The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culturing: Dissection of the Brain and Preparation of Murine Neural Stem and Progenitor Cells (NSPCs)
2.2. Experimental Design
2.2.1. Synthetic Cathinone αPHP
2.2.2. NSPCs Exposure to NPS: Cell Morphology, Viability, and Proliferation
- (i)
- Phase-Contrast Microscopy: NSPCs Morphology Characterization
- (ii)
- Proliferation assay
- (iii)
- MTT Assay: cell metabolic activity study
- (iv)
- Clonal cell survival assay
2.3. Immunofluorescence Reactions
2.3.1. Fluorescence Microscopy
2.3.2. Confocal Fluorescence Microscopy
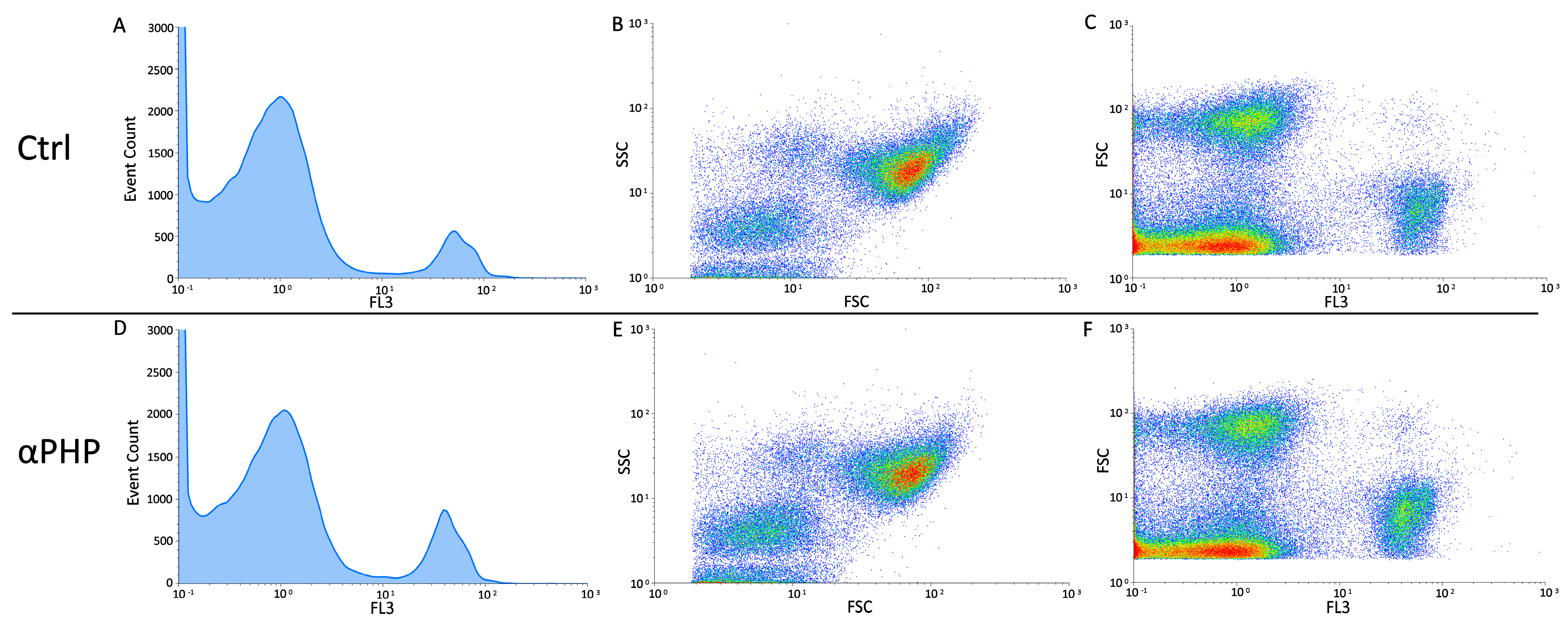
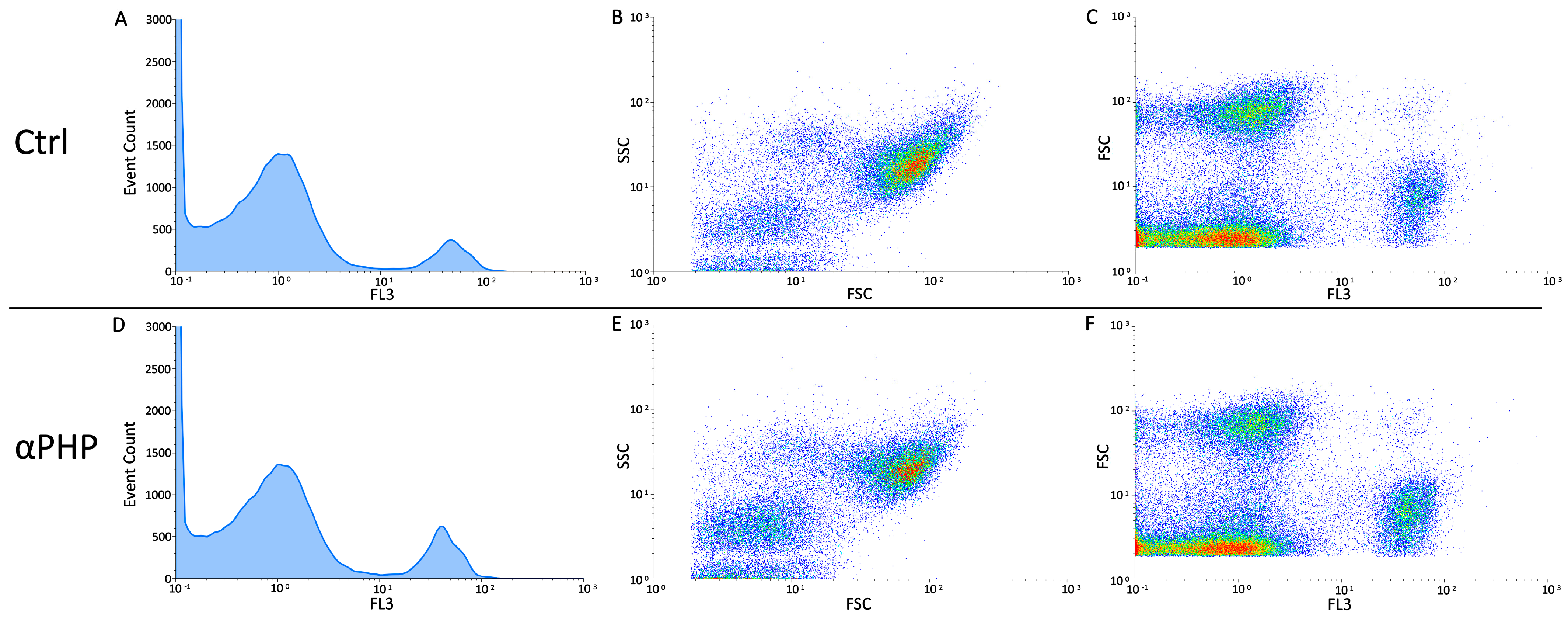
2.4. Flow Cytometry
Cell Cycle Analysis and Identification of Dead Cell
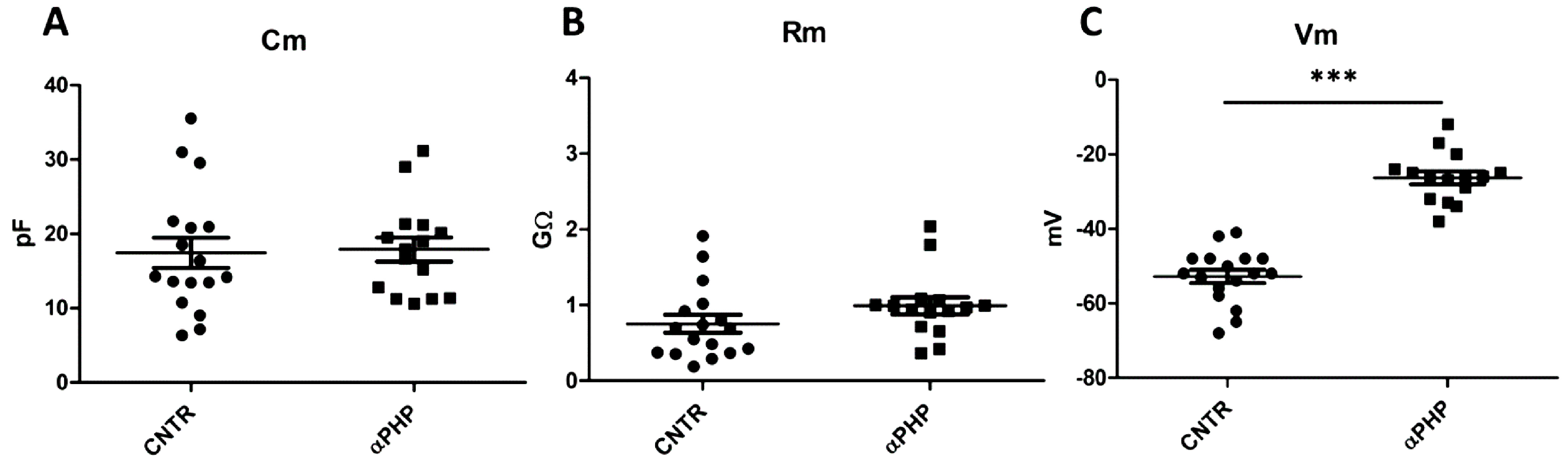
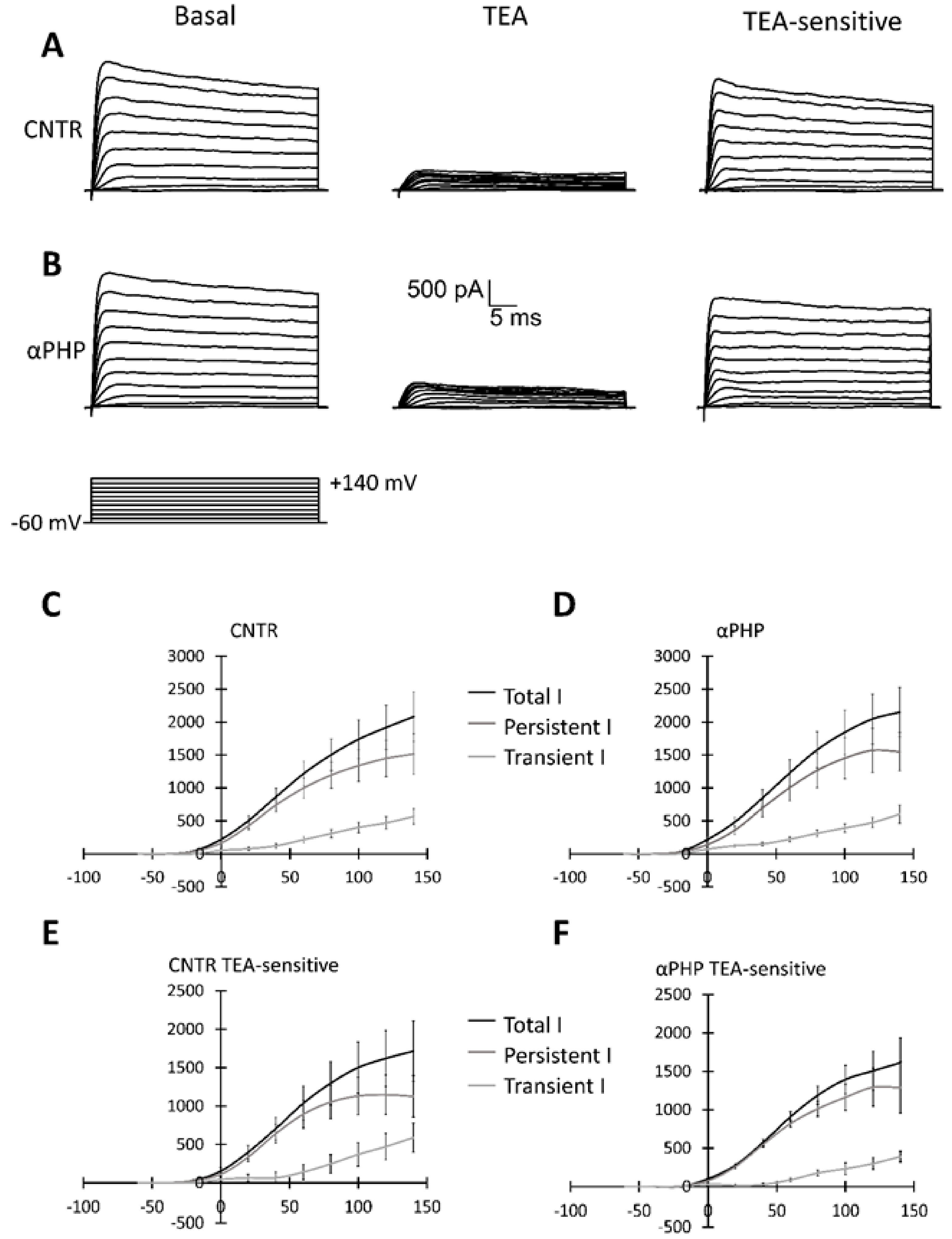
2.5. Patch Clamp Experiments
2.6. Transmission Electron Microscopy (TEM): UA and LC Staining
2.7. Statistical Analysis
3. Results
3.1. αPHP Alters NSPCs Proliferation
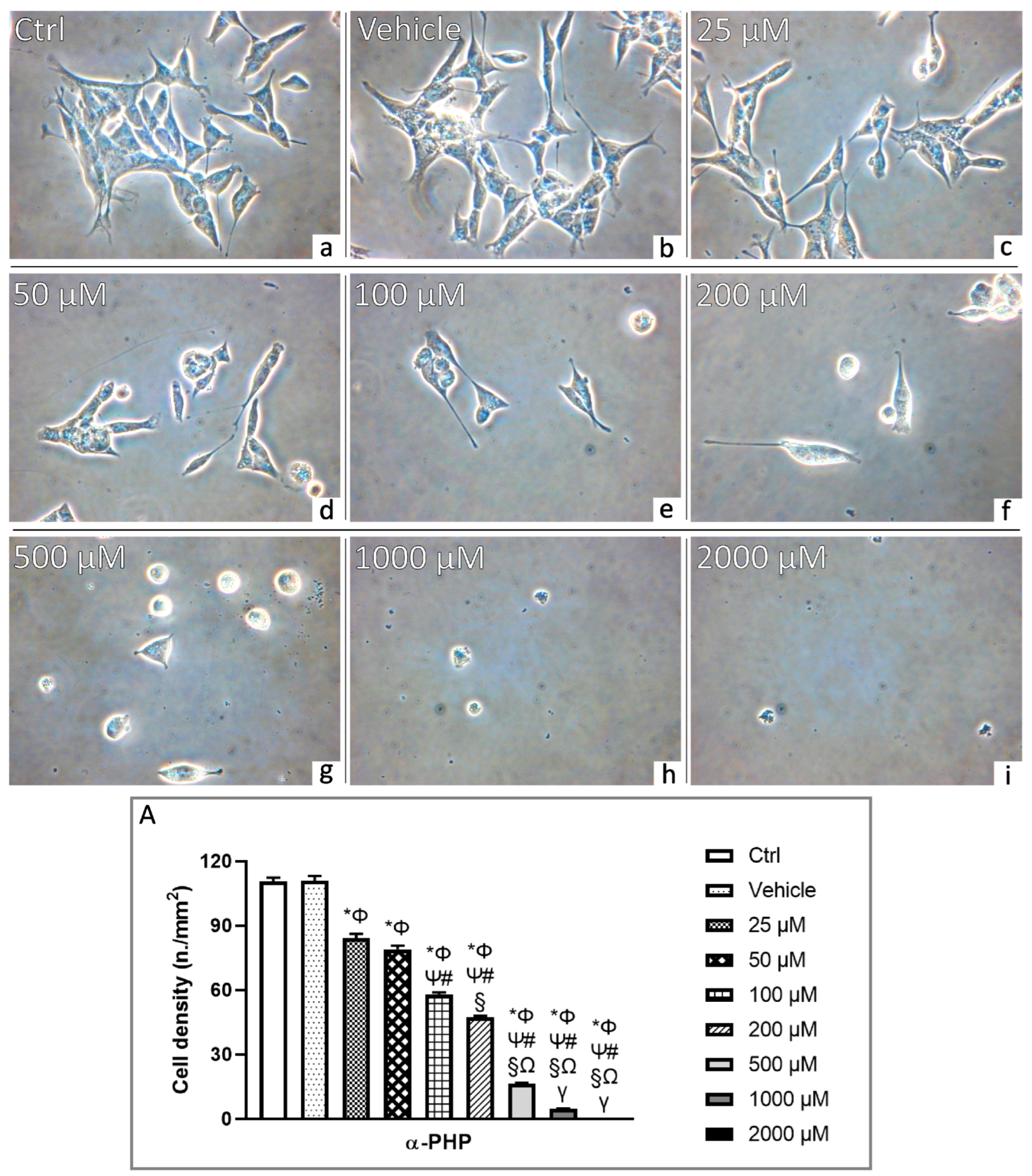
3.2. αPHP Affects Cell Density and Morphology
3.3. αPHP Affects Cell Metabolism
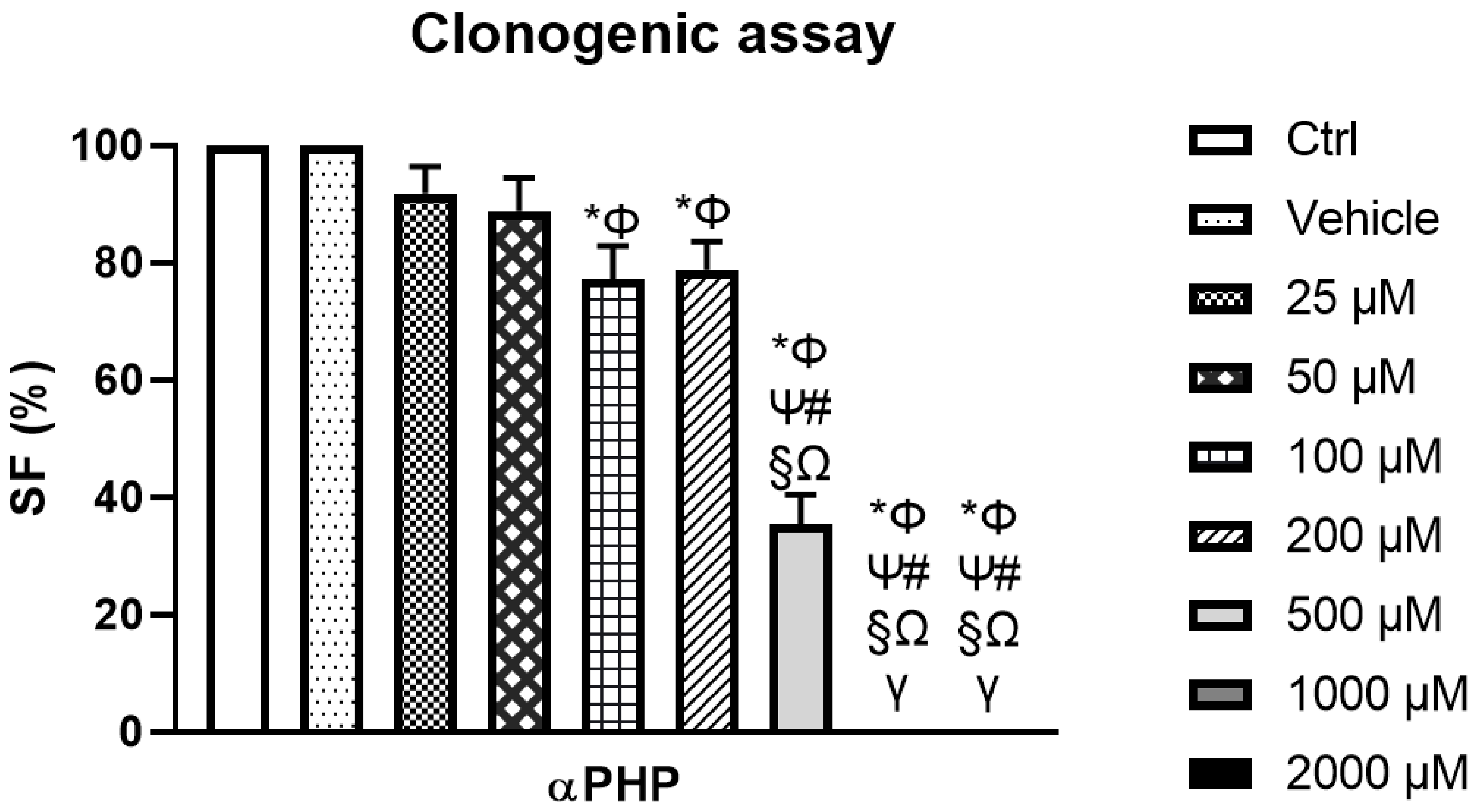
3.4. αPHP Modified NSCs Clonogenic Capability
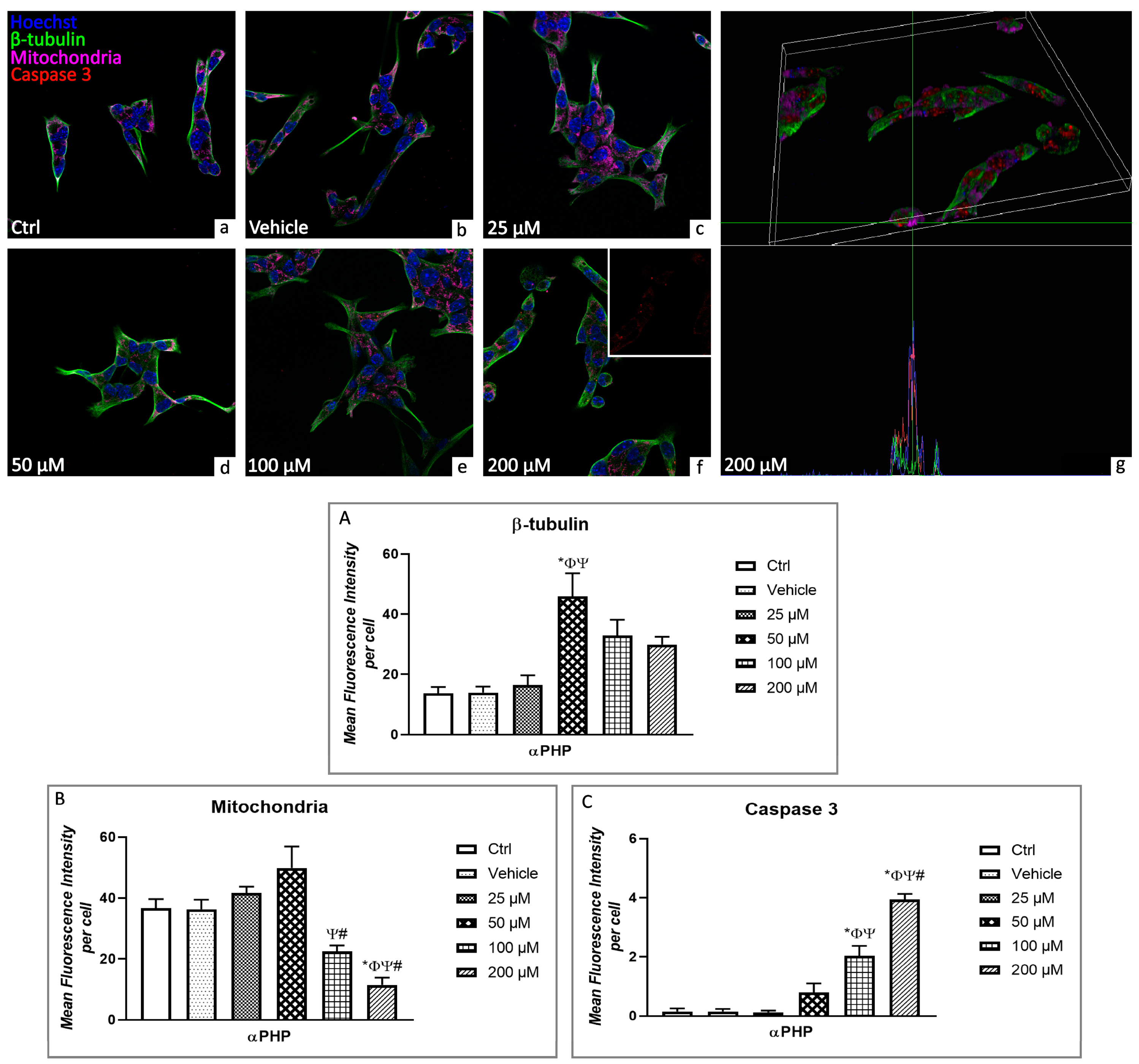
