Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Potential Mother Tree
2.2. Selection of Potential Mother Trees Based on Astragalin (AG) Content
2.3. Anti-Inflammatory Bioassay of Moringa Genotypes with Chemicals Possessing the Highest Astragalin Content
2.3.1. Lipoxygenase Inhibitory Assay (LOX)
2.3.2. Xanthine Oxidase Inhibitory Assay (XO)
2.3.3. Hyaluronidase Inhibitory Assay (HYA)
2.4. Collection and Preparation of Branch Cuttings
2.5. Effect of Hormone Treatment on Rooting Ability and Survival of M. oleifera Cuttings
2.6. Effects of Cutting Length and Size on Rooting Ability and Survival of M. oleifera Cuttings
2.7. Effects of Rooting Substrates on Rooting Ability and Survival of M. oleifera Cuttings
2.8. Growth Conditions and Observations
2.9. Acclimatisation of Rooted Cuttings in the Nursery
2.10. Growth Performance of Clones in the Field under the Same Environmental Conditions
2.11. Data Collection and Analysis
3. Results and Discussion
3.1. Genotype Screening and Quantification of Astragalin (3-O-Glucoside of Kaempferol) Content in Selected Moringa oleifera Mother Trees
3.2. Anti-Inflammatory Bioassay of Selected Moringa oleifera Genotypes
3.3. Development of Vegetative Propagation Techniques through Branch Cuttings of Selected Moringa oleifera Genotypes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- WHO. World Health Organization General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. Available online: https://apps.who.int/iris/bitstream/handle/10665/66783/WHO_EDM_TRM_2000.1.pdf (accessed on 18 June 2021).
- Salmeron-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, T.M.; Tahira, M.; Ikram, U.K. Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar] [CrossRef]
- Fahey, J.; Olson, M.; Stephenson, K.; Wade, K.; Chodur, G.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.; et al. The Diversity of Chemoprotective Glucosinolates in Moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [PubMed]
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of Drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Fahey, J.W. Moringa oleifera: A review of the medicinal evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005, 1, 5. [Google Scholar]
- Mbikay, M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: A review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561. [Google Scholar] [CrossRef]
- Hamza, M.; Khan, S.; Ahmed, S.; Attique, Z.; Rehman, S.; Khan, A.; Ali, A.; Rizwan, M.; Munir, A.; Mehmood, K.A.; et al. nCOV-19 peptides mass fingerprinting identification, binding, and blocking of inhibitors flavonoids and anthraquinone of Moringa oleifera and hydroxychloroquine. J. Biomol. Struct. Dyn. 2020, 39, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guine, R.P.F.; Correia, P.M.R.; Mehra, R.; Kumar, H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Muni, S.G.; Ramesh, G.; Devi, P.R.; Meriga, B. Astragalin, (3-O-glucoside of kaempferol), isolated from Moringa oleifera leaves modulates leptin, adiponectin secretion and inhibits adipogenesis in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 2020, 27, 1–7. [Google Scholar]
- Azad, A.K.; Rasul, M.G.; Khan, M.M.K.; Sharma, S.C.; Islam, R. Prospect of Moringa seed oil as a sustainable biodiesel fuel in Australia: A review. Procedia Eng. 2015, 105, 601–606. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Freire, C.C.; Brandao, P.E.B.; Mendes, A.A.; Pereira, M.M.; Lima, A.S.; Soares, C. Biolubricant production under zero-waste Moringa oleifera Lam biorefinery approach for boosting circular economy. Ind. Crops Prod. 2021, 167, 113542. [Google Scholar] [CrossRef]
- Escobar, O.D.S.; Azevedo, C.F.; Swarowsky, A.A.M.A.; Netto, M.S.; Machado, F.M. Utilization of different parts of Moringa oleifera Lam. seeds as biosorbents to remove Acid Blue 9 synthetic dye. J. Environ. Chem. Eng. 2021, 9, 105553. [Google Scholar] [CrossRef]
- Rajeswari, M.; Pushpa, A.; Nagashree, N.R.; Ashwani, S.; Lingayya, H.; Tippareddy, K.S.; Shivandappa. Modelling and efficiency assessment of the up-flow fixed bed process packed with Moringa oleifera for continuous Cd (II) removal from drinking water. J. Mol. Struct. 2021, 1236, 130328. [Google Scholar] [CrossRef]
- Ahuja, K.; Mamtani, K. Moringa Ingredient Market Size by Product. Research Report in Global Market Insights. 2019. Report ID: GM14352. Available online: https://www.gminsights.com/industry-analysis/moringa-ingredients-market (accessed on 18 June 2021).
