Cell Migration–Proliferation Dichotomy in Cancer: Biological Fact or Experimental Artefact?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Principles
- The possible hypothetical alternative migration–proliferation models for the MPD model are (i) simultaneous migration and proliferation at the individual cancer cell level (the SMP model) [19,20] and (ii) the two-separate-tumour-cell-clones model, in which one tumour cell clone is migration-proficient and proliferation-refractory, while the other clone has the reverse phenotype (the phenotype–refractory [PR] model).
- The implication of the MPD model in cancer cells is that the proliferation and migration phenotypes would either exist in a mutually exclusive relationship at the individual cancer cell level [3,4,5] or in an inverse relationship if measured by gene expression indices in a tumour body that also includes stromal and inflammatory cells. The SMP model implies that proliferation and migration phenotypes exist in direct correlation since individual cells can migrate and proliferate simultaneously [19,20]. In the PR model, proliferation and migration are independent of each other since proliferative cells are migration-refractory or migration-deficient, and vice versa. However, cell proliferation promotes the migratory phenotype because the higher the proliferation of cancer cells, the worse the depletion of nutrients and oxygen in the tumour microenvironment, and hence, the stronger the cues for migration [21,22].
- The body of a tumour in progression comprises subpopulations of cancer cells that either differentially or simultaneously exhibit proliferative and migratory traits at the cellular level. The dominant characteristic of a tumour body, i.e., proliferation versus migration/invasion at the cell population level, is dependent on the proportion of cancer cells with either cancer trait. However, this dominance would be absent if tumour cell proliferation and invasion are coupled together or occur simultaneously at the level of individual cancer cells.
