Simple Summary
It has been established that a variety of factors, including the environment, influence craniofacial growth. Environmental alterations are regarded to have the potential to impact maxillary and mandibular growth. Numerous studies have partially addressed the question of how diet consistency influences maxillary and mandibular growth in one generation of rats by suggesting that diet consistency may lead to variable masticatory forces that affect mandibular growth. There has not been any research carried out yet that looks at potential qualitative and quantitative growth changes in the maxilla and mandible throughout the course of different generations using 3D imaging technology and wistar rats as the experimental animal of choice. The current study assessed the effects of various meal compositions on the growth of the mandible across three generations. The findings of this study suggest that a soft diet may be to blame for decreased maxillary and mandibular growth, and this genetic information may be passed down through the generations.
Abstract
Background: In this study, wistar rats were used to examine the impact of diet consistency on maxillary and mandibular growth over three generations. Methods: In this investigation, a breeding sample of 60 female and 8 male wistar rats was used. Measuring was only performed on female animals. The first generation’s primary breeding sample consisted of 20 female wistar rats that were 30 days old and 4 male rats that were also 30 days old; two subsequent generations were created from these animals. At the age of 100 days, CBCTs were collected of all male rats. Twenty-eight craniofacial landmarks were selected for the linear measurements on stl format extracted from the DICOM files. A Bonferroni test was performed for the statistical analysis. Results: Means of measurements of all soft diet groups compared to corresponding measurements of the hard diet groups were significantly different. According to linear measurements, there was statistical difference on the maxillary measurements between the soft diet groups of the first and third generation, while the rest did not appear to have any statistical difference. There was significant difference for the mandibular dimensions only when the first generation soft diet group was compared with the third generation soft diet group. Conclusions: Food consistency has a significant impact on the growth and development of the maxilla and mandible. Soft diet habits may result in retrognathic mandible, and narrower maxilla.
1. Introduction
The craniofacial system grows as a result of environmental and inherited variables [1,2]. It is widely acknowledged that muscle loading forces contribute to bone formation and growth [3]. The orofacial region is one of the most important muscular systems since it is required for feeding in vertebrates. Jaw movement and stresses on the orofacial region are intimately related to mandibular growth [4]. A number of developmental alterations in the craniofacial area, especially the mandible, appear to be caused by mastication as one of the environmental influences [5,6]. This could be the explanation for the average rise in malocclusions observed in industrial cultures throughout the 20th century [7,8]. According to recent research, the maxilla’s morphology, which deforms less in those who can generate proportionately bigger temporalis muscle forces, controls how much force is transmitted from the mastication process to the rest of the skull [9]. Additionally, recent studies have revealed a connection between mandibular muscle force and shape. Consequently the total shape of the mandible may be due to some extend masticatory muscle force and, mandibular loading [10]. It is suggested that the decline in functional constraints has led to the increased form variation seen in modern/urban populations [11].
Craniofacial growth is primarily influenced by genetic factors, but it also can be affected to a significant degree by environmental factors such as dietary conditions, status of physical activity and health condition. The major contemporary genetic theories, trying to explain the determinants of craniofacial growth, imply that either the bone itself, the cartilage or the soft tissue matrix surrounding the hard tissues mainly control craniofacial growth, but it is generally accepted that environmental factors contribute to the final expression of gene information. The main genetic and, to a lesser extent, functional and environmental, variables affect the growth of the face. It is believed that force and function have a close relationship. The growth process is influenced by a variety of factors, including genetic control, differential cellular responses, neurotrophic control, membrane control, oxygen tension, bioelectric potentials, pH levels, temperature effects, chalone-like inhibitors and stimulators, cyclic AMP, vasomotor control, nutrition, and enzymatic and hormonal factors. It is crucial to understand the difference between primary and secondary, or supporting, elements. Facial growth is thought to be influenced by specific combinations of all the aforementioned elements, which regulate the size, shape, and position of the faces in the craniofacial skeleton [12]. Compared to populations from non-industrialized societies, the human population of a contemporary civilization experiences more severe malocclusions. Modern food practices put less strain on the masticatory muscles than earlier dietary practices, which has been linked to an increase in malocclusion frequency. Researchers are therefore interested in how food consistency affects craniofacial growth. Rats were used as experimental animals in the majority of experimental studies to examine the relationship between diet consistency and growth. Rats are frequently chosen as experimental animals because they are tiny, readily kept, express little social anxiety, have a brief lifespan, and have well-known genetic backgrounds and growth patterns that are similar to those of humans [13,14,15,16,17,18,19,20,21,22,23]. Genetics has a significant and well-established effect on both the occlusion and craniofacial growth. Similar craniofacial growth trends have been observed in many family generations. This led to the conclusion that some heritability exists for craniofacial growth. Additionally, it is acknowledged that the environment can influence and modify genetic information [24,25].