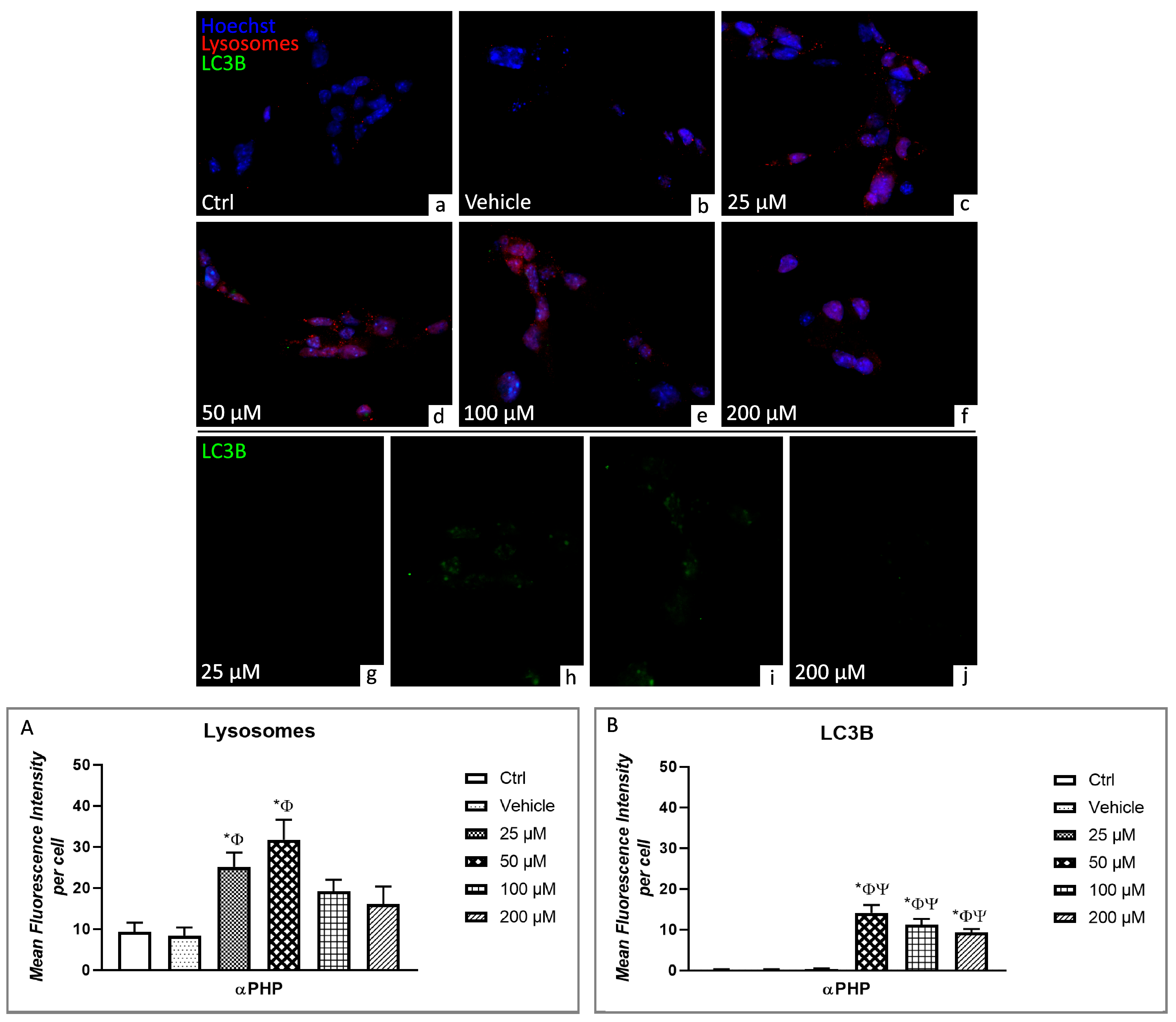
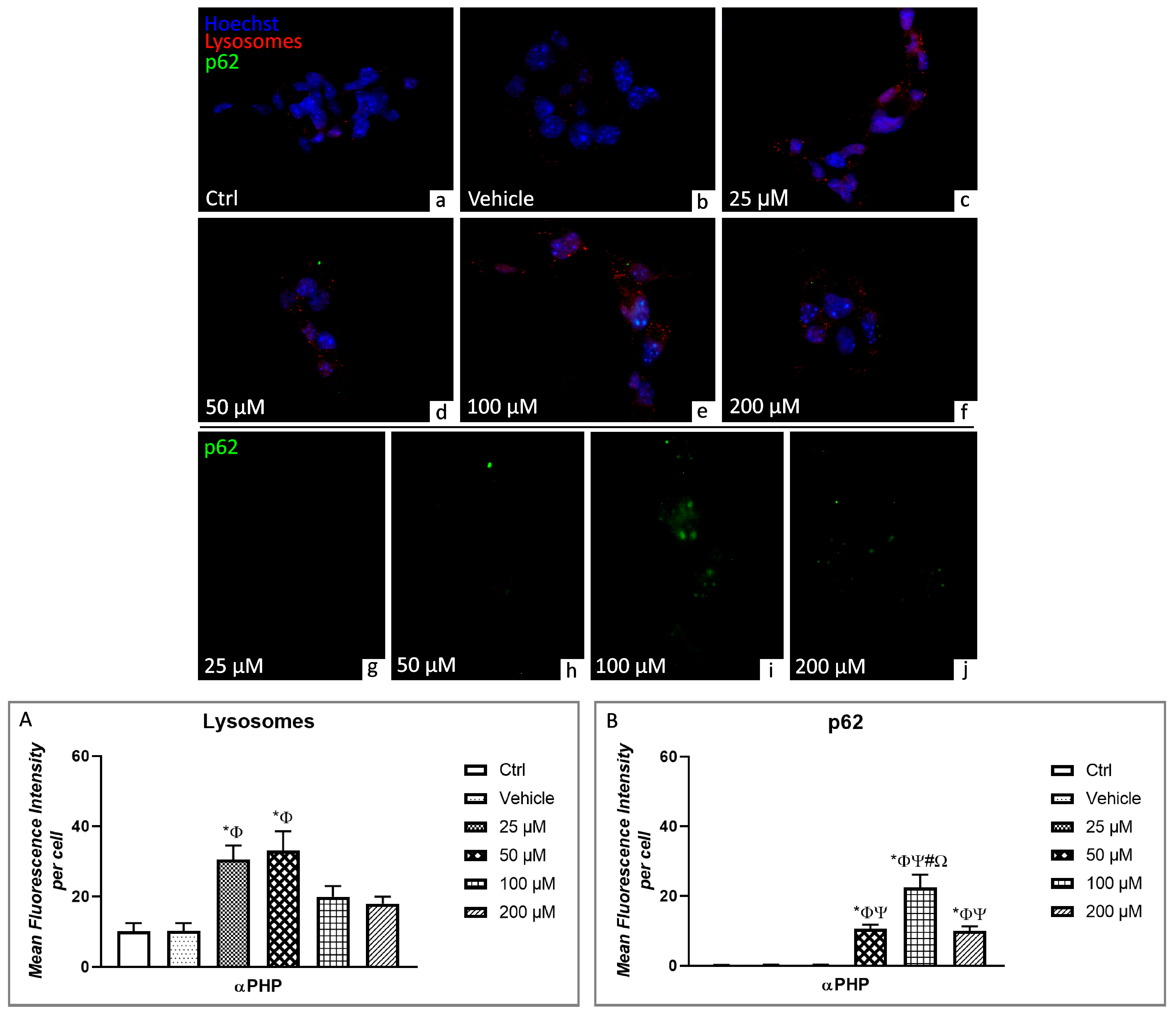
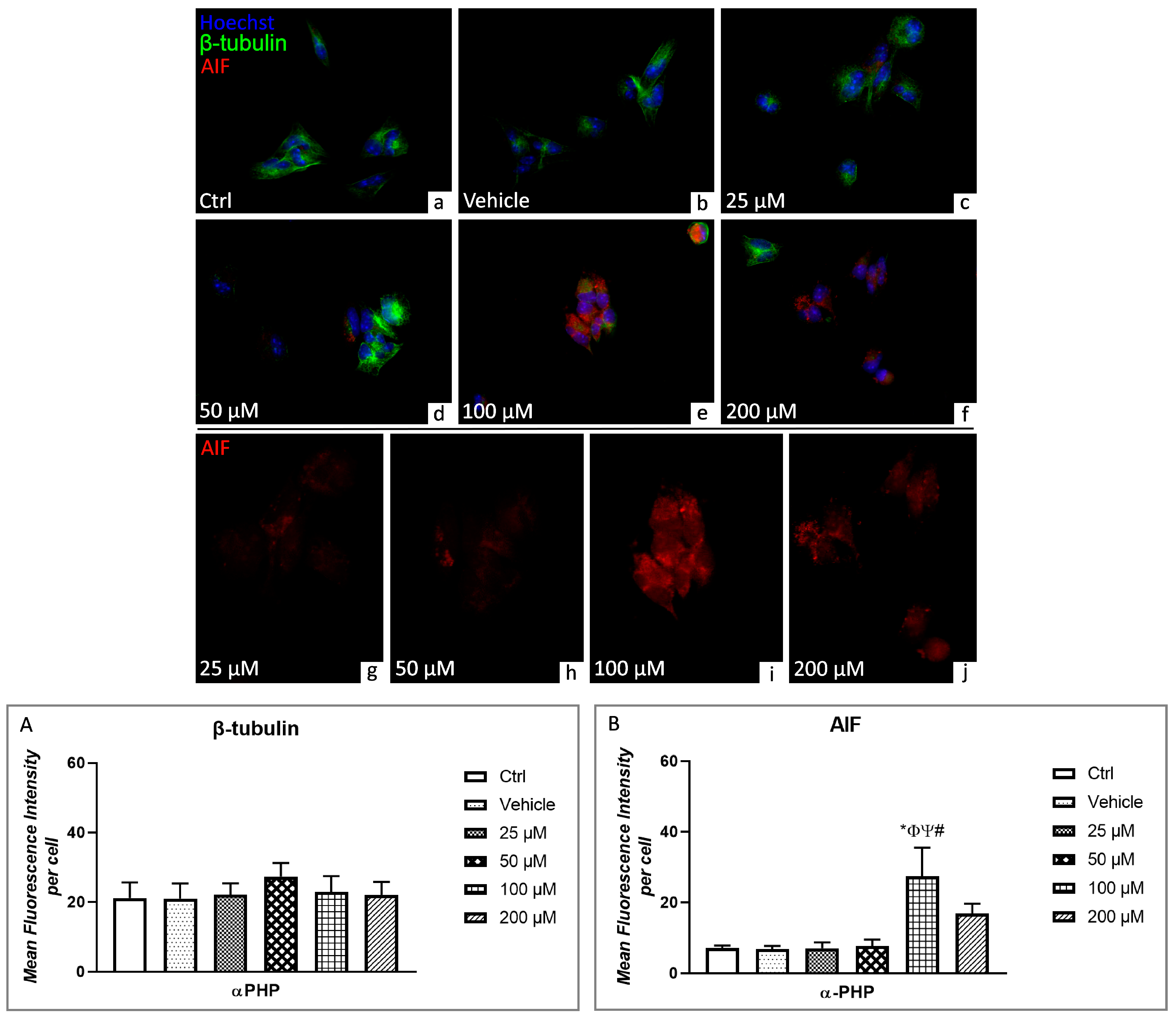
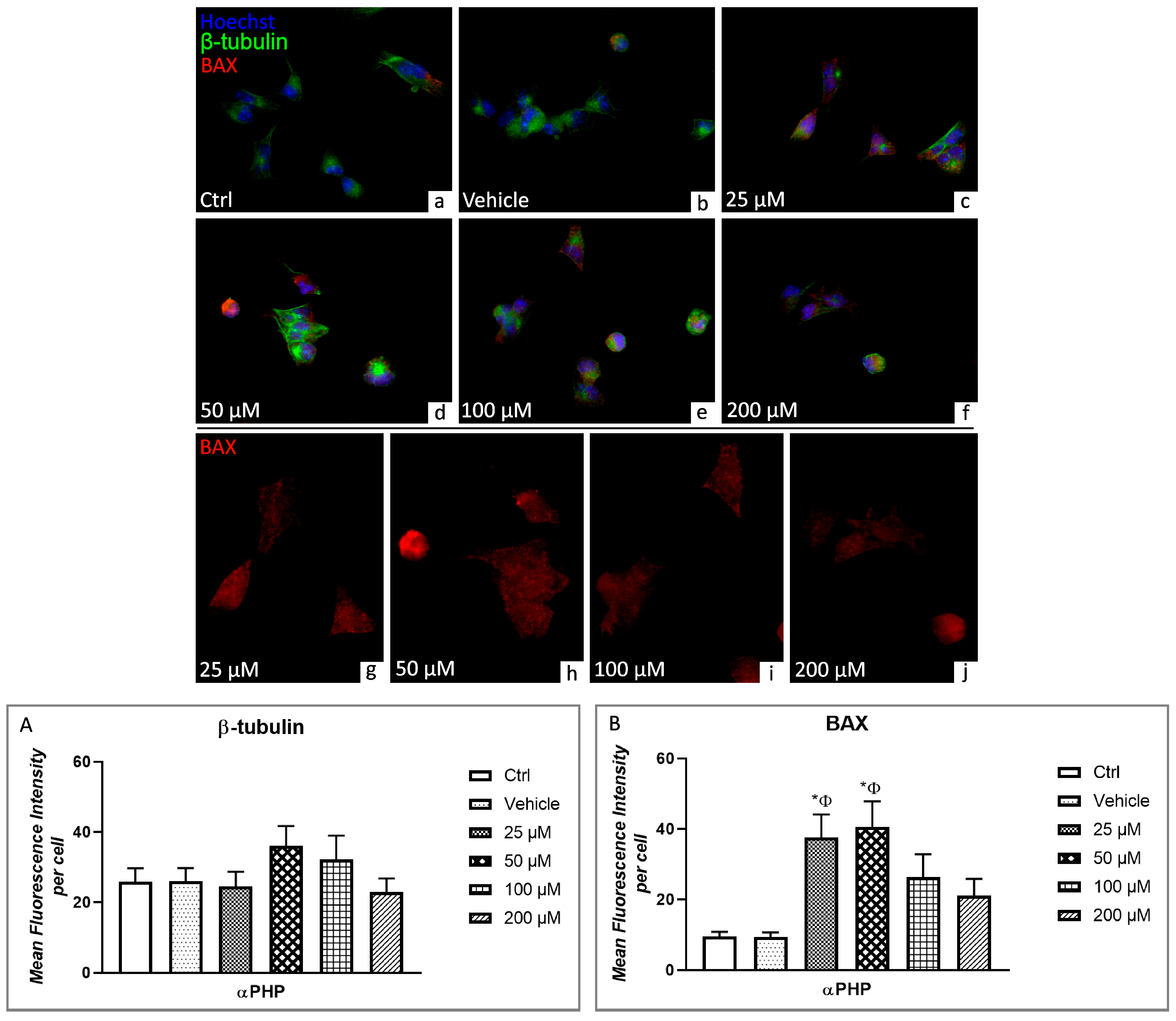
3.5. αPHP Altered Cytoplasmic Structures and Cell Death Pathways
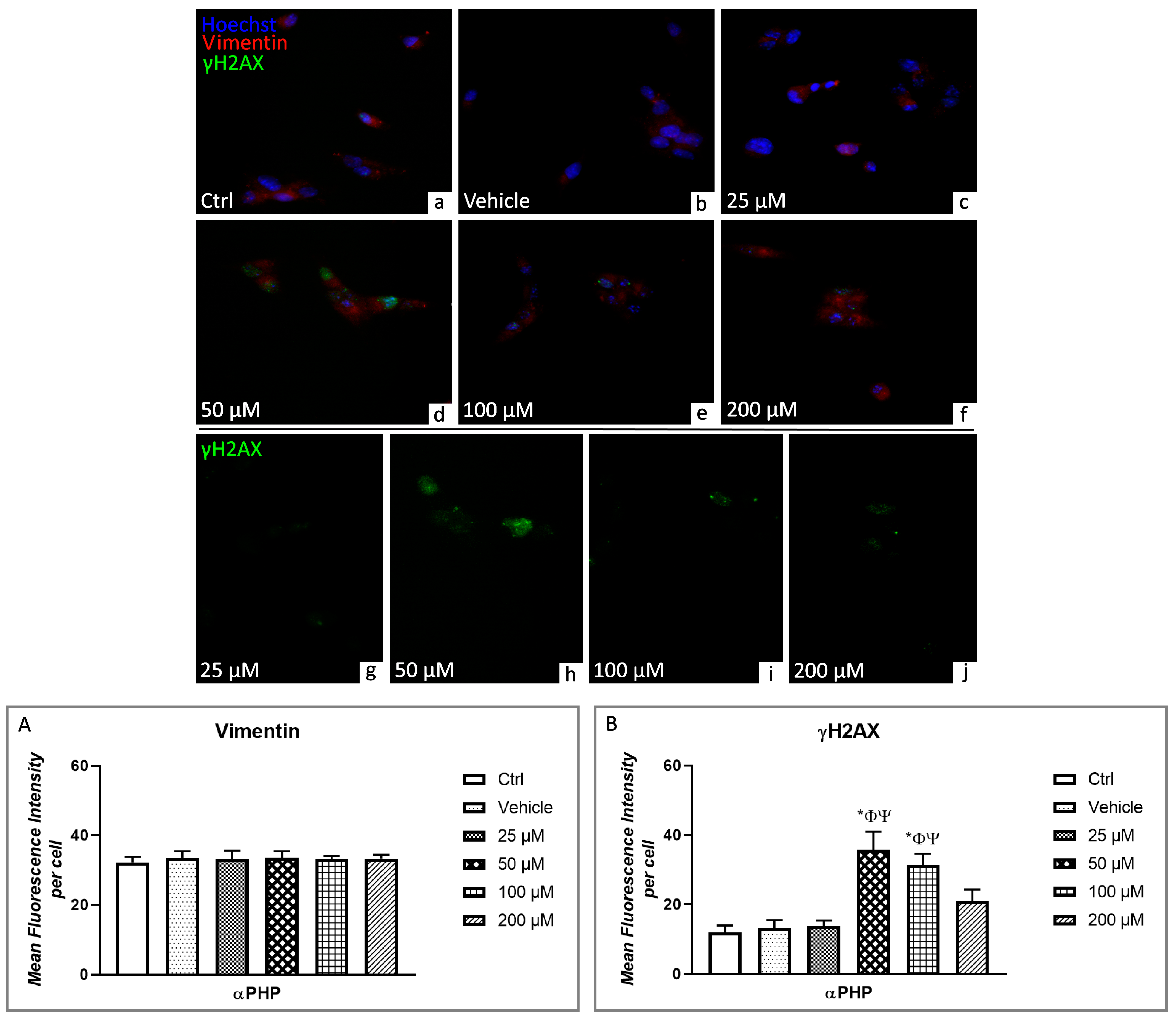
3.6. Cell Cycle Distribution and Cell Death
3.7. Electrophysiological Recordings
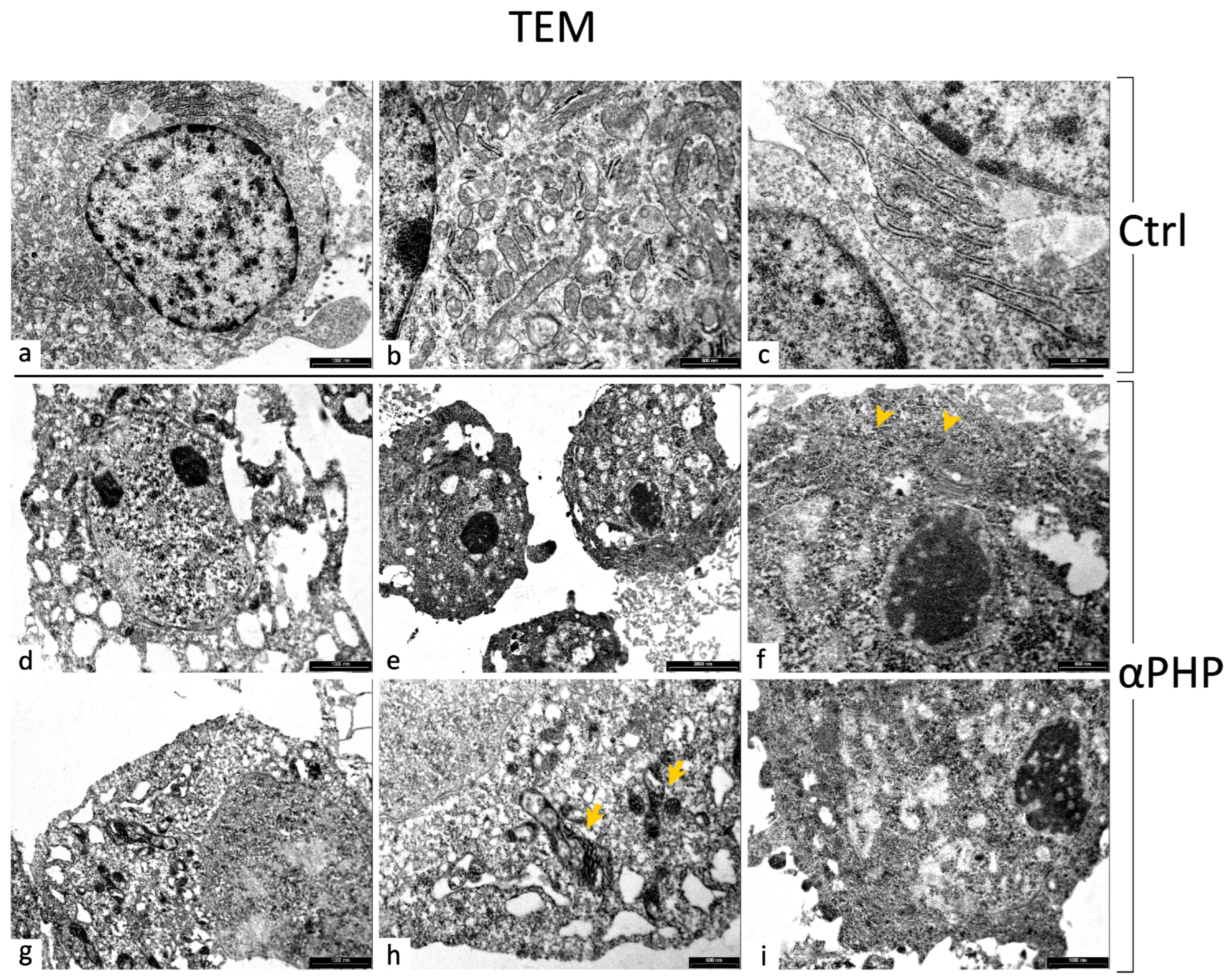
3.8. Ultrastructural Features by TEM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Miliano, C.; Serpelloni, G.; Rimondo, C.; Mereu, M.; Marti, M.; De Luca, M.A. Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 2016, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Wojcieszak, J. An Expanding World of New Psychoactive Substances—Designer Benzodiazepines. NeuroToxicology 2019, 73, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Busardò, F.P.; Pichini, S. Molecular Insights on New Psychoactive Substances (NPSs). Int. J. Mol. Sci. 2022, 23, 3282. [Google Scholar] [CrossRef]
- Daziani, G.; Lo Faro, A.F.; Montana, V.; Goteri, G.; Pesaresi, M.; Bambagiotti, G.; Montanari, E.; Giorgetti, R.; Montana, A. Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6230. [Google Scholar] [CrossRef]