- Yerima, B.P.K.; Ayuk, G.M.; Enang, R.K.; Guehjung, N.; Tiamgne, Y.A. Germination and Early Seedling Growth of Moringa oleifera Lam with Different Seeds Soaking Time and Substrates at the Yongka Western Highlands Research Garden Park (YWHRGP) Nkwen-Bamenda, North-West Cameroon. Am. J. Plant Sci. 2016, 7, 2173–2185. [Google Scholar] [CrossRef]
- Gupta, N.; Manimurugan, C.; Singh, P.M.; Kumar, R.; Mishra, M.; Sagar, V. Standardization of seed germination testing protocol in Moringa oleifera Lam. Veg. Sci. 2019, 46, 148–151. [Google Scholar] [CrossRef]
- Islam, S.; Jahan, M.A.A.; Khatun, R. In vitro regeneration and multiplication of year-round fruit-bearing Moringa oleifera L. J. Biol. Sci. 2005, 5, 145–148. [Google Scholar] [CrossRef]
- El-nagish, A.; Hassanein, A.; Salem, J.; Faheed, F. Some important aspects in Moringa oleifera Lam. micropropagation. Acta Agric. Slov. 2019, 113, 13–27. [Google Scholar] [CrossRef]
- Palada, M.; Ebert, A. Adaptability and horticultural characterization of Moringa accessions under Central Philippines conditions. In Proceedings of the Conference: Regional Symposium on High-Value Vegetables in Southeast Asia: Production, Supply and Demand (SEAVEG2012), Chiang Mai, Thailand, 24–26 January 2012. [Google Scholar]
- Dantata, I.J.; Omoayena, B.O.; Peter, E.; Aminu, S.A. Appraising Some Agronomic Characteristics of Moringa oleifera: A lost Indigenous Leafy Vegetable Crop in Northern Nigeria. J. Environ. Technol. Sustain. Agric. 2015, 1, 8–21. [Google Scholar]
- Rufal, S.; Hanafi, M.M.; Rafii, M.Y.; Mohidin, H.; Omar, S.R.S. Growth and development of moringa (Moringa oleifera L.) stem cuttings as affected by diameter magnitude, growth media, and Indole-3-butyric acid. Ann. For. Res. 2016, 59, 209–218. [Google Scholar] [CrossRef][Green Version]
- Coles, Z.; Elsa, D.T. Open air-layering of Moringa oleifera utilizing seedling plug containers. S. Afr. J. Bot. 2019, 129, 225–228. [Google Scholar] [CrossRef]
