 2017, 184, 350–357. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Peng, J.; Sun, Y.; Liu, Q.; Yang, Y.; Zhou, J.; Feng, W.; Zhang, X.; Li, F. Up conversion nanoparticles dramatically promote plant growth without toxicity. Nano Res. 2012, 5, 770–782. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kim, B.; Barrios, A.C.; Medina-Velo, I.A.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.A.; Gardea-Torresdey, J.L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-A review. Environ. Pollut. 2018, 241, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Asif, N.; Ahmad, R.; Fatima, S.; Shehzadi, S.; Siddiqui, T.; Zaki, A.; Fatma, T. Toxicological assessment of Phormidium sp. derived copper oxide nanoparticles for its biomedical and environmental applications. Sci. Rep. 2023, 13, 6246. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Z.; Zhao, H.; Lai, F.; Han, Y.; Lin, X. Effects of copper oxide nanoparticles on soil diazotrophic communities in maize rhizosphere. J. Soils Sediments 2023, 23, 1760–1774. [Google Scholar] [CrossRef]
- Di, X.; Fu, Y.; Huang, Q.; Xu, Y.; Zheng, S.; Sun, Y. Comparative effects of copper nanoparticles and copper oxide nanoparticles on physiological characteristics and mineral element accumulation in Brassica chinensis L. Plant Physiol. Biochem. 2023, 196, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.I.; Zafar, I.; Ain, Q.U.; Noreen, A.; Nazir, A.; Javed, R.; Sehgal, S.A.; Khan, A.A.; Rahman, M.M.; Rashid, S.; et al. Synthesis and characterization of copper oxide nanoparticles: Its influence on corn (Z. mays) and wheat (Triticum aestivum) plants by inoculation of Bacillus subtilis. Environ. Sci. Pollut. Res. 2023, 30, 37370–37385. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper nanoparticles induced genotoxicity, oxidative stress, and changes in superoxide dismutase (sod) gene expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef]
- Shoman, N.; Solomonova, E.; Akimov, A.; Rylkova, O.; Meger, Y. Thecomprehensive effect of copper oxide nanoparticles on the physiology of the diatom microalga Thalassiosiraweissflogii. Funct. Plant Biol. 2023, 50, 612–622. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Liaquat, F.; Gulzar, S.; Chang, L.; Zhang, Y. Phytotoxic effects of chemically synthesized copper oxide nanoparticles induce physiological, biochemical, and ultrastructural changes in Cucumis melo. Environ. Sci. Pollut. Res. 2023, 30, 51595–51606. [Google Scholar] [CrossRef]
- Omar, S.A.; Elsheery, N.I.; Pashkovskiy, P.; Kuznetsov, V.; Allakhverdiev, S.I.; Zedan, A.M. Impact of titanium oxide nanoparticles on growth, pigment content, membrane stability, DNA damage, and stress-related gene expression in Vicia faba under Saline Conditions. Horticulturae 2023, 9, 1030. [Google Scholar] [CrossRef]
- Siddiqui, S.; Al-Rumman, S. Exposure of Pisum sativum L. seeds to methomyl and imidacloprid cause genotoxic effects in pollen-mother cells. Biology 2022, 11, 1549. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alrumman, S.A. Methomyl has clastogenic and aneugenic effects and alters the mitotic kinetics in Pisum sativum L. Caryologia 2022, 75, 91–99. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alrumman, S.A. Methomyl, imbraclaobrid and clethodim induced cytomixis and syncytes behaviors in PMCs of Pisum sativum L: Causes and outcomes. Saudi J. Biol. Sci. 2022, 29, 103390. [Google Scholar] [CrossRef]
- Siddiqui, S. Phenthoate toxicity evaluation in root meristem of Pisum sativum L. Caryologia 2023, 76, 57–66. [Google Scholar] [CrossRef]
- Davies, D.R.; Berry, G.J.; Heath, M.C.; Dawkins, T.C.K. Pea (Pisum sativum L.). In Grain Legume Crops; Collins: London, UK, 1985; Volume 147, p. 198. [Google Scholar]
- Qian, X.W. Improvement on experiment method of micronucleus in root tip cell of Vicia faba. J. Wenzhou Norm. Coll. 1998, 19, 64–65. [Google Scholar]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen, H.C.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat. Res. 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y. Effects of graphene on seed germination and seedling growth. J. Nanoart. Res. 2015, 17, 78. [Google Scholar] [CrossRef]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef]
- Duran, N.M.; Savassa, S.M.; Lima, R.G.D.; de Almeida, E.; Linhares, F.S.; van Gestel, C.A.; Pereira de Carvalho, H.W. X-ray spectroscopy uncovering the effects of Cu based nanoparticle concentration and structure on Phaseolus vulgaris germination and seedling develop ment. J. Agric. Food Chem. 2017, 65, 7874–7884. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Zhao, J.; Wang, X.; White, J.C.; Xing, B. CuO nano particle interaction with Arabidopsis thaliana: Toxicity parent progeny transfer and gene expression. Environ. Sci. Technol. 2016, 50, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Huang, L.; Head, J.; Chen, D.R.; Kong, I.C.; Tang, Y.J. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J. Pet. Environ. Biotechnol. 2012, 3, 126–130. [Google Scholar]