- Prosser, J.M.; Nelson, L.S. The Toxicology of Bath Salts: A Review of Synthetic Cathinones. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- Hohmann, N.; Mikus, G.; Czock, D. Effects and Risks Associated with Novel Psychoactive Substances: Mislabeling and Sale as Bath Salts, Spice, and Research Chemicals. Dtsch. Arztebl. Int. 2014, 111, 139–147. [Google Scholar] [CrossRef][Green Version]
- Karila, L.; Billieux, J.; Benyamina, A.; Lançon, C.; Cottencin, O. The Effects and Risks Associated to Mephedrone and Methylone in Humans: A Review of the Preliminary Evidences. Brain Res. Bull. 2016, 126, 61–67. [Google Scholar] [CrossRef]
- Pantano, F.; Tittarelli, R.; Mannocchi, G.; Pacifici, R.; Luca, A.d.; Busardò, F.P.; Marinelli, E. Neurotoxicity Induced by Mephedrone: An up-to-Date Review. Curr. Neuropharmacol. 2017, 15, 738. [Google Scholar] [CrossRef]
- Kelly, J.P. Cathinone Derivatives: A Review of Their Chemistry, Pharmacology and Toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath Salts and Synthetic Cathinones: An Emerging Designer Drug Phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef]
- Paillet-Loilier, M.; Cesbron, A.; Le Boisselier, R.; Bourgine, J.; Debruyne, D. Emerging Drugs of Abuse: Current Perspectives on Substituted Cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52. [Google Scholar] [CrossRef]
