Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Attention Deficit-Hyperactivity Disorder (ADHD)
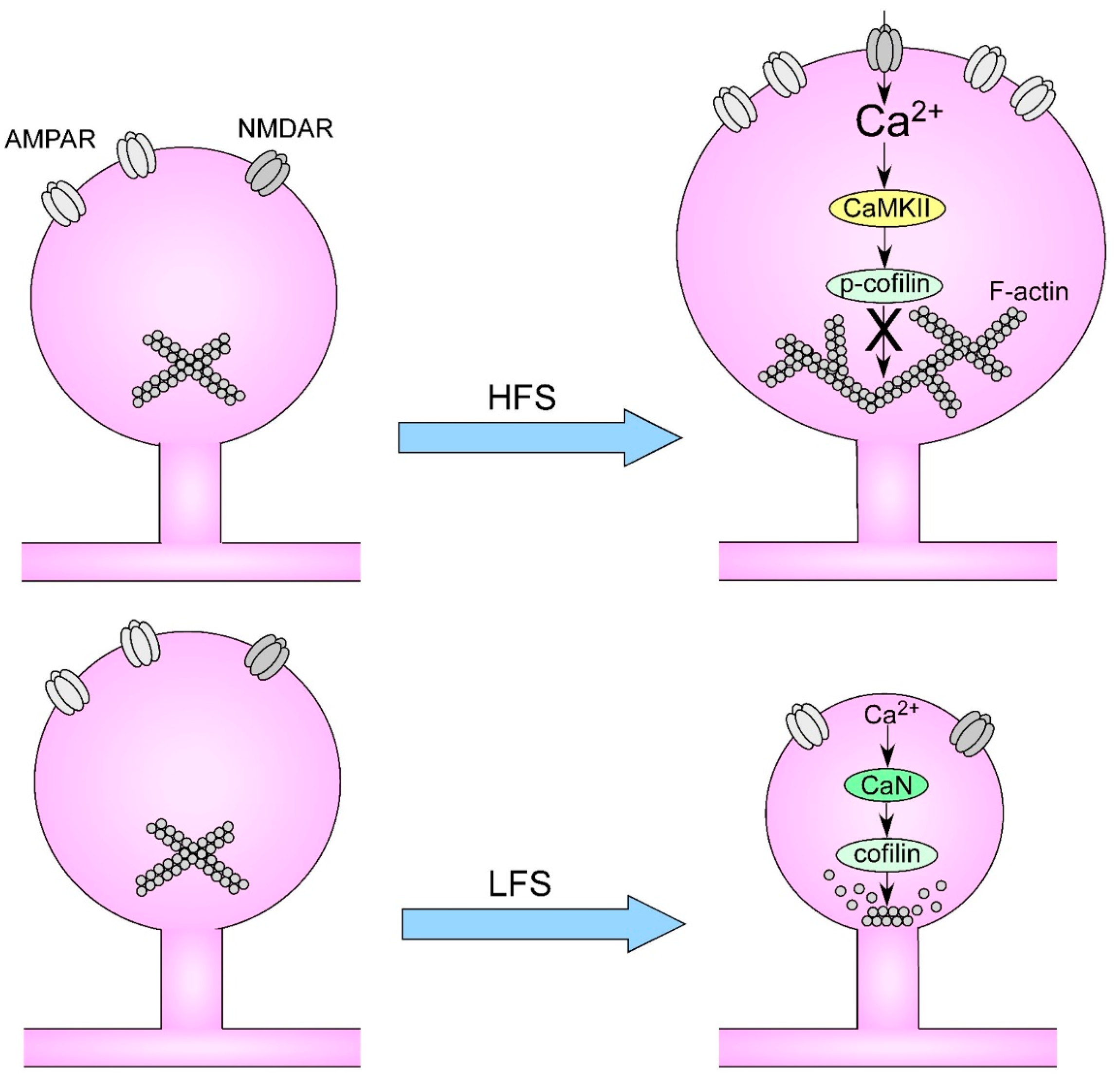
3. Long-Term Potentiation and ADHD
4. Dendritic Spines and ADHD
5. Dendritic Remodeling and Learning
6. Discussion
7. Conclusions and Futures Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purpura, D.P. Dendritic Spine “Dysgenesis” and Mental Retardation. Science 1974, 186, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Hutsler, J.J.; Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 2018, 19, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef]
- Hwang, F.J.; Roth, R.H.; Wu, Y.W.; Sun, Y.; Kwon, D.K.; Liu, Y.; Ding, J.B. Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron 2022, 110, 2790–2801.e5. [Google Scholar] [CrossRef]
- Attardo, A.; Fitzgerald, J.E.; Schnitzer, M.J. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature 2015, 523, 592–596. [Google Scholar] [CrossRef]
- Thompson, P.M.; Jahanshad, N.; Ching, C.R.K.; Salminen, L.E.; Thomopoulos, S.I.; Bright, J.; Baune, B.T.; Bertolín, S.; Bralten, J.; Bruin, W.B.; et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 2020, 10, 100. [Google Scholar] [CrossRef]
- Feldman, H.M.; Reiff, M.I. Attention Deficit–Hyperactivity Disorder in Children and Adolescents. N. Engl. J. Med. 2014, 370, 838–846. [Google Scholar] [CrossRef]
- Braun, J.M.; Kahn, R.S.; Froehlich, T.; Auinger, P.; Lanphear, B.P. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ. Health Perspect. 2006, 114, 1904–1909. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Anixt, J.S.; Loe, I.M.; Chirdkiatgumchai, V.; Kuan, L.; Gilman, R.C. Update on Environmental Risk Factors for Attention-Deficit/Hyperactivity Disorder. Curr. Psychiatry Rep. 2011, 13, 333–344. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Swanson, J.M. Adult Attention Deficit–Hyperactivity Disorder. N. Engl. J. Med. 2013, 369, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Smalley, S.L.; McCracken, J.T.; Yang, M.; Del’Homme, M.; Lynn, D.E.; Loo, S. Psychiatric Comorbidity in Adult Attention Deficit Hyperactivity Disorder: Findings From Multiplex Families. Am. J. Psychiatry 2005, 162, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.L.; Howes, M.J.; Secnik, K.; et al. The Prevalence and Correlates of Adult ADHD in the United States: Results From the National Comorbidity Survey Replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef] [PubMed]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the Attention-Deficit/Hyperactivity Disorder; Patel, Y.; Parker, N.; Shin, J.; Howard, D.; French, L.; Thomopoulos, S.I.; Pozzi, E.; Abe, Y.; Abé, C.; et al. Virtual Histology of Cortical Thickness and Shared Neurobiology in 6 Psychiatric Disorders. JAMA Psychiatry 2021, 78, 47–63. [Google Scholar] [CrossRef]
- Li, C.S.; Chen, Y.; Ide, J.S. Gray matter volumetric correlates of attention deficit and hyperactivity traits in emerging adolescents. Sci. Rep. 2022, 12, 11367. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef]