- Mutual exclusivity between proliferation and migration per cell implies that an inverse relationship exists between proliferation and invasion/migration gene expression indices at the cell population level, both within each cancer stage and between early and late cancer stages. It also implies that differential enrichment of the proliferation and invasion gene sets must also exist between early- and late-stage diseases, inasmuch as early-stage disease is predominantly proliferative, whereas late-stage disease may or may not be largely invasive [4,8].
2.2. Study Cohorts
2.3. Data Handling
2.4. Study Approach
2.5. Statistical Analysis
3. Results
3.1. Demographics of Cancer Cohorts
3.2. Migration–Proliferation Correlation in CRC
3.3. Migration–Proliferation Correlation in GC
3.4. Migration–Proliferation Correlation in PCa
3.5. Migration–Proliferation Correlation within Molecular Subtypes of Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lattmann, E.; Deng, T.; Hajnal, A. To Divide or Invade: A Look Behind the Scenes of the Proliferation-Invasion Interplay in the Caenorhabditis elegans Anchor Cell. Front. Cell Dev. Biol. 2021, 8, 616051. [Google Scholar] [CrossRef] [PubMed]
- Hatzikirou, H.; Basanta, D.; Simon, M.; Schaller, K.; Deutsch, A. ‘Go or grow’: The key to the emergence of invasion in tumour progression? Math. Med. Biol. 2012, 29, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.; Kolobov, A. Investigation of solid tumor progression with account of proliferation/migration dichotomy via Darwinian mathematical model. J. Math. Biol. 2019, 80, 601–626. [Google Scholar] [CrossRef]
- Al Shamsi, H. Migration and Proliferation Dichotomy: A Persistent Random Walk of Cancer Cells. Fractal Fract 2023, 7, 318. [Google Scholar] [CrossRef]
- Marchal, M.A.; Moose, D.L.; Varzavand, A.; Jordan, N.E.; Taylor, D.; Tanas, M.R.; Brown, J.A.; Henry, M.D.; Stipp, C.S. Abl kinases can function as suppressors of tumor progression and metastasis. Front. Oncol. 2023, 13, 1241056. [Google Scholar] [CrossRef]
- Patsialou, A.; Wang, Y.; Pignatelli, J.; Chen, X.; Entenberg, D.; Oktay, M.; Condeelis, J.S. Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFβ in claudin-low breast tumor cells. Oncogene 2014, 34, 2721–2731. [Google Scholar] [CrossRef]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Sohn, B.H.; Hwang, J.-E.; Jang, H.-J.; Lee, H.-S.; Oh, S.C.; Shim, J.-J.; Lee, K.