- Hadlock, G.C.; Webb, K.M.; McFadden, L.M.; Chu, P.W.; Ellis, J.D.; Allen, S.C.; Andrenyak, D.M.; Vieira-Brock, P.L.; German, C.L.; Conrad, K.M.; et al. 4-Methylmethcathinone (Mephedrone): Neuropharmacological Effects of a Designer Stimulant of Abuse. J. Pharmacol. Exp. Ther. 2011, 339, 530–536. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine Transporter and Receptor Interaction Profiles of a New Series of Designer Cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef]
- Matsunaga, T.; Morikawa, Y.; Kamata, K.; Shibata, A.; Miyazono, H.; Sasajima, Y.; Suenami, K.; Sato, K.; Takekoshi, Y.; Endo, S.; et al. α-Pyrrolidinononanophenone Provokes Apoptosis of Neuronal Cells through Alterations in Antioxidant Properties. Toxicology 2017, 386, 93–102. [Google Scholar] [CrossRef]
- Karila, L.; Lafaye, G.; Scocard, A.; Cottencin, O.; Benyamina, A. MDPV and α-PVP Use in Humans: The Twisted Sisters. Neuropharmacology 2018, 134, 65–72. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Gasperini, S.; Arfè, R.; Marti, M.; Hrelia, P. Evaluation of Cytotoxic and Mutagenic Effects of the Synthetic Cathinones Mexedrone, α-PVP and α-PHP. Int. J. Mol. Sci. 2021, 22, 6320. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J. α-Pyrrolidinophenones: A New Wave of Designer Cathinones. Forensic Toxicol. 2017, 35, 201–216. [Google Scholar] [CrossRef]