- Johnson, E.C.; Demontis, D.; Thorgeirsson, T.E.; Walters, R.K.; Polimanti, R.; Hatoum, A.S.; Sanchez-Roige, S.; Paul, S.E.; Wendt, F.R.; Clarke, T.-K.; et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 2020, 7, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Sollis, E.; Deriziotis, P.; Saitsu, H.; Miyake, N.; Matsumoto, N.; Hoffer, M.J.V.; Ruivenkamp, C.A.L.; Alders, M.; Okamoto, N.; Bijlsma, E.K.; et al. Equivalent missense variant in the FOXP2 and FOXP1 transcription factors causes distinct neurodevelopmental disorders. Hum. Mutat. 2017, 38, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.; Stavsky, A.; Kadir, R.; Drabkin, M.; Wormser, O.; Yogev, Y.; Dolgin, V.; Proskorovski-Ohayon, R.; Perez, Y.; Nudelman, H.; et al. CDH2 mutation affecting N-cadherin function causes attention-deficit hyperactivity disorder in humans and mice. Nat. Commun. 2021, 12, 6187. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S. Pharmacologic Treatment of Attention Deficit–Hyperactivity Disorder. N. Engl. J. Med. 2020, 383, 1050–1056. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Newcorn, J.; Telang, F.; Solanto, M.V.; Fowler, J.S.; Logan, J.; Ma, Y.; Schulz, K.; Pradhan, K.; et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2007, 64, 932–940. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2022, 132, 621–637. [Google Scholar] [CrossRef]
- Contreras, D.; Piña, R.; Carvallo, C.; Godoy, F.; Ugarte, G.; Zeise, M.; Rozas, C.; Morales, B. Methylphenidate Restores Behavioral and Neuroplasticity Impairments in the Prenatal Nicotine Exposure Mouse Model of ADHD: Evidence for Involvement of AMPA Receptor Subunit Composition and Synaptic Spine Morphology in the Hippocampus. Int. J. Mol. Sci. 2022, 23, 7099. [Google Scholar] [CrossRef]
- Piña, R.; Rozas, C.; Contreras, D.; Hardy, P.; Ugarte, G.; Zeise, M.L.; Rojas, P.; Morales, B. Atomoxetine Reestablishes Long Term Potentiation in a Mouse Model of Attention Deficit/Hyperactivity Disorder. Neuroscience 2020, 439, 268–274. [Google Scholar] [CrossRef]
- Schneider, T.; Ilott, N.; Brolese, G.; Bizarro, L.; Asherson, P.J.; Stolerman, I.P. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 2011, 36, 1114–1125. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Xu, Y.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J. Neurosci. 2012, 32, 9410–9418. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, K.P.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J. Neurosci. 2014, 34, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Huganir, R.L.; Nicoll, R.A. AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.C.; Derkach, V.A.; Guire, E.S.; Soderling, T.R. Extrasynaptic Membrane Trafficking Regulated by GluR1 Serine 845 Phosphorylation Primes AMPA Receptors for Long-term Potentiation. J. Biol. Chem. 2006, 281, 752–758. [Google Scholar] [CrossRef]
- Vanhoose, A.M.; Clements, J.M.; Winder, D.G. Novel blockade of protein kinase A-mediated phosphorylation of AMPA receptors. J. Neurosci. 2006, 26, 1138–1145. [Google Scholar] [CrossRef]
- Buonarati, O.R.; Hammes, E.A.; Watson, J.F.; Greger, I.H.; Hell, J.W. Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci. Signal. 2019, 12, eaar6889. [Google Scholar] [CrossRef]
- Groc, L.; Choquet, D. Linking glutamate receptor movements and synapse function. Science 2020, 368, eaay4631. [Google Scholar] [CrossRef]
- Carvallo, C.; Contreras, D.; Ugarte, G.; Delgado, R.; Pancetti, F.; Rozas, C.; Piña, R.; Constandil, L.; Zeise, M.L.; Morales, B. Single and repeated administration of methylphenidate modulates synaptic plasticity in opposite directions via insertion of AMPA receptors in rat hippocampal neurons. Front. Pharmacol. 2018, 9, 1485. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Georgiou, J.; Sanderson, T.M.; Ko, K.H.; Kang, H.; Kim, J.-L.; Bradley, C.A.; Bortolotto, Z.A.; Zhuo, M.; Kaang, B.K.; et al. PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus. Nat. Commun. 2021, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef]