Intracortical bone remodeling (or simply remodeling) is the process of existing cortical bone being resorbed and replaced as a result of the coordinated actions of osteoclasts and osteoblasts. As a result of this procedure, secondary osteons, which are cylinder-shaped structures, are produced. Cross-sectional images of secondary osteons show concentric lamellae and a cement line surrounding them. The resorption phase of the remodeling process releases mineral reserves to maintain mineral homeostasis, but it also causes mechanical deformation that results in the formation of microcracks. Microcracks have been associated with both large mechanical deformations (high strain) and repetitive cycles of loading. As a result, regions of the skeleton under more demanding loading conditions ought to have higher rates of remodeling since those regions ought to experience more microdamage. It is less clear if greater remodeling may be caused by high strain or cyclical loading when the load situation is unclear. The current body of literature contains controversial findings that support the theory that increased remodeling may be more likely to occur under conditions of cyclical loading rather than high strain [26,27,28,29,30,31,32].
With the aid of molecular biology, different epigenetic mechanisms that extend from the cell membrane to the nucleus and are made up of intracellular macromolecular chain reactions are now understood. The extracellular setting and the nucleus exchange information in this manner. As a result of its ability to recognize and react to mechanical stimuli, the osteocyte network is crucial in initiating bone remodeling. In addition, the form of the cells can be altered by loading the tissues. As a result, processes are engaged that even alter the methods by which the genome acts, leading to deformation of the intracellular material, including the cytoskeleton. Through this mechanism the epigenetic information can be inherited [33,34,35].
As documented by Odman et al. in 2008, a 7-month interval with reduced masticatory requirements in a soft diet group throughout adolescence and early adulthood led to smaller mandibles. Through morphometric research, it was discovered that the angular process area and the condylar process inclination differ significantly. Sprague Dawley rats were utilized as the experimental animals in this study [36]. Trabecular bone had a higher degree of mineralization than cortical bone, according to a 2007 Tanaka et al. study on wistar rats. The anterior mandibular region has higher levels of mineralization than the posterior mandibular region. The soft diet group showed a higher level of mineralization than the hard diet group in those two locations. The trabecular bone of the condyle in the hard diet group had more mineralization than the soft diet group [37]. Similar hypotheses were made by Grunheid et al. in 2011, but they used New Zealand White rabbits as their experimental animals. Their findings showed that the remodeling rate is not significantly affected by a moderate reduction in masticatory functional load [38]. By utilizing wistar rats as their experimental animals in 2022, Tsolakis et al. pointed out that a soft diet led to a smaller condyle and a decreased angle of the jaw as well as the body of the mandible [39].
The existing literature demonstrates that, compared to chewing soft food, chewing hard food improves practically all physiological masticatory parameters, muscular coordination, and masticatory side changes. The association between mastication and general health problems, such as obesity and diminished cognitive function, as well as a more variable and symmetrical weight on the craniofacial structures that affects their growth and wellness, may be explained in part by these findings [40].
Robles RA developed Cone Beam Computer Tomography (CBCT) in 1982 for use in angiography [41]. In a manner similar to Computed Tomography (CT), CBCT rotates around the object of interest while taking several images, creating a three-dimensional (3D) volume. However, CBCT uses a volumetric approach that only requires one rotation, resulting in lower radiation exposure. Recent advancements in CBCT imaging techniques have made it possible to expose patients to less radiation than with conventional two-dimensional radiography. This machine gave the ability to represent the skull with less radiation but with high accuracy [42].
Numerous studies with varying outcomes from that expectation have addressed the subject of how diet consistency influences maxillary and mandibular growth within one generation. There is not much research that has examined potential maxillary and mandibular alterations throughout generations, and only one equivalent study in rats was conducted in the past.
The aim of this study is to examine the impact of different food consistencies on maxillary and mandibular growth within three generations through 3D imaging technology using Cone Beam Computed Tomography (CBCT).
2. Materials and Methods
Prior to beginning this investigation, the General Directorate of Veterinary Policy of the prefecture of Attica in the Hellenic Republic received approval from the Institutional Review Board. 1405 is the approval code. Subjects were chosen from the National and Kapodistrian University of Athens School of Medicine’s Laboratory of Experimental Surgery and Surgical Research in Athens, Greece. Eight male and sixty female Wistar rats were employed in the study’s total sample. Male rats were solely utilized in this study for reproduction; all measurements were performed on female rats. The identification of the specimen was not able during all measurements for blinding reasons.