-W.; Kim, E.H.; Yim, S.Y.; Lee, S.H.; et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin. Cancer Res. 2017, 23, 4441–4449. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, J.; Vazquez, F.; McFarland, J.M. Bridging the gap between cancer cell line models and tumours using gene expression data. Br. J. Cancer 2021, 125, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.L.; Bodmer, W.F. Cancer Cell Lines for Drug Discovery and Development. Cancer Res. 2014, 74, 2377–2384. [Google Scholar] [CrossRef]
- Harbhajanka, A.; Lamzabi, I.; Bitterman, P.; Reddy, V.B.; Ghai, R.; Gattuso, P. Correlation of p16 Expression on Cancer and Stromal Cells With Clinicopathologic and Immunohistochemical Features of Lobular Breast Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 658–662. [Google Scholar] [CrossRef]
- Jiang, Z.; Generoso, S.F.; Badia, M.; Payer, B.; Carey, L.B. A conserved expression signature predicts growth rate and reveals cell & lineage-specific differences. PLoS Comput. Biol. 2021, 17, e1009582. [Google Scholar] [CrossRef]
- Chida, K.; Oshi, M.; An, N.; Kanazawa, H.; Roy, A.M.; Mann, G.K.; Yan, L.; Endo, I.; Hakamada, K.; Takabe, K. Gastric cancer with enhanced myogenesis is associated with less cell proliferation, enriched epithelial-to-mesenchymal transition and angiogenesis, and poor clinical outcomes. Am. J. Cancer Res. 2024, 14, 355–367. [Google Scholar] [CrossRef]
- Haass, N.K.; Beaumont, K.A.; Hill, D.S.; Anfosso, A.; Mrass, P.; Munoz, M.A.; Kinjyo, I.; Weninger, W. Real-time cell cycle imaging during melanoma growth, invasion, and drug response. Pigment. Cell Melanoma Res. 2014, 27, 764–776. [Google Scholar] [CrossRef]
- Vittadello, S.T.; McCue, S.W.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. Examining Go-or-Grow Using Fluorescent Cell-Cycle Indicators and Cell-Cycle-Inhibiting Drugs. Biophys. J. 2020, 18, 1243–1247. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, C.; Goding, C.R. Starvation and Pseudo-Starvation as Drivers of Cancer Metastasis through Translation Reprogramming. Cell Metab. 2019, 29, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sethi, N.S.; Hinoue, T.; Schneider, B.G.; Cherniack, A.D.; Sanchez-Vega, F.; Seoane, J.A.; Farshidfar, F.; Bowlby, R.; Islam, M.; et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell 2018, 33, 721–735.e8. [Google Scholar] [CrossRef] [PubMed]