- Berry, K.P.; Nedivi, E. Spine Dynamics: Are They All the Same? Neuron 2017, 96, 43–55. [Google Scholar] [CrossRef]
- Grutzendler, J.; Kasthuri, N.; Gan, W.-B. Long-term dendritic spine stability in the adult cortex. Nature 2002, 420, 812–816. [Google Scholar] [CrossRef]
- Holtmaat, A.J.G.D.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Lin, A.; Chang, P.; Gan, W.B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 2005, 46, 181–189. [Google Scholar] [CrossRef]
- Ofer, N.; Berger, D.R.; Kasthuri, N.; Lichtman, J.W.; Yuste, R. Ultrastructural analysis of dendritic spine necks reveals a continuum of spine morphologies. Dev. Neurobiol. 2021, 81, 746–757. [Google Scholar] [CrossRef]
- Zito, K.; Scheuss, V.; Knott, G.; Hill, T.; Svoboda, K. Rapid Functional Maturation of Nascent Dendritic Spines. Neuron 2009, 61, 247–258. [Google Scholar] [CrossRef]
- Helm, M.S.; Dankovich, T.M.; Mandad, S.; Rammner, B.; Jähne, S.; Salimi, V.; Koerbs, C.; Leibrandt, R.; Urlaub, H.; Schikorski, T.; et al. A large-scale nanoscopy and biochemistry analysis of postsynaptic dendritic spines. Nat. Neurosci. 2021, 24, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Béïque, J.C.; Lin, D.T.; Kang, M.G.; Aizawa, H.; Takamiya, K.; Huganir, R.L. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA 2006, 103, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.M.; Wilkinson, K.A. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 2016, 17, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Yasuda, R. Plasticity of spine structure: Local signaling, translation and cytoskeletal reorganization. Front. Synaptic Neurosci. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Castro, J.; Saneyoshi, T.; Matsuno, H.; Sur, M.; Hayashi, Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 2014, 82, 444–459. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Ellis-Davies, G.C.R.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001, 4, 1086–1092. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef]
- Lee, H.K.; Kirkwood, A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Semin. Cell Dev. Biol. 2011, 22, 514–520. [Google Scholar] [CrossRef]
- Lee, S.J.R.; Escobedo-Lozoya, Y.; Szatmari, E.M.; Yasuda, R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 2009, 458, 299–304. [Google Scholar] [CrossRef]
- Kallergi, E.; Daskalaki, A.D.; Kolaxi, A.; Camus, C.; Ioannou, E.; Mercaldo, V.; Haberkant, P.; Stein, F.; Sidiropoulou, K.; Dalezios, Y.; et al. Dendritic autophagy degrades postsynaptic proteins and is required for long-term synaptic depression in mice. Nat. Commun. 2022, 13, 680. [Google Scholar] [CrossRef]
- Ucar, H.; Watanabe, S.; Noguchi, J.; Morimoto, Y.; Iino, Y.; Yagishita, S.; Takahashi, N.; Kasai, H. Mechanical actions of dendritic-spine enlargement on presynaptic exocytosis. Nature 2021, 600, 686–689. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Perlik, A.J.; Tobin, W.F.; Zweig, J.A.; Tennant, K.; Jones, T.; Zuo, Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 2009, 462, 915–919. [Google Scholar] [CrossRef]
- Yang, G.; Pan, F.; Gan, W.-B. Stably maintained dendritic spines are associated with lifelong memories. Nature 2009, 462, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yu, X.; Lu, J.; Zuo, Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 2012, 483, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Hayashi-Takagi, A.; Yagishita, S.; Nakamura, M.; Shirai, F.; Wu, Y.I.; Loshbaugh, A.L.; Kuhlman, B.; Hahn, K.M.; Kasai, H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 2015, 525, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Albarran, E.; Raissi, A.; Jáidar, O.; Shatz, C.J.; Ding, J.B. Enhancing motor learning by increasing the stability of newly formed dendritic spines in the motor cortex. Neuron 2021, 109, 3298–3311.e4. [Google Scholar] [CrossRef]