- Elavarasan, K.; Sumathi, T.; Pugalendhi, L.; Ravichandran, V. Graft compatibility of different perennial moringa rootstocks on the annual scion. J. Pharmacogn. Phytochem. 2021, 10, 2855–2857. [Google Scholar] [CrossRef]
- Azhar-UI-Haq; Malik, A.; Anis, I.; Khan, S.B.; Ahmed, E.; Ahmed, Z.; Nawaz, S.A.; Choudhary, M.I. Enzymes Inhibiting Lignans from Vitex negundo. Chem. Pharm. Bull. 2004, 52, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Oda, Y.; Miyase, T.; Ueno, A.; Fukushima, S. Inhibitors of xanthine oxidase from flowers and buds of Daphne genkwa. Chem. Pharm. Bull. 1983, 31, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Takashi, T.; Isao, K. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by β-Glucosidase in the presence of amino acids. Biol. Pharm. Bull. 2003, 26, 352–356. [Google Scholar] [CrossRef]
- Guevara, A.P.; Vargas, C.; Sakurai, H.; Fujiwara, Y.; Hashimoto, K.; Maoka, T.; Kozuka, M.; Ito, Y.; Tokuda, H.; Nishino, H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res. 1999, 44, 181–188. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef]
- Aja, P.M.; Nwachukwu, N.; Ibiam, U.A.; Igwenyi, I.O.; Offor, C.E.; Orji, U.J. Chemical constituents of Moringa oleifera leaves and seeds from Abakaliki, Nigeria. Am. J. Phytomed. Clin. Ther. 2014, 2, 310–321. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compost. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. HPLC Quantitative Analysis of Three Major Antioxidative Components of Moringa oleifera Leaf Extracts. Planta Med. 2012, 78, PJ15. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of Polyphenolics, Nutrients and Antioxidant Potential of Germplasm’s Leaves from Seven Cultivars of Moringa oleifera Lam. Ind. Crops Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Kaushal, R.; Gulabrao, Y.A.; Tewari, S.K.; Chaturvedi, S.; Chaturvedi, O.P. Rooting behavior and survival of bamboo species propagated through branch cuttings. Indian J. Soil Conserv. 2011, 39, 171–175. [Google Scholar]
- Chiann, L.P.; Kumar, S.M.; Shukor, N.A.A. Rooting Ability of Gigantochloa scortechinii through Branch Cuttings. Malaysian For. 2020, 83, 64–72. [Google Scholar]