A power analysis was carried out to determine the results’ power significance based on a prior study [43]. According to the power analysis, a minimum sample size of 10 animals per group will result in a 95% confidence level. Thus, it was decided to choose 20 animals for each generation of this study, 10 for the soft diet group and 10 for the hard diet group. The main sample consisted of 20 female Wistar rats that were 30 days old and 4 male rats. The first generation is made up of the 20 females. By using computer-generated randomness, these were divided into two equal groups of 10 females each. The female rats were fed a soft diet for 30 days in the first group (S1) and a hard diet for 30 days in the second group (H1). Additionally, two males of the same age were fed hard food, while the other two received soft food. Rats from each relevant group were combined on experimental day 31 to facilitate reproduction. After the ablactation phase, the female rats of the first generation were separated from their offspring, and on the 70th day of the experiment, CBCTs were taken before the sacrifice. As a result, we produced two more groups of the second generation, each of which had ten female rats picked at random from the first generation’s progeny. The new groups were distinguished from the old ones using new indicators denoting those who had a soft diet (S2) and those who received a hard diet (H2). Additionally, for the goal of research reproduction, two male rats from each descendant group of the first generation’s soft and hard diets were kept in the experiment. The second generation male and female rats were merged to enhance reproduction at 41 days following each relevant group. After the ablactation phase, the female rats of the second generation were separated from their offspring, and on the 150th day of the experiment, CBCTs were taken before the sacrifice. Two groups of female rats, representing the third generation, were created from the offspring of the second generation, each group consisting of 10 randomly chosen animals. The hard diet group (H3) and the soft diet group (S3) made up the third generation. Additionally, two male rats from each descendant group of the second generation’s soft and hard diets were kept in the experiment for the purpose of breeding. Next, 41 days after the third generation’s relevant groups’ female and male rats were mixed to facilitate reproduction. After the ablactation phase and before euthanasia (on the 230th trial day), the female rats of the third generation were isolated and randomly chosen from their offsprings. CBCTs were also taken (Figure 1 and Figure 2).
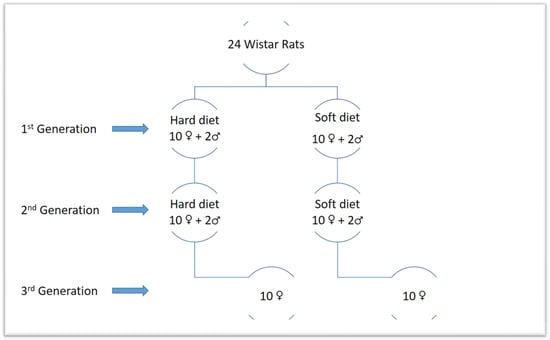
Figure 1.
Graphic scheme of the generations.
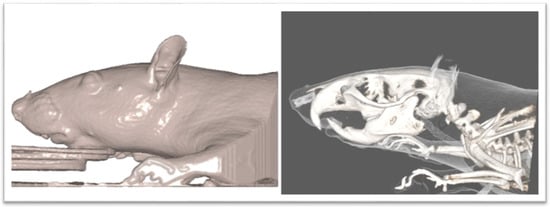
Figure 2.
CBCT image of rat.
The standard rat diet (R34; Lactamin) was administered to the hard diet groups as hard pellets. For the soft diet groups, the standard diet was crushed and mixed in specified ratios with water (2 parts food:5 parts water). To eliminate bulky objects that would induce excessive chewing, the bedding material in the cages of this group was sorted.
The Νewtom VGi evoTM Cone Beam Computed Tomography (Newtom, Bologna, Emilia-Romagna, Italy) was used. All the animals were sedated in order to be stable during the procedure. Since the head of the wistar rats are small objects, we wanted the size of FOV to be as close as it could be in the skull size, we used the option of the Newtom VGi evoTM to scan smaller objects like a dental cast (Figure 1). Once the DICOM files were created they were uploaded to the Viewbox software (Version 4.1.0.12). From those DICOM files we were able to extract stl files of each skull (Figure 3 and Figure 4). Color mapping was used in order to extract maxilla and mandible from the skull. Twenty-eight craniofacial landmarks were selected for the linear measurements. (Table 1) Eleven measurements were selected for the transverse analysis of the maxilla and one measurement for the analysis on the vertical plane. For the mandible, fourteen measurements showed the differences on the horizontal plane, four measurements showed the differences on the vertical plane, and four measurements indicated the differences on the transverse plane. Once the stl files of each jaw were extracted, they were digitized. (Table 2, Figure 3) All data were imported into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) and statistical analysis was performed using SPSS Version 22 (IBM Corporation, Armonk, NY, USA).
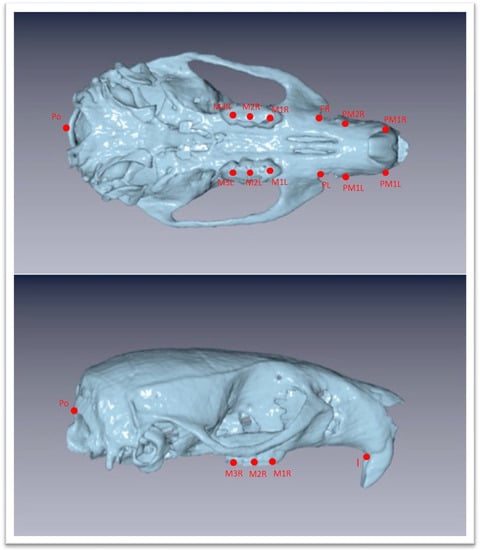
Figure 3.