- Xu, Z.; Adler, A.; Li, H.; Pérez-Cuesta, L.M.; Lai, B.; Li, W.; Gan, W.B. Fear conditioning and extinction induce opposing changes in dendritic spine remodeling and somatic activity of layer 5 pyramidal neurons in the mouse motor cortex. Sci. Rep. 2019, 9, 4619. [Google Scholar] [CrossRef]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef]
- Harward, S.C.; Hedrick, N.G.; Hall, C.E.; Parra-Bueno, P.; Milner, T.A.; Pan, E.; Laviv, T.; Hempstead, B.L.; Yasuda, R.; McNamara, J.O. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 2016, 538, 99–103. [Google Scholar] [CrossRef]
- Tu, X.; Yasuda, R.; Colgan, L.A. Rac1 is a downstream effector of PKCα in structural synaptic plasticity. Sci. Rep. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Tanaka, J.I.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.R.; Kasai, H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.M.; Pothula, S.; Liu, R.-J.; Wu, M.; Li, X.-Y.; Girgenti, M.J.; Taylor, S.R.; Duman, C.H.; Delpire, E.; Picciotto, M.; et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Investig. 2020, 130, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Kavalali, E.T.; Monteggia, L.M. Ketamine and rapid antidepressant action: New treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology 2023. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef]
- Tu, X.; Jain, A.; Bueno, P.P.; Decker, H.; Liu, X.; Yasuda, R. Local autocrine plasticity signaling in single dendritic spines by insulin-like growth factors. Sci. Adv. 2023, 9, eadg0666. [Google Scholar] [CrossRef]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; Vanleeuwen, J.-E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef]
- Kolluri, N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Lamina-Specific Reductions in Dendritic Spine Density in the Prefrontal Cortex of Subjects With Schizophrenia. Am. J. Psychiatry 2005, 162, 1200–1202. [Google Scholar] [CrossRef]
- Glantz, L.A.; Lewis, D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 2000, 57, 65–73. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yalcin, E.; Presumey, J.; Aw, E.; Ma, M.; Whelan, C.W.; Stevens, B.; McCarroll, S.A.; Carroll, M.C. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat. Neurosci. 2021, 24, 214–224. [Google Scholar] [CrossRef]
- Sekar, A.; Bialas, A.R.; De Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Barsotti, N.; Bertero, A.; Trakoshis, S.; Ulysse, L.; Locarno, A.; Miseviciute, I.; De Felice, A.; Canella, C.; Supekar, K.; et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat. Commun. 2021, 12, 6084. [Google Scholar] [CrossRef]
- Kim, Y.; Teylan, M.A.; Baron, M.; Sands, A.; Nairn, A.C.; Greengard, P. Methylphenidate-induced dendritic spine formation and ΔFosB expression in nucleus accumbens. Proc. Natl. Acad. Sci. USA 2009, 106, 2915–2920. [Google Scholar] [CrossRef]