- Fragoso, R.O.; Stuepp, C.A.; Rickli, H.C.; Ribas, K.C.Z.; Koehler, H.S. Maximum efficiency concentration of indole butyric acid in promoting the rooting of Japanese Flowering Cherry. Cienc. Rural. Santa Maria 2017, 47, e20150894. [Google Scholar] [CrossRef]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS analysis, determination of total phenolics, flavonoid content, and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef]
- Akinyele, A.O. Effects of growth hormones, rooting media and leaf size on juvenile stem cuttings of Buchholzia coriacea Engler. Ann. For. Res. 2010, 53, 127–133. [Google Scholar]
- Yang, F.O.; Wang, J.; Li, Y. Effects of cutting size and exogenous hormone treatment on rooting of shoot cuttings in Norway spruce [Picea abies (L.) Karst.]. New For. 2015, 46, 91–105. [Google Scholar] [CrossRef]
- Reuveni, O.; Raviv, M. Importance of leaf retention to rooting of avocado cuttings. J. Am. Soc. Hortic. Sci. 1980, 106, 127–130. [Google Scholar] [CrossRef]
- Muniandi, S.K.; Muhammad, N.; Md Ariff, F.F.; Taheri, Y. Improved Clonal Propagation through Rejuvenation of MaturBranch Cutting of Four Important Acacia Species. Forests 2022, 13, 1403. [Google Scholar] [CrossRef]
- Friml, J.; Palme, K. Polar Auxin Transport-Old Questions and New Concepts? Auxin Molecular Biology; Springer: New York, NY, USA, 2002; pp. 273–284. [Google Scholar]
- Bollmark, M.; Eliasson, L. Ethylene accelerates the breakdown of cytokinins and thereby stimulates rooting in Norway spruce hypocotyl cuttings. Physiol. Plant. 1990, 80, 534–540. [Google Scholar] [CrossRef]
- Aminah, H.; Naimah, C.L.; Raja Barizan, R.S.; Mohd Noor, M. Effect of light intensity and fertiliser levels on the stock plants of Chengal (neobalanocarpus heimii) and rooting of its subsequent cuttings. Sains Malays. 2013, 42, 257–263. [Google Scholar]