- Ko, K.S.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity seed germination and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol. Plant. 2015, 37, 1719. [Google Scholar] [CrossRef]
- Baskar, V.; Nayeem, S.; Kuppuraj, S.P.; Muthu, T.; Ramalingam, S. Assessment of the effects of metal oxide nanoparticles on the growth physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech 2018, 8, 362–373. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Venkidasamy, B.; Thiruvengadam, M. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut. 2019, 230, 48. [Google Scholar] [CrossRef]
- Tiwari, P.K.; Shweta, S.A.K.; Singh, V.P.; Prasad, S.M.; Ramawat, N.; Tripathi, D.K.; Chauhan, D.K.; Rai, A.K. Liquid assisted pulsed laser ab lation synthesized copper oxide nanoparticles (CuO-NPs) and their differential impact on rice seedlings. Ecotoxicol. Environ. Saf. 2019, 176, 321–329. [Google Scholar] [CrossRef]
- Nair, P.G.M.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignicaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant responses to nanoparticle stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S. Ultrastructural and molecular implications of ecofriendly made silver nanoparticles treatments in pea (Pisum sativum L.). J. Genet. Eng. Biotechnol. 2022, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S.C. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, H.; Zhang, Y.; Liu, Y.; Li, H. Wheat morphological and biochemical responses to copper oxide nanoparticle treatment in two soils. Pedosphere 2023, 34, 814–825. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and beneficial trace elements in plants and their transport in roots: A review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Yanik, F.; Vardar, F. Toxic effects of aluminum oxide (Al2O3) nano particles on root growth and development in Triticum aestivum. Water Air Soil Pollut. 2015, 226, 296. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem and phloem-based transport of CuO nanoparticles in maize (Zea mays L). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 1366–1375. [Google Scholar] [CrossRef]
- Ogunkunle, C.O.; Bornmann, B.; Wagner, R.; Fatoba, P.O.; Frahm, R.; Lützenkirchen-Hecht, D. Copper uptake, tissue partitioning and biotransformation evidence by XANES in cowpea (Vigna unguiculata L.) grown in soil amended with nanosized copper parti cles. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100231. [Google Scholar]
- Nair, P.M.G.; Chung, I.M. Evaluation of stress effects of copper oxide nanoparticles in Brassica napus L. seedlings. 3 Biotech 2017, 7, 293. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Kavitha, S.; Alex, S.A.; Kumar, D.; Mukherjee, A.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip effects of oxidative stress generation and biouptake. Environ. Sci. Pollut. Res. 2015, 22, 11057–11066. [Google Scholar] [CrossRef]
- Liman, R.; Ali, M.M.; Istifli, E.S.; Cigerci, I.H.; Bonciu, E. Genotoxic and cytotoxic effects of pethoxamid herbicide on Allium cepa cells and its molecular docking studies to unravel genotoxicity mechanism. Environ. Sci. Pollut. Res. 2022, 29, 63127–63140. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Su, T.; Wei, D.; Wu, D.; Zhang, G.; Shen, Q. Copper oxide nanoparticles alleviate cadmium toxicity in cereal crops. Environ. Sci. Nano 2022, 9, 3502–3513. [Google Scholar] [CrossRef]
- Ozkul, M.; Ozel, C.A.; Yuzbaşıoglu, D.; Unal, F. Does 2, 4-dichlorophenoxyacetic acid (2, 4-D) induce genotoxic effects in tissue cultured Allium roots? Cytotechnology 2016, 68, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Paul, J.A.J.; Kumar, P.; Archana, J.; Begam, H.F.; Karmegam, N.; Biruntha, M. Impact of biosynthesized CuO nanoparticles on seed germination and cyto-physiological responses of Trigonella foenum-graecum and Vigna radiata. Mater. Lett. 2022, 313, 131756. [Google Scholar] [CrossRef]
- Mehrian, S.K.; De Lima, R. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016, 6, 184–193. [Google Scholar]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Chen, C.; Zhao, Y.; Jia, L.; Wang, P.C. Biopharmaceutics and therapeutic potential of engineered nanomaterials. Curr. Drug Metab. 2008, 9, 697–709. [Google Scholar] [CrossRef]
- Yan, J.J. Study on organic wastewater monitoring of laboratory using MCN test of Vicia faba root tips. J. Anhui Agric. Sci. 2011, 17. [Google Scholar]