Three-dimensional images of the maxilla extracted as stl file from the DICOM files with chosen landmarks. Landmarks are defined in Table 1.
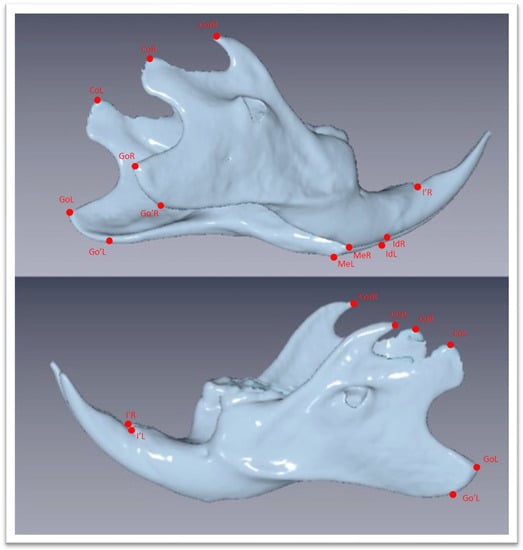
Figure 4.
Three-dimensional images of the mandible extracted as stl file from the DICOM files with chosen landmarks. Landmarks are defined in Table 1.

Table 1.
Craniofacial landmarks.

Table 2.
Reliability performed on 20 randomly selected subjects re-measured 2 weeks apart.
Statistical Analysis
Utilizing intraclass correlation on 20 randomly chosen participants, whose data were re-measured three weeks later, the operator’s reliability was determined.
Regression analysis were utilized for the linear measurements to assess differences linked to diet and generation. On each variable, the effects of diet, generation, and their interactions were regressed. When the normality requirement for the residuals was violated, quantile regression was used. Multiple comparisons were adjusted for using the Bonferroni technique. A statistical significance level of =5% was used for the analysis.
3. Results
The effect of food consistency on maxillary and mandibular growth throughout one, two, and three generations was examined in this study. Twenty-four rats were divided into two groups for the current study: the hard diet group and the soft diet group. All rat species reproduced after 30 days of growth. This process took place in order to create three generations. Using CBCTs, we examined how dietary physical consistency affected the growth of the maxilla and mandible. Sixty female rats made up the sample. An excellent level of agreement between all measurements was produced by the intra-observer variability.
3.1. Maxillary Measurements
The linear measurements that indicated the transverse analysis of the maxilla were PR–PL, PM1R–PM1L, PM2R–PM2L, M1R–M1L, and M2R–M2L, and M3R–M3L. The horizontal differences were expressed from the I-Po measurement (Table 3 and Table 4). As shown in Table 5, there were no significant differences between all soft diet groups when they were compared with the hard diet groups in PR–PL, PM1R–PM1L, PM2R–PM2L linear measurements. There were no significant changes on M1R–M1L of the first generation soft diet group when they were compared to the hard diet groups, while there were significant changes on M2R–M2L, M3R–M3L. There were significant differences in M1R–M1L, M2R–M2L, M3R–M3L measurements of second and third generation soft diet groups in comparison with hard diet groups. There were no significant changes between all hard diet generations due to measurements. Between the first and second generations of the soft diet groups, as well as between the second and third generations of the soft diet groups, the linear measurements did not reveal any significant differences. Between the first generation and the third generation of the soft diet group, there were significant alterations in M1R–M1L, M2R–M2L, M3R–M3L measurements (Table 5).

Table 3.
Results for soft diet groups variables measured, means, and standard deviations.

Table 4.
Results for hard diet groups variables measured, means, and standard deviations.

Table 5.
Bonferroni corrected (multiple comparisons). p values.
3.2. Mandibular Measurements
The mandibular measurements that indicated horizontal differences were Go’R–MeR, GoR–MeR, CorR–MeR, CoR\go’R–MeR. CoR–MeR, CoR–IdR, CoR–I’R, Go’L–MeL, GoL–MeL, CorL–MeL, CoL\Go’L–MeL, CoL–MeL, CoL–IdL, CoL–I’L. The measurements of vertical analysis were CoR–GoR, CoR–Go’R, CoL–GoL, CoL–Go’L and for the transverse analysis were CoL–CoR, Go’L–Go’R, MeL–MeR, IdL–IdR. (Table 3 and Table 4) There were significant differences between all soft diet groups when they were compared with the hard diet groups. There were no significant changes between all hard diet generations due to linear measurements. The measurements showed significant differences only on CoR–MeR, CoR–IdR, CoR–I’R, CoL–MeL, CoL–IdL, CoL–I’L and CoL\Go’L–MeL between all possible comparisons of the soft diet groups. The rest of the linear measurements for soft diet groups showed no significant differences between the first and second generation; conversely, the statistical analysis showed differences between the first and the third generation of the soft diet group as significant as between the second and third generations (Table 5).