- Ebili, H.O.; Agboola, A.O.; Rakha, E. MSI-WES: A Simple approach for microsatellite instability testing using whole exome sequencing. Future Oncol. 2021, 17, 3595–3606. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, C.; Qi, X.; Zhou, L.; Liu, D.; Lv, H.; Li, T.; Sun, D.; Zhang, X. Distinctive targetable genotypes of younger patients with lung adenocarcinoma: A cBioPortal for cancer genomics data base analysis. Cancer Biol. Ther. 2020, 21, 26–33. [Google Scholar] [CrossRef]
- Alfahed, A.; Ebili, H.O.; Almoammar, N.E.; Alasiri, G.; AlKhamees, O.A.; Aldali, J.A.; Al Othaim, A.; Hakami, Z.H.; Abdulwahed, A.M.; Waggiallah, H.A. Prognostic Values of Gene Copy Number Alterations in Prostate Cancer. Genes 2023, 14, 956. [Google Scholar] [CrossRef]
- Alfahed, A.; Ebili, H.O.; Waggiallah, H.A. Chromosome-specific segment size alterations are determinants of prognosis in prostate cancer. Saudi J. Biol. Sci. 2023, 30, 103629. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Maleki, F.; Ovens, K.; Hogan, D.J.; Kusalik, A.J. Gene Set Analysis: Challenges, Opportunities, and Future Research. Front. Genet. 2020, 11, 654. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Spurr, L.F.; Martinez, C.A.; Katipally, R.R.; Iyer, S.C.; Pugh, S.A.; Bridgewater, J.A.; Primrose, J.N.; Domingo, E.; Maughan, T.S.; D’angelica, M.I.; et al. A proliferative subtype of colorectal liver metastases exhibits hypersensitivity to cytotoxic chemotherapy. npj Precis. Oncol. 2022, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Pfister, K.; Pipka, J.L.; Chiang, C.; Liu, Y.; Clark, R.A.; Keller, R.; Skoglund, P.; Guertin, M.J.; Hall, I.M.; Stukenberg, P.T. Identification of Drivers of Aneuploidy in Breast Tumors. Cell Rep. 2018, 23, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kang, Y. Multilayer control of the EMT master regulators. Oncogene 2014, 33, 1755–1763. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, J.; Hu, Y.; Lu, P.; Luo, Q.; Wang, L. Gli promotes tumor progression through regulating epithelial-mesenchymal transition in non-small-cell lung cancer. J. Cardiothorac. Surg. 2020, 15, 18. [Google Scholar] [CrossRef]