- Carlier, J.; Diao, X.; Giorgetti, R.; Busardò, F.P.; Huestis, M.A. Pyrrolidinyl Synthetic Cathinones α-PHP and 4F-α-PVP Metabolite Profiling Using Human Hepatocyte Incubations. Int. J. Mol. Sci. 2020, 22, 230. [Google Scholar] [CrossRef]
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Shimokawa, Y.; Kikura-Hanajiri, R.; Aritake, K.; Urade, Y.; Goda, Y. Characterization of Four New Designer Drugs, 5-Chloro-NNEI, NNEI Indazole Analog, α-PHPP and α-POP, with 11 Newly Distributed Designer Drugs in Illegal Products. Forensic Sci. Int. 2014, 243, 1–13. [Google Scholar] [CrossRef]
- Roberts, A.L.; Fisher, A.; Smith, L.; Heinrich, M.; Potts, H.W.W. Digital Health Behaviour Change Interventions Targeting Physical Activity and Diet in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Cancer Surviv. Res. Pract. 2017, 11, 704–719. [Google Scholar] [CrossRef]
- Dragogna, F.; Oldani, L.; Buoli, M.; Altamura, A.C. A Case of Severe Psychosis Induced by Novel Recreational Drugs. F1000Research 2014, 3, 21. [Google Scholar] [CrossRef]