4. Discussion
Previous studies have looked over the influence of diet consistency on growth but only two of them exceeded the one-generation as period of time. All of the studies used lateral cephalometric X-rays or 3D imaging (micro-CT). Three-dimensional imaging cone beam computed tomography gave us the opportunity to have a 3D image of the whole skull of live animals with no risk of losing any hard tissue due to the process of sacrificing the rats and extracting the skull from the body. Furthermore, it is impossible to examine the facial dimensions of a live rat in a micro-CT.
Yamamoto found that food consistency affects the bone appositional pattern at growth site in palatal region of the maxilla [44]. In 1997 Ulgen et al. found that maxillary width have been reduced by examining dry skulls [45]. In 2002 Katsaros et al. examined dry skulls and found that the dental arch was affected. Soft diet groups had narrower arch in the third molar region. Premaxilla and frontal bones were narrower as well [46]. Our study showed that premaxilla measurements had no significant changes. The anterior part of maxilla showed no significant differences between all possible comparisons. The dental arch was becoming narrower in all three generations of soft diet group.
Kiliaridis et al. found that the growth rate in the gonial angle of mandible was increased in the hard diet groups but the angle between occlusal and mandibular plane was decreased in the hard diet groups [47]. In 2002, Maki et al. employed linear measurements on X-rays and discovered that the hard diet groups had higher ramus heights as well as condylar process and coronoid process heights. They found that there was no discernible change in mandibular length between the hard diet and soft diet groups. [48]. Five years later, Abed et al. reported that the anterior corpus length, the ramus height, and the bigonial width were increased in the hard diet groups [49]. Hichijo et al. concurred with the earlier investigations that the hard diet groups’ ramus height increased. While on the contrary the gonial angle and the ramus angle were decreased in the hard diet groups. Mandibular length, mandibular base length, and coronoid process height did not significantly differ between the hard diet and soft diet groups [50]. Recent research by Tsolakis et al. revealed that all measurements corresponding to the mandibular posterior height and length showed significant differences. In the soft diet group, there was a reduction in length and posterior height and any differences in the condyle, the mandibular angle, and the mandibular body were revealed by the morphometric superimposition [39]. The present three dimensional study showed that mandibular morphology changed on horizontal and vertical planes when soft diet groups were compared to hard diet groups, respectively but there was no difference on the transverse plane. From the comparisons between the soft diet groups, it was found that there was significant differences mainly on the horizontal linear measurements that included condylar points between the first generation and the second generation. From the comparison of second and third generation, there were significant differences for all horizontal measurements. At last, comparison of the first and third generations of the soft diet groups showed differences on horizontal as long as on vertical plane.
There are only two studies in the literature that looked over the maxillary and mandibular changes within different generations. Only one of these studies used 3D imaging technology (microCT) to represent the anatomical structures but in this study, mice were used as experimental animals. To be more specific, Hassan et al. examined the variations in craniofacial morphology over 15 generations of mice fed either a soft diet or a hard diet in 2020 [51]. The animals were sacrificed before their skull dimensions were examined with the micro-CT technique. This is in contrast to our method that examined skull dimensions in live rats, avoiding any bony and muscular distraction as a result of skull separation. They found that short-term soft diet consumption resulted in a number of morphological alterations as well as a significant reduction in craniofacial size. However, shape analysis revealed that in their study, mice had shorter mandibles and craniums in the anteroposterior dimension. Consumption of a soft diet for 15 generations in a row did not modify the size of the craniofacial structure. Further evidence that changes in shape and size due to different functional loads appeared to be independent was provided by the fact that changes in shape persisted after diets were switched for one generation while size dropped and then returned to baseline. The authors did not mention any changes in the maxilla as a separate anatomic area but they emphasized on cranium [51]. In contrast, Tsolakis et al. [43] used wistar rats as their experimental animals in 2023, and their findings showed that the soft diet groups differed statistically significantly from the hard diet groups in all length measurements. The comparison of all length measurements between the first generation and the third generation of the soft diet group revealed statistically significant changes, despite the fact that there were no differences between the first and second generations of the soft diet groups or between the second and third generations of the soft diet groups. Additionally, in all posterior height linear measurements, there were statistically significant differences between the hard diet groups and all soft diet groups. Furthermore, there were no statistically significant variations in posterior height measurements between the second and third generations of the soft diet groups or between the first and second generations of the soft diet groups. Nevertheless, there were statistically significant differences between the first generation and the third generation of the soft diet groups when all posterior height data were compared. Since there were changes between all generations but the differences were statistically significant two generations later, it was revealed that the long-term mastication of a soft food was capable of affecting the morphology of the mandible. The current study shown that, when comparing soft diet groups to hard diet groups, respectively, mandibular morphology changed on the horizontal and vertical planes, but there was no difference on the transverse plane. It was found out that there were substantial variations between the soft diet groups, mostly in the horizontal linear mandibular measures that comprised condylar points from the first to the second generation. There were significant variations between the second and third generations for all horizontal mandibular measurements. Finally, there were variations between the first and third generations of soft diet groups on both a horizontal and vertical axis for the mandible.