- Leakey, R.; Coutts, M. The dynamics of rooting in Triplochiton scleroxylon cuttings: Their relation to leaf area, node position, dry weight accumulation, leaf water potential, and carbohydrate composition. Tree Physiol. 1989, 5, 135–146. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davis, J.R.F.T. Plant Propagation––Principals and Practices, 5th ed.; Prentice-Hall International Editions: Hoboken, NJ, USA, 1990. [Google Scholar]
- Hossain, M.A.; Kumar, S.M.; Seca, G.; Maheran, A.A.; Nor Aini, A.S. Mass Propagation of Dendrocalamus asper by branch cutting. J. Trop. For. Sci. 2018, 30, 82–88. [Google Scholar] [CrossRef]


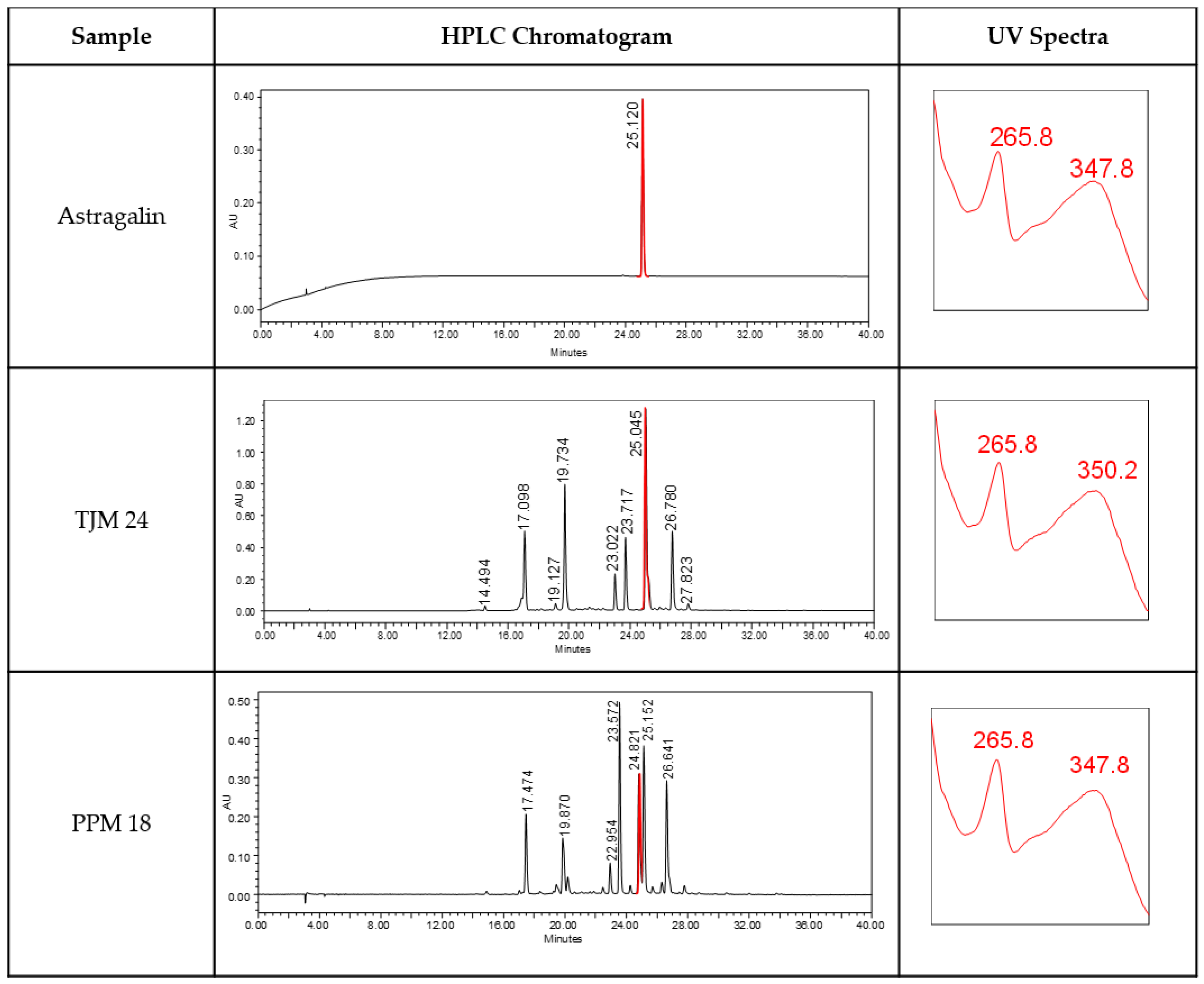




| (Samples) | Average Concentration of Astragalin ± RSD (ppm) | Average Percentage of Astragalin in the Sample ± RSD (w/w) |
|---|---|---|
| TJM 24 | 489.26 ± 0.61 | 0.49 ± 0.61 |
| TJM 23 | 408.40 ± 2.30 | 0.41 ± 2.30 |
| TJM 27 | 182.57 ± 1.86 | 0.18 ± 1.86 |
| TJM 30 | 176.93 ± 0.56 | 0.18 ± 0.56 |
| NSM 22 | 159.89 ± 1.86 | 0.16 ± 1.86 |
| NSM 23 | 149.54 ± 0.50 | 0.15 ± 0.50 |
| TJM 21 | 121.32 ± 0.60 | 0.12 ± 0.57 |
| TJM 5 | 111.08 ± 2.65 | 0.11 ± 2.65 |
| TJM 26 | 99.99 ± 0.30 | 0.10 ± 0.30 |
| NSM 16 | 95.81 ± 1.38 | 0.10 ± 1.38 |
| Extracts (Samples) | IC50 (µg/mL) | ||
|---|---|---|---|
| Lipoxygenase Inhibition | Xanthine Oxidase Inhibition | Hyaluronidase Inhibition | |
| TJM5 | 340.70 ± 9.08 c,d,m | NA | 424.33 ± 39.87 a,b,c,d,e,k |
| TJM21 | 423.43 ± 4.36 h,j,m | 336.60 ± 174.52 | 633.65 ± 59.81 a,b,c,k |
| TJM23 | 396.90 ± 0.55 a,g,m | NA | 196.10 ± 8.45 d |
| TJM24 | 344.87 ± 3.73 c,d,e,m | NA | 292.32 ± 19.81 d,k |
| TJM27 | 396.23 ± 14.74 a,b,g,m | NA | 319.99 ± 21.45 a,d,k |
| TJM26 | 357.47 ± 14.73 m | 507.57 ± 361.40 | 299.62 ± 19.65 a,d |
| TJM30 | 245.67 ± 2.42 m | >1000.00 | 476.27 ± 90.79 a,b,c,e,k |
| NSM16 | 340.80 ± 13.46 c,d,m | >1000.00 | 108.96 ± 6.15 |
| NSM22 | 401.93 ± 16.88 j,m | 450.73 ± 106.84 | 167.30 ± 14.61 |
| NSM23 | 307.37 ±5.71 c,d,e,m | 417.70 ± 40.26 | 216.84 ± 7.65 k |
| Positive controls | |||
| NDGA | 6.75 ± 0.58 | ||
| Allopurinol | 1.00 ± 0.01 | ||
| Apigenin | 100.59 ± 5.70 | ||
| Pearson Correlation | Hyaluronidase Inhibitory Activity (IC50) | Lipoxygenase Inhibitory Activity (IC50) | Xanthine Oxidase Inhibitory Activity (IC50) |