- Ervin, E.-H.; French, R.; Chang, C.-H.; Pauklin, S. Inside the stemness engine: Mechanistic links between deregulated transcription factors and stemness in cancer. Semin. Cancer Biol. 2022, 87, 48–83. [Google Scholar] [CrossRef]
- Vallania, F.; Zisman, L.; Macaubas, C.; Hung, S.-C.; Rajasekaran, N.; Mason, S.; Graf, J.; Nakamura, M.; Mellins, E.D.; Khatri, P. Multicohort Analysis Identifies Monocyte Gene Signatures to Accurately Monitor Subset-Specific Changes in Human Diseases. Front. Immunol. 2021, 12, 659255. [Google Scholar] [CrossRef]
- Feys, S.; Heylen, J.; Carvalho, A.; Van Weyenbergh, J.; Wauters, J.; Variomic Study Group. A signature of differential gene expression in bronchoalveolar lavage fluid predicts mortality in influenza-associated pulmonary aspergillosis. Intensiv. Care Med. 2023, 49, 254–257. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef]
- Buruiană, A.; Gheban, B.-A.; Gheban-Roșca, I.-A.; Georgiu, C.; Crișan, D.; Crișan, M. The Tumor Stroma of Squamous Cell Carcinoma: A Complex Environment That Fuels Cancer Progression. Cancers 2024, 16, 1727. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.-T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Murphy, K.J.; Chambers, C.R.; Herrmann, D.; Timpson, P.; Pereira, B.A. Dynamic Stromal Alterations Influence Tumor-Stroma Crosstalk to Promote Pancreatic Cancer and Treatment Resistance. Cancers 2021, 13, 3481. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Ansieau, S.; Hinkal, G.; Thomas, C.; Bastid, J.; Puisieux, A. Early origin of cancer metastases: Dissemination and evolution of premalignant cells. Cell Cycle 2008, 7, 3659–3663. [Google Scholar] [CrossRef][Green Version]
- Bai, L.; Yang, H.H.; Hu, Y.; Shukla, A.; Ha, N.-H.; Doran, A.; Faraji, F.; Goldberger, N.; Lee, M.P.; Keane, T.; et al. An Integrated Genome-Wide Systems Genetics Screen for Breast Cancer Metastasis Susceptibility Genes. PLoS Genet. 2016, 12, e1005989. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Almagro, J.; Messal, H.A.; Elosegui-Artola, A.; van Rheenen, J.; Behrens, A. Tissue architecture in tumor initiation and progression. Trends Cancer 2022, 8, 494–505. [Google Scholar] [CrossRef]