Premaxilla measurements did not alter significantly, according to our study. Between all conceivable comparisons, the anterior portion of the maxilla did not exhibit any appreciable variations. All three generations of the soft diet group showed a narrowing of the dental arch. Only the first generation displayed noticeable alterations on the third molar, while the next two generations displayed noticeable changes on all three molars. This may be explained as a result of the different timing in molars’ eruption. It is known that the first rat molar typically erupts around day 17, the second rat molar on day 20, and the third rat molar on day 33. Since our primary subjects were 30 days of age, it seems logical that only the third molar could be affected by the diet of the first generation. Lastly, our study showed no significant differences on the horizontal plane for maxillary measurements.
Mastication, speech, and respiration are all impacted by craniofacial morphology, specifically jaw morphology. The current study suggests that nutrition can significantly affect craniofacial shape. The soft diet mastication may cause the mandible to become more retrognathic and the maxilla narrower. It appears that genetic information is passed down through the generations and will likely appear to have a substantial impact on mandibular growth. This allowed the mandible to develop more vertically. These findings might imply that the reduced mandible was part of the craniofacial alterations that occurred during human evolution. The current body of literature makes the argument that a smaller mandible may result in crowded teeth, insufficient room for the third molars, and restricted airways. It is crucial to note that recent research has linked the activity of the less-toned muscles in the oropharynx and craniofacial region to a number of chronic conditions, including cardiovascular issues, ADHD, and obstructive sleep apnea [52].
5. Conclusions
In conclusion, the results of this study indicate that diet consistency has a significant influence on maxillary and mandibular growth. According to our findings, soft diet habits may result in narrower dental arches, a narrower maxilla, and a retrognathic mandible. The maxilla is mostly affected on the transverse plane by becoming narrower and smaller. On the contrary, the mandible is mostly affected on the horizontal plane by becoming retrognathic and on the vertical plane by becoming shorter in height. This information may be possibly carried over different generations, and it might take more than two generations to be expressed.
Author Contributions
Conceptualization, A.I.T., I.A.T., C.V. and D.P.; methodology, I.A.T. and A.I.T.; software, I.A.T.; validation, I.A.T. and C.V.; formal analysis, D.P., K.-E.A. and Z.Y.; investigation, I.A.T., D.P. and A.I.T.; resources, D.P., M.G. and E.P.; data curation, I.A.T., K.-E.A. and P.P.; writing—original draft preparation, I.A.T., C.V., D.P., K.-E.A., Z.Y., P.P., I.L., M.G., E.P. and A.I.T.; writing—review and editing, I.A.T. and I.L.; supervision, A.I.T. All authors have read and agreed to the published version of the manuscript.
Funding
The Department of Orthodontics at the National and Kapodistrian University of Athens, Greece, funded this study.
Institutional Review Board Statement
Prior to beginning this study, the Institutional Review Board gave their consent. The Institutional Review Board gave their approval to the animal study protocol. The General Directorate of Veterinary Policy of the prefecture of Attica in the Hellenic Republic gave its approval to the research. 1405, the Acceptance Code. The study was carried out at the Laboratory of Experimental Surgery and Surgical Research at the National and Kapodistrian University of Athens School of Medicine in Athens, Greece.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author will provide the datasets used and/or analyzed during the current work upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dias, G.J.; Cook, R.B.; Mirhosseini, M. Influence of food consistency on growth and morphology of the mandibular condyle. Clin. Anat. 2011, 24, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Yang, L.Y.; Chen, K.T.; Chiu, W.C. The influence of masticatory hypofunction on developing rat craniofacial structure. Int. J. Oral. Maxillofac. Surg. 2010, 39, 593–598. [Google Scholar] [CrossRef]
- Rabey, K.N.; Green, D.J.; Taylor, A.B.; Begun, D.R.; Richmond, B.G.; McFarlin, S.C. Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. J. Hum. Evol. 2015, 78, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R.; Fields, H.W.; Sarver, D.M. Contemporary orthodontic appliances. In Contemporary Orthodontics; Elsiever: St. Louis, MO, USA, 2012; pp. 348–350. [Google Scholar]