|---|---|---|---|
| r | −0.1839 | 0.08076 | −0.5052 |
| p-squared | 0.03381 | 0.006522 | 0.2552 |
| p-value (two-tailed) | 0.6111 | 0.8245 | 0.1364 |
| p-value summary | ns | ns | ns |
| IBA Level (ppm) | Rooting (%) | Shooting (%) | Shoot No | Root No | Shoot Length (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|
| 0 (T0) | 80.0 ± 5.77 a | 90.0 ± 4.08 a | 2.29 ± 0.29 a | 4.14 ± 1.16 a | 25.31 ± 5.35 ab | 8.86 ± 4.29 ab | 4.14 ± 0.91 ab | 1.00 ± 0.21 b |
| 1000 (T1) | 80.0 ± 4.08 a | 95.0 ± 2.88 a | 3.17 ± 0.98 a | 3.67 ± 0.71 a | 19.97 ± 9.33 ab | 20.51 ± 7.66 a | 8.41 ± 1.42 a | 3.17 ± 0.44 a |
| 3000 (T2) | 55.0 ± 6.46 b | 80.0 ± 2.40 b | 3.0 ± 0.62 a | 2.57 ± 0.69 a | 6.26 ± 1.15 b | 5.87 ± 1.92 b | 0.99 ± 0.36 b | 1.48 ± 0.43 b |
| 5000 (T3) | 27.5 ± 7.50 c | 75.0 ± 2.88 b | 3.33 ± 0.49 a | 3.0 ± 1.13 a | 29.18 ± 7.84 a | 6.6 ± 2.73 ab | 4.23 ± 2.10 b | 0.73 ± 0.33 b |
| Diameter of Cuttings (cm) | Rooting Percentage (%) | Shoot Number | Root Number | Shoot Length (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| 2.5–≤3.5 (D0) | 80.00 ± 5.77 a | 2.43 ± 0.30 b | 5.29 ± 1.11 ab | 33.14 ± 2.90 a | 10.66 ± 4.07 ab | 3.23 ± 0.78 c | 0.37 ± 0.04 b |
| 3.5–≤4.5 (D1) | 56.67 ± 6.67 b | 3.57 ± 0.57 b | 5.42 ± 0.37 ab | 33.50 ± 3.92 a | 5.45 ± 0.56 b | 10.96 ± 1.75 b | 1.08 ± 0.21 b |
| 4.5–≤5.5 (D2) | 53.33 ± 3.33 b | 3.33 ± 0.68 b | 4.50 ± 0.50 b | 27.08 ± 5.17 a | 15.11 ± 3.02 a | 13.23 ± 1.84 b | 2.46 ± 0.17 a |
| 5.5–≤6.5 (D3) | 60.00 ± 5.77 b | 9.83 ± 0.77 a | 7.83 ± 0.44 a | 37.58 ± 3.77 a | 13.09 ± 0.79 ab | 24.47 ± 1.46 a | 3.69 ± 0.55 a |
| Length of Cuttings (cm) | Rooting Percentage (%) | Shoot Number | Root Number | Shoot Length (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| 30 (L0) | 70.00 ± 5.77 a | 2.50 ± 0.22 b | 3.20 ± 0.51 b | 34.00 ± 2.09 a | 9.20 ± 3.01 a | 3.26 ± 0.63 b | 0.34 ± 0.03 b |
| 40 (L1) | 56.67 ± 6.66 a | 5.88 ± 1.39 a | 4.63 ± 0.92 ab | 22.15 ± 4.36 a | 3.05 ± 0.65 b | 10.68 ± 2.64 a | 1.16 ± 0.20 b |