- Periakaruppan, R.; Vanathi, P.; Priyanka, G.; Vidhya, D. Toxicity in plants by metal oxide nanoparticles. In Nanometal Oxides in Horticulture and Agronomy; Academic Press: Cambridge, MA, USA, 2023; pp. 241–273. [Google Scholar]
- Das, D.; Bisht, K.; Chauhan, A.; Gautam, S.; Jaiswal, J.P.; Salvi, P.; Lohani, P. Morpho-physiological and Biochemical responses in wheat foliar sprayed with zinc-chitosan-salicylic acid nanoparticles during drought stress. Plant Nano Biol. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Fenech, M.; Krisch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Noppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanism of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Tasar, N. Mitotic effects of copper oxide nanoparticle on root development and root tip cells of Phaseolus vulgaris L. seeds. Microsc. Res. Tech. 2022, 85, 3895–3907. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Datta, A.K.; Gupta, S.; Ghosh, B. Copper oxide nanoparticles induced fertile desynaptic mutant line in Coriandrum sativum L. (Apiaceae). Cytologia 2018, 83, 103–107. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Ilhan, E.; Özkan, G.; Öztürk, H.İ.; Haliloglu, K.; Cinisli, K.T. Plant growth-promoting bacteria (PGPBs) and copper (II) oxide (CuO) nanoparticle ameliorates DNA damage and DNA methylation in wheat (Triticum aestivum L.) exposed to NaCl stress. J. Plant Biochem. Biotechnol. 2022, 31, 751–764. [Google Scholar] [CrossRef]
- Shobha, G.; Shashidhara, K.S.; Naik, C. Cuprous oxide nanoparticles induced antioxidant response and genotoxicity in Lycopersicum esculentum. Bio Nanosci. 2020, 10, 1128–1137. [Google Scholar] [CrossRef]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G. Investigation of the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Bapi, G.; Kumar, D.A.; Debadrito, D.; Vishambhar, K.D.; Ankita, P. Assessment of nanoparticles (copper, cadmium sulphide, copper oxide and zinc oxide) mediated toxicity in a plant system (Indigofera tinctoria L.; Fabaceae). Res. J. Chem. Environ. 2018, 22, 34–48. [Google Scholar]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum—Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Kisin, E.R.; Murray, A.R.; Keane, M.J.; Shi, X.C.; Schwegler-Berry, D.; Gorelik, O.; Arepalli, S.; Castranova, V.; Wallace, W.E.; Kagan, V.E.; et al. Single-walled carbon nanotubes: Geno and cytotoxic Page 17/27 effects in lung fibroblast V79 cells. J. Toxicol. Environ. Health Part A 2007, 70, 2071–2079. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. The responses of germinating seedlings of green peas to copper oxide nanoparticles. Biol. Plant. 2015, 59, 591–595. [Google Scholar] [CrossRef]
- Nagdalian, A.; Askerova, A.; Blinov, A.; Shariati, M.A. Evaluation of the Toxicity of Copper Oxide Nanoparticles toward Pea Seeds. World J. Environ. Biosci. 2024, 13, 23–30. [Google Scholar]
- Ochoa, L.; Medina-Velo, I.A.; Barrios, A.C.; Bonilla-Bird, N.J.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total Environ. 2017, 598, 513–524. [Google Scholar] [CrossRef] [PubMed]



| CuO NP Concentration | LG | DB | STC | CNi | PS | SB | SA | TA |
|---|---|---|---|---|---|---|---|---|
| 00.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| 25 ppm | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.15 ± 0.003 * | 0.25 ± 0.03 | 0.40 ± 0.033 |
| 50 ppm | 0.00 ± 0.00 | 0.38 ± 0.01 * | 0.34 ± 0.01 * | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.26 ± 0.09 * | 0.35 ± 0.01 | 1.33 ± 0.12 |
| 75 ppm | 0.65 ± 0.06 * | 0.55 ± 0.05 * | 0.96 ± 0.05 * | 0.25 ± 0.03 * | 0.35 ± 0.03 * | 0.46 ± 0.03 * | 0.75 ± 0.03 | 3.97 ± 0.28 |
| 100 ppm | 0.97 ± 0.05 * | 0.76 ± 0.02 * | 1.66 ± 0.63 * | 0.98 ± 0.10 * | 0.68 ± 0.05 * | 0.66 ± 0.05 * | 0.98 ± 0.21 * | 6.68 ± 1.11 |
| 125 ppm | 1.24 ± 0.20 * | 0.98 ± 0.10 * | 1.50 ± 0.99 * | 0.97 ± 0.23 * | 0.91 ± 0.23 * | 0.86 ± 0.11 | 10 ± 0.42 ** | 7.56 ± 2.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S. DNA Damage, Cell Death, and Alteration of Cell Proliferation Insights Caused by Copper Oxide Nanoparticles Using a Plant-Based Model. Biology 2024, 13, 805. https://doi.org/10.3390/biology13100805
Siddiqui S. DNA Damage, Cell Death, and Alteration of Cell Proliferation Insights Caused by Copper Oxide Nanoparticles Using a Plant-Based Model. Biology. 2024; 13(10):805. https://doi.org/10.3390/biology13100805
Chicago/Turabian StyleSiddiqui, Sazada. 2024. "DNA Damage, Cell Death, and Alteration of Cell Proliferation Insights Caused by Copper Oxide Nanoparticles Using a Plant-Based Model" Biology 13, no. 10: 805. https://doi.org/10.3390/biology13100805
APA StyleSiddiqui, S. (2024). DNA Damage, Cell Death, and Alteration of Cell Proliferation Insights Caused by Copper Oxide Nanoparticles Using a Plant-Based Model. Biology, 13(10), 805. https://doi.org/10.3390/biology13100805