- Moss, M.L. The functional matrix hypothesis revisited. 1. The role of mechanotransduction. Am. J. Orthod. Dentofac. Orthop. 1997, 112, 8–11. [Google Scholar] [CrossRef]
- Moss, M.L. The functional matrix hypothesis revisited. 3. The genomic thesis. Am. J. Orthod. Dentofac. Orthop. 1997, 112, 338–342. [Google Scholar] [CrossRef]
- Rose, J.C.; Roblee, R.D. Origins of dental crowding and malocclusions: An anthropological perspective. Compend. Contin. Educ. Dent. 2009, 30, 292–300. [Google Scholar] [PubMed]
- Evensen, J.P.; Øgaard, B. Are malocclusions more prevalent and severe now? A comparative study of medieval skulls from Norway. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 710–716. [Google Scholar] [CrossRef]
- Toro-Ibacache, V.; Zapata Muñoz, V.; O’Higgins, P. The relationship between skull morphology, masticatory muscle force and cranial skeletal deformation during biting. Ann. Anat. 2016, 203, 59–68. [Google Scholar] [CrossRef]
- Sella-Tunis, T.; Pokhojaev, A.; Sarig, R.; O’Higgins, P.; May, H. Human mandibular shape is associated with masticatory muscle force. Sci. Rep. 2018, 8, 6042. [Google Scholar] [CrossRef]
- Eyquem, A.P.; Kuzminsky, S.C.; Aguilera, J.; Astudillo, W.; Toro-Ibacache, V. Normal and altered masticatory load impact on the range of craniofacial shape variation: An analysis of pre-Hispanic and modern populations of the American Southern Cone. PLoS ONE 2019, 14, e0225369. [Google Scholar] [CrossRef]
- Enlow, D.H.; McNamara, J.A., Jr. The neurocranial basis for facial form and pattern. Angle Orthod. 1973, 43, 256–270. [Google Scholar] [PubMed]
- Herring, S.W. TMJ anatomy and animal models. J. Musculoskelet. Neuronal Interact. 2003, 3, 391–394. [Google Scholar] [PubMed]
- Suzuki, A.; Iwata, J. Mouse genetic models for temporomandibular joint development and disorders. Oral. Dis. 2016, 22, 33–38. [Google Scholar] [CrossRef]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal. Interact. 2001, 1, 193–207. [Google Scholar] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Roach, H.I.; Mehta, G.; Oreffo, R.O.C.; Clarke, N.M.P.; Cooper, C. Cooper Temporal Analysis of Rat Growth Plates: Cessation of Growth with Age Despite Presence of a Physis. J. Histochem. Cytochem. 2003, 3, 373–383. [Google Scholar] [CrossRef]
- Gomes, P.S.; Fernandes, M.H. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Verikokos, C.; Perrea, D.; Bitsanis, E.; Tsolakis, A.I. Effects of diet consistency on mandibular growth. A review. J. Hell. Vet. Med. Soc. 2019, 70, 1603–1610. [Google Scholar] [CrossRef]
- Dontas, I.; Tsolakis, A.I.; Khaldi, L.; Patra, E.; Lyriritis, G.P. Malocclusion in Aging Wistar Rats. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 22–26. [Google Scholar]
- Karamani, I.I.; Tsolakis, I.A.; Makrygiannakis, M.A.; Georgaki, M.; Tsolakis, A.I. Impact of Diet Consistency on the Mandibular Morphology: A Systematic Review of Studies on Rat Models. Int. J. Environ. Res. Public Health 2022, 19, 2706. [Google Scholar] [CrossRef]
- Nicholson, E.K.; Stock, S.R.; Hamrick, M.W.; Ravosa, M.J. Biomineralization and adaptive plasticity of the temporomandibular joint in myostatin knockout mice. Arch. Oral. Biol. 2006, 51, 37–49. [Google Scholar] [CrossRef]
- Kufley, S.; Scott, J.E.; Ramirez-Yanez, G. The effect of the physical consistency of the diet on the bone quality of the mandibular condyle in rats. Arch. Oral. Biol. 2017, 77, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ohnuki, Y.; Yamane, A.; Saeki, Y. Effects of diet consistency on the myosin heavy chain mRNAs of rat masseter muscle during postnatal development. Arch. Oral. Biol. 2002, 47, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Epigenetic consequences of a changing human diet. Proc. Nutr. Soc. Nov. 2013, 72, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Enlow, D. Functions of the Haversian system. Am. J. Anat. 1962, 110, 269–305. [Google Scholar] [CrossRef]
- Burr, D.B. Targeted and nontargeted remodeling. Bone 2002, 30, 2–4. [Google Scholar] [CrossRef]
- Bouvier, M.; Hylander, W.L. The mechanical or meta-bolic function of secondary osteonal bone in the monkeyMac-aca fascicularis. Arch. Oral. Biol. 1996, 41, 941–950. [Google Scholar] [CrossRef]
- Lad, S.E.; Pampush, J.D.; McGraw, W.S.; Daegling, D.J. The influence of leaping frequency on secondary bone incercopithecid primates. Anat. Rec. 2019, 302, 1116–1126. [Google Scholar] [CrossRef]
- Terhune, C.E.; Sylvester, A.D.; Scott, J.E.; Ravosa, M.J. Trabecular architecture of the mandibular condyle of rabbits isrelated to dietary resistance during growth. J. Experiment. Biol. 2020, 223, jeb220988s. [Google Scholar]