- Klavž, J.; Gorenjak, M.; Marinšek, M. Suicide Attempt with a Mix of Synthetic Cannabinoids and Synthetic Cathinones: Case Report of Non-Fatal Intoxication with AB-CHMINACA, AB-FUBINACA, Alpha-PHP, Alpha-PVP and 4-CMC. Forensic Sci. Int. 2016, 265, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Umebachi, R.; Aoki, H.; Sugita, M.; Taira, T.; Wakai, S.; Saito, T.; Inokuchi, S. Clinical Characteristics of α-Pyrrolidinovalerophenone (α-PVP) Poisoning. Clin. Toxicol. Phila. Pa 2016, 54, 563–567. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine Transporter and Receptor Interaction Profiles of Novel Psychoactive Substances: Para-Halogenated Amphetamines and Pyrovalerone Cathinones. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Senior, T.; Botha, M.J.; Kennedy, A.R.; Calvo-Castro, J. Understanding the Contribution of Individual Amino Acid Residues in the Binding of Psychoactive Substances to Monoamine Transporters. ACS Omega 2020, 5, 17223–17231. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Berardinelli, D.; Montanari, E.; Sirignano, A.; Di Trana, A.; Busardò, F.P. 3F-α-Pyrrolydinovalerophenone (3F-α-PVP) in Vitro Human Metabolism: Multiple in Silico Predictions to Assist in LC-HRMS/MS Analysis and Targeted/Untargeted Data Mining. J. Chromatogr. B 2022, 1193, 123162. [Google Scholar] [CrossRef] [PubMed]
- Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F. Cognitive Deficits and Neurotoxicity Induced by Synthetic Cathinones: Is There a Role for Neuroinflammation? Psychopharmacology 2019, 236, 1079–1095. [Google Scholar] [CrossRef]
- Leong, H.S.; Philp, M.; Simone, M.; Witting, P.K.; Fu, S. Synthetic Cathinones Induce Cell Death in Dopaminergic SH-SY5Y Cells via Stimulating Mitochondrial Dysfunction. Int. J. Mol. Sci. 2020, 21, 1370. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Swanson, T.; Johnson, R.A.; Janowsky, A. Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J. Pharmacol. Exp. Ther. 2017, 360, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Mottarlini, F.; Bilel, S.; Targa, G.; Tirri, M.; Maggi, C.; Marti, M.; Fumagalli, F. Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain. Int. J. Mol. Sci. 2021, 22, 7397. [Google Scholar] [CrossRef]
- Selig, T.M.; Pierre, K.; Arja, R.D.; Dagra, A.; Gold, M.S.; Kobeissy, F.; Raghavan, V.; Yang, Z.; Wang, K.K.W. Effects of Cathinones (Bath Salts) on Cultured Primary Neurons/Astroglia Cells and Neurobehavioral Functions in Mice. SSRN 2022. Available online: https://ssrn.com/abstract=4139214 (accessed on 29 June 2023).