| 50 (L2) | 63.33 ± 3.33 a | 5.27 ± 0.40 a | 5.33 ± 0.67 ab | 31.90 ± 5.08 a | 5.01 ± 0.64 ab | 14.02 ± 2.71 a | 1.96 ± 0.15 ab |
| 60 (L3) | 66.66 ± 3.33 a | 6.23 ± 0.62 a | 6.27 ± 0.53 a | 23.00 ± 2.56 a | 3.74 ± 0.56 ab | 18.00 ± 2.13 a | 3.24 ± 1.05 a |
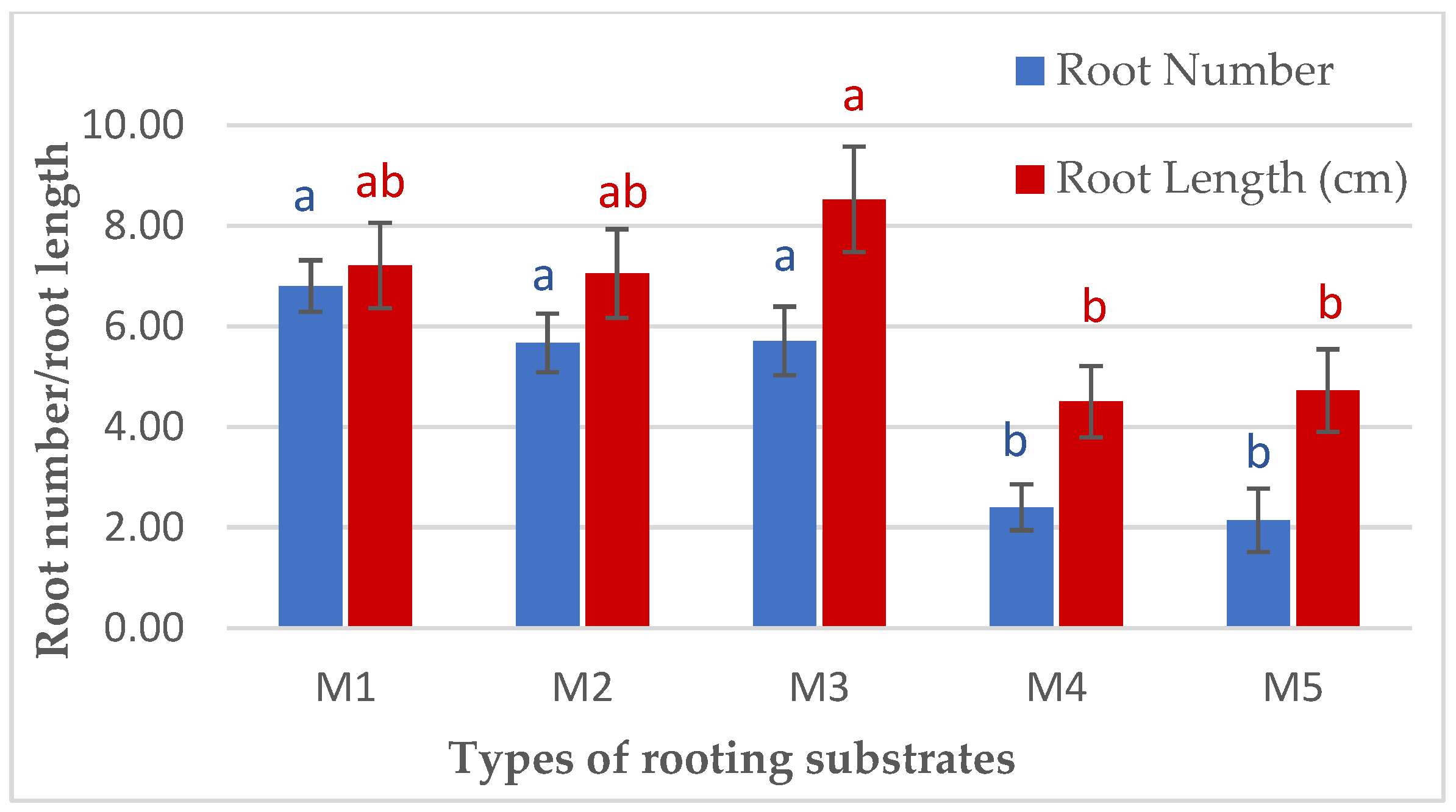
| Rooting Substrates | Rooting Percentage (%) | Shoot Number | Shoot Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|
| M1 | 76.67 ± 3.33 a | 10.93 ± 1.03 a | 50.77 ± 4.92 a | 37.86 ± 4.48 a | 3.02 ± 0.55 a |
| M2 | 66.67 ± 3.33 ab | 5.44 ± 0.52 b | 45.56 ± 6.33 bc | 25.30 ± 3.48 ab | 1.54 ± 0.28 ab |
| M3 | 53.33 ± 3.33 b | 6.00 ± 0.95 b | 60.02 ± 9.71 a | 30.21 ± 3.72 a | 1.46 ± 0.32 ab |
| M4 | 53.33 ± 3.33 b | 6.40 ± 0.74 b | 20.78 ± 3.06 b | 11.61 ± 1.94 b | 2.33 ± 0.35 a |
| M5 | 56.67 ± 8.82 b | 5.43 ± 1.05 b | 21.04 ± 6.53 b | 12.89 ± 3.32 b | 0.65 ± 0.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniandi, S.K.; Ariff, F.F.M.; Pisar, M.M.; Harun, S.T.; Abdullah, M.Z.; Abdullah, F.; Hashim, S.N.A.M.; Bahari, S.N.S.; Saffie, N. Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia. Biology 2024, 13, 785. https://doi.org/10.3390/biology13100785
Muniandi SK, Ariff FFM, Pisar MM, Harun ST, Abdullah MZ, Abdullah F, Hashim SNAM, Bahari SNS, Saffie N. Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia. Biology. 2024; 13(10):785. https://doi.org/10.3390/biology13100785
Chicago/Turabian StyleMuniandi, Sures Kumar, Farah Fazwa Md Ariff, Mazura Md Pisar, Samsuri Toh Harun, Mohd Zaki Abdullah, Fauziah Abdullah, Siti Nur Aisyah Mohd Hashim, Syafiqah Nabilah Samsul Bahari, and Norhayati Saffie. 2024. "Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia" Biology 13, no. 10: 785. https://doi.org/10.3390/biology13100785
APA StyleMuniandi, S. K., Ariff, F. F. M., Pisar, M. M., Harun, S. T., Abdullah, M. Z., Abdullah, F., Hashim, S. N. A. M., Bahari, S. N. S., & Saffie, N. (2024). Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia. Biology, 13(10), 785. https://doi.org/10.3390/biology13100785