- Mitchell, D.R.; Wroe, S.; Ravosa, M.J.; Menegaz, R.A. More challenging diets sustain feeding performance: Applications toward the captive rearing of wildlife. Integr. Org. Biol. 2021, 3, obab030. [Google Scholar] [CrossRef]
- Lad, S.E.; Anderson, R.J.; Cortese, S.A.; Alvarez, C.E.; Danison, A.D.; Morris, H.M.; Ravosa, M.J. Bone remodeling and cyclical loading in maxillae of New Zealand white rabbits (Oryctolagus cuniculus). Anat. Rec. 2021, 304A, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puck, T.T.; Krystosek, A. Role of the cytoskeleton in genome regulation and cancer. Int. Rev. Cytol. 1992, 132, 75–108. [Google Scholar]
- Murshid, S.A. The role of osteocytes during experimental orthodontic tooth movement: A review. Arch. Oral. Biol. 2017, 73, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Matyas, J.; Edwards, P.; Miniaci, A.; Shrive, N.; Wilson, J.; Bray, R.; Frank, C. Ligament tension affects nuclear shape in situ: An in vitro study. Connect. Tissue Res. 1994, 31, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Odman, A.; Mavropoulos, A.; Kiliaridis, S. Do masticatory functional changes influence the mandibular morphology in adult rats. Arch. Oral. Biol. 2008, 53, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Sano, R.; Kawai, N.; Langenbach, C.E.J.A.; Brugman, P.; Tanne, K.; Theo, M.G.J.; Van Eijden, T.M.G.J. Effect of Food Consistency on the Degree of Mineralization in the Rat Mandible. Ann. Biomed. Eng. 2007, 35, 1617–1621. [Google Scholar] [CrossRef]
- Grünheid, T.; Langenbach, G.E.J.; Brugman, P.; Vincent Everts, V.; Zentner, A. The masticatory system under varying functionalload. Part 2: Effect of reduced masticatory load on the degree and distribution of mineralization in the rabbit mandible. Eur. J Orthod. 2011, 33, 365–371. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Verikokos, C.; Perrea, D.; Alexiou, K.; Gizani, S.; Tsolakis, A.I. Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study. Biology 2022, 11, 901. [Google Scholar] [CrossRef]
- Tonni, I.; Riccardi, G.; Piancino, M.G.; Stretti, C.; Costantinides, F.; Paganelli, C. The influence of food hardness on the physiological parameters of mastication: A systematic review. Arch. Oral. Biol. 2020, 120, 104903. [Google Scholar] [CrossRef]
- Ludlow, J.B.; Walker, C. Assessment of phantom dosimetry and image quality of i-CAT FLX cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 802–817. [Google Scholar] [CrossRef]
- Govila, S.; Gundappa, M. Cone beam computed tomography—An overview. J. Conserv. Dent. 2007, 10, 53–58. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Verikokos, C.; Papaioannou, W.; Alexiou, K.-E.; Yfanti, Z.; Perrea, D.; Tsolakis, A.I. Long-Term Effect of Diet Consistency on Mandibular Growth within Three Generations: A Longitudinal Cephalometric Study in Rats. Biology 2023, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Kiliaridis, S.; Thilander, B.; Kjellberg, H.; Topouzelis, N.; Zafiriadis, A. Effect of low masticatory function on condylar growth: Amorphometric study in the rat. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S. The effects of food consistency on maxillary growth in rat. Eur. J. Orthod. 1996, 18, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, C.; Berg, R.; Kiliaridis, S. Influence of masticatory muscle function or transversal skull dimension in the growing rat. J. Orofac. Orthop. 2002, 63, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kiliaridis, S.; Engstrdm, C.; Thilander, B. The relationship between masticatory function and craniofacial morphology I. A cephalometric longitudinal analysis in the growing rat fed a soft diet. Eur. J. Orthod. 1985, 7, 273–283. [Google Scholar] [CrossRef]
- Maki, K.; Nishioka, T.; Shioiri, E.; Takahashi, T.; Kimura, M. Effects of Dietary Consistency on the Mandible of Rats at the Growth Stage: Computed X-ray Densitometric and Cephalometric Analysis. Angle Orthod. 2002, 72, 468–475. [Google Scholar]
- Abed, G.S.; Buschang, P.H.; Taylor, R.; Hinton, R.J. Maturational and functional related differences in rat craniofacial growth. Arch. Oral. Biol. 2007, 52, 1018–1025. [Google Scholar] [CrossRef]
- Hichijo, N.; Kawai, N.; Mori, H.; Sano, R.; Ohnuki, Y.; Okumura, S.; Langenbach, G.E.J.; Tanaka, E. Effects of the masticatory demand on the rat mandibular development. J. Oral Rehabil. 2014, 41, 581–587. [Google Scholar] [CrossRef]
- Hassan, M.G.; Kaler, H.; Zhang, B.; Cox, T.C.; Young, N.; Jheon, A.H. Effects of Multi-Generational Soft Diet Consumption on Mouse Craniofacial Morphology. Front. Physiol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Kahn, S.; Ehrlich, P.; Feldman, M.; Sapolsky, R.; Wong, S. The Jaw Epidemic: Recognition, Origins, Cures, and Prevention. Bioscience 2020, 70, 759–771. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).