- Ilina, O.; Gritsenko, P.G.; Syga, S.; Lippoldt, J.; La Porta, C.A.M.; Chepizhko, O.; Grosser, S.; Vullings, M.; Bakker, G.-J.; Starruß, J.; et al. Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 2020, 22, 1103–1115. [Google Scholar] [CrossRef]
- Kang, W.; Ferruzzi, J.; Spatarelu, C.-P.; Han, Y.L.; Sharma, Y.; Koehler, S.A.; Mitchel, J.A.; Khan, A.; Butler, J.P.; Roblyer, D.; et al. A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation. iScience 2021, 24, 103252. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Ganguli, S.; Plak, K.; Taubenberger, A.V.; Win, Z.; Williamson, M.; Piel, M.; Guck, J.; Baum, B. Oncogenic Signaling Alters Cell Shape and Mechanics to Facilitate Cell Division under Confinement. Dev. Cell 2020, 52, 563–573.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, M.; Cui, L.; Gao, M.; Zhang, M.; Yue, F.; Shi, T.; Yang, X.; Pan, Y.; Zheng, X.; et al. Chemotherapy exacerbates ovarian cancer cell migration and cancer stem cell-like characteristics through GLI1. Br. J. Cancer 2020, 122, 1638–1648. [Google Scholar] [CrossRef]
- Raudenská, M.; Petrláková, K.; Juriňáková, T.; Fialová, J.L.; Fojtů, M.; Jakubek, M.; Rösel, D.; Brábek, J.; Masařík, M. Engine shutdown: Migrastatic strategies and prevention of metastases. Trends Cancer 2023, 9, 293–308. [Google Scholar] [CrossRef] [PubMed]

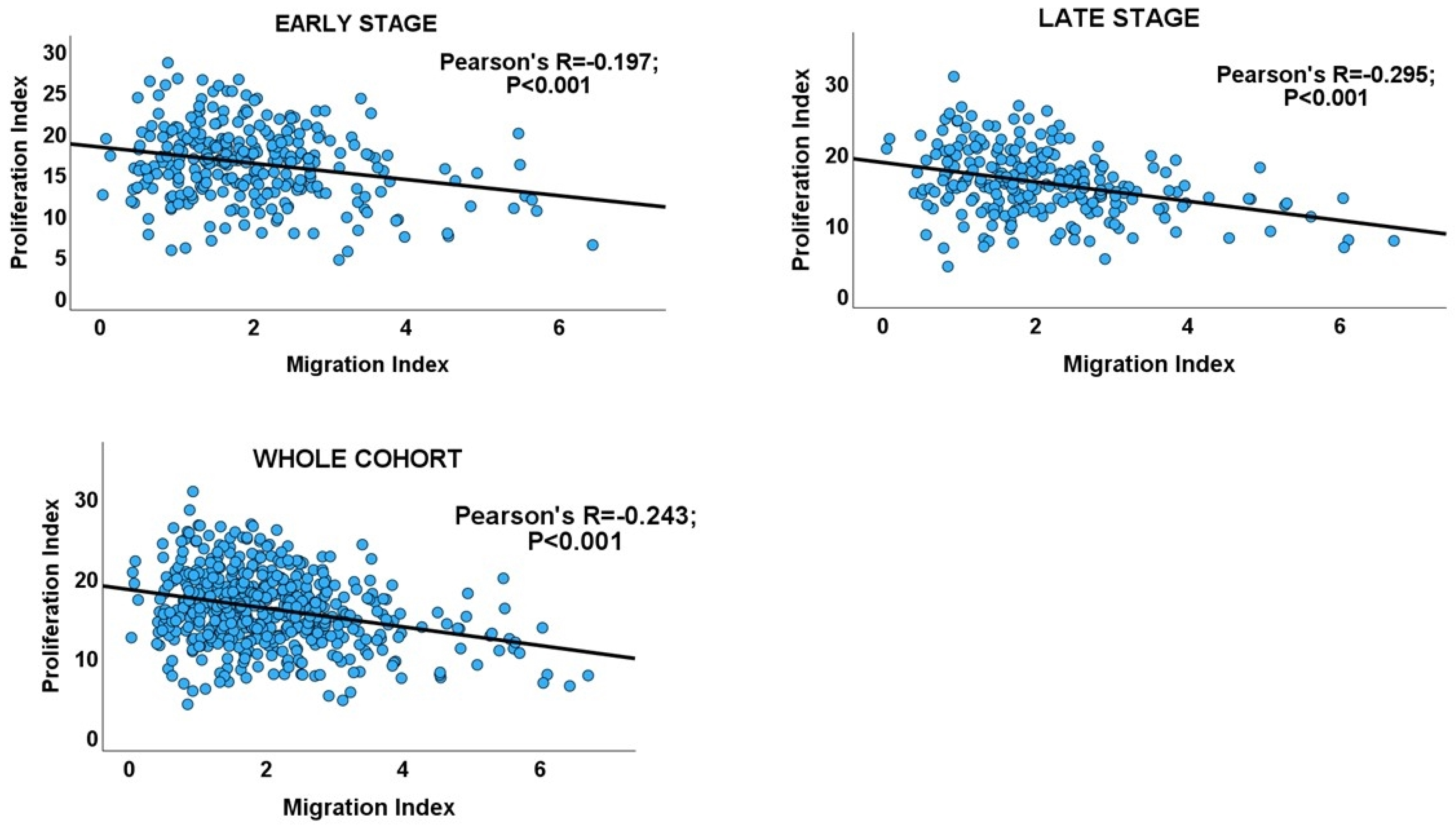

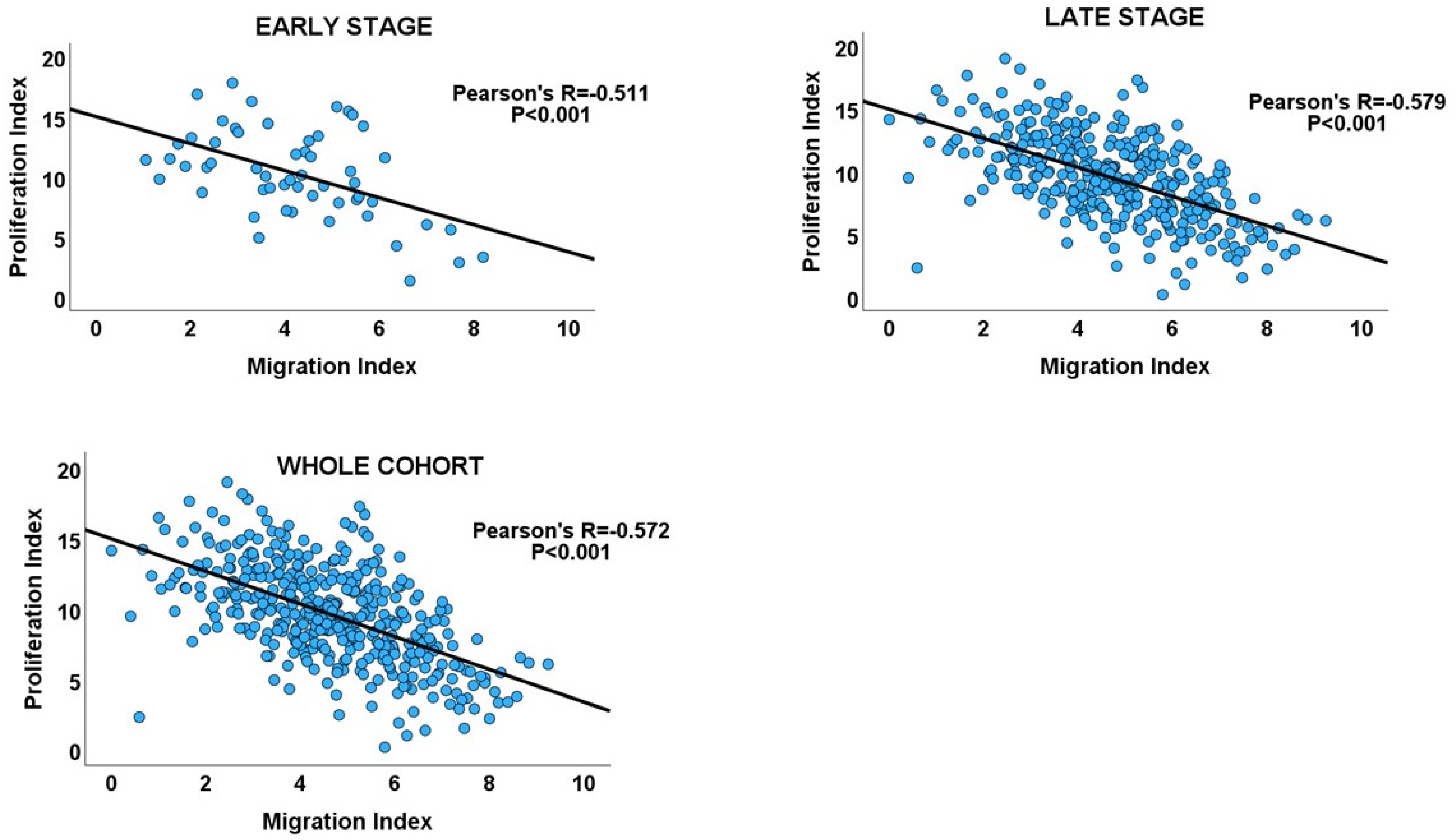
| Cancer Stage | Number of Cases | Pearson’s Correlation (R) | 95% Confidence Interval for R | p Value | Adjusted p Value |
|---|---|---|---|---|---|
| CRC cohort | |||||
| Early stage | 290 | −0.197 | −0.305 to −0.083 | <0.001 | <0.001 |
| Late stage | 242 | −0.295 | −0.406 to −0.175 | <0.001 | <0.001 |
| Overall | 532 | −0.243 | −0.321 to −0.161 | <0.001 | - |
| GC cohort | |||||
| Early stage | 55 | −0.511 | −0.681 to −0.280 | <0.001 | <0.001 |
| Late stage | 352 | −0.579 | −0.644 to −0.504 | <0.001 | <0.001 |
| Overall | 407 | −0.572 | −0.633 to −0.502 | <0.001 | - |
| TCGA PCa cohort | |||||
| Early stage | 135 | −0.396 | −0.506 to −0.213 | <0.001 | <0.001 |
| Late stage | 258 | −0.294 | −0.402 to −0.178 | <0.001 | <0.001 |
| Unclassified | 104 | −0.206 | −0.384 to −0.013 | <0.037 | <0.037 |
| Overall | 497 | −0.297 | −0.358 to −0.96 | <0.001 | |