- Zehle, S.; Bock, J.; Jezierski, G.; Gruss, M.; Braun, K. Methylphenidate treatment recovers stress-induced elevated dendritic spine densities in the rodent dorsal anterior cingulate cortex. Dev. Neurobiol. 2007, 67, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Sorensen, S.A.; Ting, J.T.; Miller, J.A.; Chartrand, T.; Buchin, A.; Bakken, T.E.; Budzillo, A.; Dee, N.; Ding, S.L.; et al. Human neocortical expansion involves glutamatergic neuron diversification. Nature 2021, 598, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Galakhova, A.A.; Hunt, S.; Wilbers, R.; Heyer, D.B.; de Kock, C.P.J.; Mansvelder, H.D.; Goriounova, N.A. Evolution of cortical neurons supporting human cognition. Trends Cogn. Sci. 2022, 26, 909–922. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Zhu, Y.; Sousa, A.M.M.; Gao, T.; Skarica, M.; Li, M.; Santpere, G.; Esteller-Cucala, P.; Juan, D.; Ferrández-Peral, L.; Gulden, F.O.; et al. Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 2018, 362, eaat8077. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Anitha, A.; Thanseem, I.; Nakamura, K.; Yamada, K.; Iwayama, Y.; Toyota, T.; Iwata, Y.; Suzuki, K.; Sugiyama, T.; Tsujii, M.; et al. Protocadherin α (PCDHA) as a novel susceptibility gene for autism. J. Psychiatry Neurosci. 2013, 38, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Chiola, S.; Yang, G.; Russell, C.; Armstrong, C.J.; Wu, Y.; Spampanato, J.; Tarboton, P.; Ullah, H.M.A.; Edgar, N.U.; et al. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. Nat. Commun. 2022, 13, 5688. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Pattabiraman, K.; Lorente-Galdos, B.; Andrijevic, D.; Kim, S.-K.; Kaur, N.; Muchnik, S.K.; Xing, X.; Santpere, G.; Sousa, A.M.M.; et al. Regulation of prefrontal patterning and connectivity by retinoic acid. Nature 2021, 598, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Pattabiraman, K.; Muchnik, S.K.; Kaur, N.; Morozov, Y.M.; Cheng, X.; Waxman, S.G.; Sestan, N. Hominini-specific regulation of CBLN2 increases prefrontal spinogenesis. Nature 2021, 598, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Pinson, A.; Xing, L.; Namba, T.; Kalebic, N.; Peters, J.; Oegema, C.E.; Traikov, S.; Reppe, K.; Riesenberg, S.; Maricic, T.; et al. Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals. Science 2022, 377, eabl6422. [Google Scholar] [CrossRef]
- Tonelli, E.; Pascale, E.; Troianiello, M.; D’Addario, C.; Adriani, W. DAT1 Gene Methylation as an Epigenetic Biomarker in Attention Deficit Hyperactivity Disorder: A Commentary. Front. Genet. 2020, 11, 444. [Google Scholar] [CrossRef]
| Drug | Type | Molecular Target |
|---|---|---|
| Methylphenidate | Stimulant | DAT and NET (−) |
| Amphetamine | Stimulant | DAT and NET (−) |
| Atomoxetine | Nonstimulant | NET (−) |
| Clonidine | Nonstimulant | α2-AR (+) |
| Guanfacine | Nonstimulant | α2-AR (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugarte, G.; Piña, R.; Contreras, D.; Godoy, F.; Rubio, D.; Rozas, C.; Zeise, M.; Vidal, R.; Escobar, J.; Morales, B. Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity. Biology 2023, 12, 1241. https://doi.org/10.3390/biology12091241
Ugarte G, Piña R, Contreras D, Godoy F, Rubio D, Rozas C, Zeise M, Vidal R, Escobar J, Morales B. Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity. Biology. 2023; 12(9):1241. https://doi.org/10.3390/biology12091241
Chicago/Turabian StyleUgarte, Gonzalo, Ricardo Piña, Darwin Contreras, Felipe Godoy, David Rubio, Carlos Rozas, Marc Zeise, Rodrigo Vidal, Jorge Escobar, and Bernardo Morales. 2023. "Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity" Biology 12, no. 9: 1241. https://doi.org/10.3390/biology12091241
APA StyleUgarte, G., Piña, R., Contreras, D., Godoy, F., Rubio, D., Rozas, C., Zeise, M., Vidal, R., Escobar, J., & Morales, B. (2023). Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity. Biology, 12(9), 1241. https://doi.org/10.3390/biology12091241