| MSKCC PCa cohort | |||||
| Early stage | 85 | −0.228 | −0.420 to −0.015 | 0.036 | 0.036 |
| Late stage | 64 | −0.407 | −0.595 to −0.177 | <0.001 | 0.002 |
| Primary | 131 | −0.240 | −0.396 to −0.070 | 0.006 | 0.010 |
| Metastasis | 18 | −0.500 | −0.784 to −0.043 | 0.013 | 0.016 |
| Overall | 149 | −0.328 | −0.465 to −0.176 | <0.001 | 0.002 |
| Cancer Stage | Number of Cases | Mean of DI | 95% Confidence Interval for Mean DI | F | p Value |
|---|---|---|---|---|---|
| CRC cohort | |||||
| Early stage | 289 | 26.206 | 18.645–33.767 | 3.418 | 0.065 |
| Late stage | 243 | 15.745 | 7.574–23.916 | ||
| Total | 532 | 21.428 | 15.878–26.977 | ||
| GC cohort | |||||
| Early stage | 17 | 14.233 | 10.852–17.615 | 23.682 | <0.001 |
| Late stage | 387 | 6.379 | 5.728−7.029 | ||
| Total | 404 | 6.709 | 6.055–7.634 | ||
| Cancer Stage | Number of Cases | Mean of DI | 95% Confidence Interval for Mean DI | F | p Value |
|---|---|---|---|---|---|
| TCGA cohort | |||||
| Early stage | 135 | 2.416 | 0.693–4.139 | 1.971 | 0.161 |
| Late stage | 258 | 4.390 | 2.600–6.180 | ||
| Total | 393 | 3.712 | 2.398–5.026 | ||
| MSKCC cohort | |||||
| Early stage | 85 | 2.073 | 1.098–3.048 | 2.620 | 0.108 |
| Late stage | 65 | 6.204 | 1.282–11.128 | ||
| Total | 150 | 3.864 | 1.667–6.060 | ||
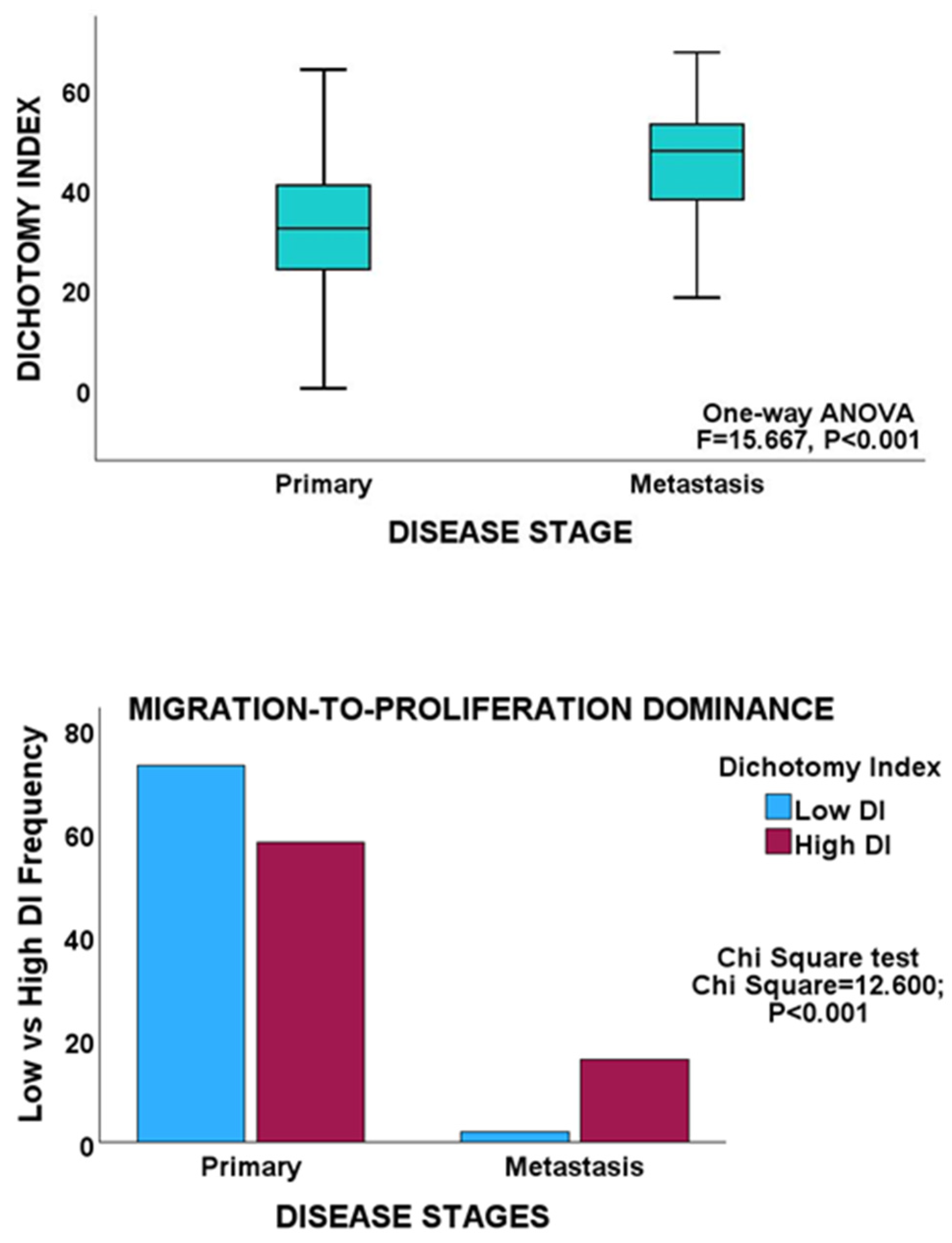
| Primary | 131 | 2.343 | 0.175–4.511 | 15.667 | <0.001 |
| Metastasis | 19 | 14.932 | 8.232–21.633 | ||
| Total | 150 | 3.864 | 1.716–6.011 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfahed, A. Cell Migration–Proliferation Dichotomy in Cancer: Biological Fact or Experimental Artefact? Biology 2024, 13, 753. https://doi.org/10.3390/biology13100753
Alfahed A. Cell Migration–Proliferation Dichotomy in Cancer: Biological Fact or Experimental Artefact? Biology. 2024; 13(10):753. https://doi.org/10.3390/biology13100753
Chicago/Turabian StyleAlfahed, Abdulaziz. 2024. "Cell Migration–Proliferation Dichotomy in Cancer: Biological Fact or Experimental Artefact?" Biology 13, no. 10: 753. https://doi.org/10.3390/biology13100753
APA StyleAlfahed, A. (2024). Cell Migration–Proliferation Dichotomy in Cancer: Biological Fact or Experimental Artefact? Biology, 13(10), 753. https://doi.org/10.3390/biology13100753