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A Review of Synthetic Cathinone–Related Fatalities From 2017 to 2020. Ther. Drug Monit. 2021, 43, 52. [Google Scholar] [CrossRef]
- Daniela, F.; Vescovi, A.L.; Bottai, D. The Stem Cells as a Potential Treatment for Neurodegeneration. Methods Mol. Biol. Clifton NJ 2007, 399, 199–213. [Google Scholar] [CrossRef]
- Bottai, D.; Fiocco, R.; Gelain, F.; Defilippis, L.; Galli, R.; Gritti, A.; Vescovi, L.A. Neural Stem Cells in the Adult Nervous System. J. Hematother. Stem Cell Res. 2003, 12, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C.F.; Repic, M.; Knoblich, J.A. Proliferation Control in Neural Stem and Progenitor Cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef]
- Adami, R.; Bottai, D. NSC Physiological Features in Spinal Muscular Atrophy: SMN Deficiency Effects on Neurogenesis. Int. J. Mol. Sci. 2022, 23, 15209. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Ming, G.-L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef]
- Alavi, M.S.; Sadeghnia, H.R.; Nikpasand, N.; Sahab Negah, S.; Rashidi, R.; Naseri, G.; Ghorbani, A. Mephedrone Induced Apoptosis and Impaired Neurogenesis in Embryonic Neural Stem/Progenitor Cells. Can. J. Physiol. Pharmacol. 2023, 101, 235–243. [Google Scholar] [CrossRef]
- Adami, R.; Pagano, J.; Colombo, M.; Platonova, N.; Recchia, D.; Chiaramonte, R.; Bottinelli, R.; Canepari, M.; Bottai, D. Reduction of Movement in Neurological Diseases: Effects on Neural Stem Cells Characteristics. Front. Neurosci. 2018, 12, 336. [Google Scholar] [CrossRef]
- Bottai, D.; Spreafico, M.; Pistocchi, A.; Fazio, G.; Adami, R.; Grazioli, P.; Canu, A.; Bragato, C.; Rigamonti, S.; Parodi, C.; et al. Modeling Cornelia de Lange Syndrome in Vitro and in Vivo Reveals a Role for Cohesin Complex in Neuronal Survival and Differentiation. Hum. Mol. Genet. 2019, 28, 64–73. [Google Scholar] [CrossRef]
- Grazioli, P.; Parodi, C.; Mariani, M.; Bottai, D.; Di Fede, E.; Zulueta, A.; Avagliano, L.; Cereda, A.; Tenconi, R.; Wierzba, J.; et al. Lithium as a Possible Therapeutic Strategy for Cornelia de Lange Syndrome. Cell Death Discov. 2021, 7, 34. [Google Scholar] [CrossRef]
- Valente, M.J.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Neurotoxicity of β-Keto Amphetamines: Deathly Mechanisms Elicited by Methylone and MDPV in Human Dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Amaral, C.; Correia-da-Silva, G.; Duarte, J.A.; Bastos, M.d.L.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Methylone and MDPV Activate Autophagy in Human Dopaminergic SH-SY5Y Cells: A New Insight into the Context of β-Keto Amphetamines-Related Neurotoxicity. Arch. Toxicol. 2017, 91, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, J.; Andrzejczak, D.; Woldan-Tambor, A.; Zawilska, J.B. Cytotoxic Activity of Pyrovalerone Derivatives, an Emerging Group of Psychostimulant Designer Cathinones. Neurotox. Res. 2016, 30, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luethi, D.; Sanvee, G.; Bouitbir, J.; Liechti, M.; Krähenbühl, S. Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts. Int. J. Mol. Sci. 2019, 20, 1561. [Google Scholar] [CrossRef]
- Coccini, T.; Vecchio, S.; Crevani, M.; De Simone, U. Cytotoxic Effects of 3,4-Catechol-PV (One Major MDPV Metabolite) on Human Dopaminergic SH-SY5Y Cells. Neurotox. Res. 2019, 35, 49–62. [Google Scholar] [CrossRef]
- Rosas-Hernandez, H.; Cuevas, E.; Lantz, S.M.; Rice, K.C.; Gannon, B.M.; Fantegrossi, W.E.; Gonzalez, C.; Paule, M.G.; Ali, S.F. Methamphetamine, 3,4-Methylenedioxymethamphetamine (MDMA) and 3,4-Methylenedioxypyrovalerone (MDPV) Induce Differential Cytotoxic Effects in Bovine Brain Microvessel Endothelial Cells. Neurosci. Lett. 2016, 629, 125–130. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Bastos, M.d.L.; Carvalho, F.; Capela, J.P. An Updated Review on Synthetic Cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Campbell, J.C.; Stipcevic, T.; Flores, R.E.; Perry, C.; Kippin, T.E. Alcohol Exposure Inhibits Adult Neural Stem Cell Proliferation. Exp. Brain Res. 2014, 232, 2775–2784. [Google Scholar] [CrossRef][Green Version]
- Di Rocco, G.; Baldari, S.; Pani, G.; Toietta, G. Stem Cells under the Influence of Alcohol: Effects of Ethanol Consumption on Stem/Progenitor Cells. Cell. Mol. Life Sci. 2019, 76, 231–244. [Google Scholar] [CrossRef]
- Vescovi, A.L.; Galli, R.; Gritti, A. Clonal Analyses and Cryopreservation of Neural Stem Cell Cultures. In Neural Stem Cells: Methods and Protocols; Methods in Molecular Biology™; Humana Press: Totowa, NJ, USA, 2002; Volume 198, pp. 115–123. [Google Scholar] [CrossRef]
- Gritti, A.; Galli, R.; Vescovi, A.L. Clonal Analyses and Cryopreservation of Neural Stem Cell Cultures. In Neural Stem Cells; Methods in Molecular Biology™; Humana Press: Totowa, NJ, USA, 2008; Volume 438, pp. 173–184. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Roda, E.; Priori, E.C.; De Luca, F.; Facoetti, A.; Ravera, M.; Brandalise, F.; Locatelli, C.A.; Rossi, P.; Bottone, M.G. A New Platinum-Based Prodrug Candidate for Chemotherapy and Its Synergistic Effect With Hadrontherapy: Novel Strategy to Treat Glioblastoma. Front. Neurosci. 2021, 15, 589906. [Google Scholar] [CrossRef]
- Grimaldi, M.; Bo, V.D.; Ferrari, B.; Roda, E.; De Luca, F.; Veneroni, P.; Barni, S.; Verri, M.; De Pascali, S.A.; Fanizzi, F.P.; et al. Long-Term Effects after Treatment with Platinum Compounds, Cisplatin and [Pt(O,O′-Acac)(γ-Acac)(DMS)]: Autophagy Activation in Rat B50 Neuroblastoma Cells. Toxicol. Appl. Pharmacol. 2019, 364, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Valente, M.J.; Carvalho, M.; Dias da Silva, D.; Gaspar, H.; Carvalho, F.; de Lourdes Bastos, M.; Guedes de Pinho, P. Raising Awareness of New Psychoactive Substances: Chemical Analysis and in Vitro Toxicity Screening of ‘Legal High’ Packages Containing Synthetic Cathinones. Arch. Toxicol. 2015, 89, 757–771. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, B.; Sundström, M.; Pelander, A.; Siltanen, A.; Ojanperä, I.; Mervaala, E.; Korpi, E.R.; Kankuri, E. Mitochondrial Respiratory Dysfunction Due to the Conversion of Substituted Cathinones to Methylbenzamides in SH-SY5Y Cells. Sci. Rep. 2015, 5, 14924. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging Roles of Caspase-3 in Apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Daugas, E.; Nochy, D.; Ravagnan, L.; Loeffler, M.; Susin, S.A.; Zamzami, N.; Kroemer, G. Apoptosis-Inducing Factor (AIF): A Ubiquitous Mitochondrial Oxidoreductase Involved in Apoptosis. FEBS Lett. 2000, 476, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.F. Apoptosis-Inducing Factor: Structure, Function, and Redox Regulation. Antioxid. Redox Signal. 2011, 14, 2545–2579. [Google Scholar] [CrossRef]
- Bano, D.; Prehn, J.H.M. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef]
- Pawlowski, J.; Kraft, A.S. Bax-Induced Apoptotic Cell Death. Proc. Natl. Acad. Sci. USA 2000, 97, 529–531. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Peña-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The Interplay between BAX and BAK Tunes Apoptotic Pore Growth to Control Mitochondrial-DNA-Mediated Inflammation. Mol. Cell 2022, 82, 933–949.e9. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. Clifton NJ 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Hansen, T.E.; Johansen, T. Following Autophagy Step by Step. BMC Biol. 2011, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Runwal, G.; Stamatakou, E.; Siddiqi, F.H.; Puri, C.; Zhu, Y.; Rubinsztein, D.C. LC3-Positive Structures Are Prominent in Autophagy-Deficient Cells. Sci. Rep. 2019, 9, 10147. [Google Scholar] [CrossRef] [PubMed]
- Schläfli, A.M.; Adams, O.; Galván, J.A.; Gugger, M.; Savic, S.; Bubendorf, L.; Schmid, R.A.; Becker, K.-F.; Tschan, M.P.; Langer, R.; et al. Prognostic Value of the Autophagy Markers LC3 and P62/SQSTM1 in Early-Stage Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 39544–39555. [Google Scholar] [CrossRef]
- Emanuele, S.; Lauricella, M.; D’Anneo, A.; Carlisi, D.; De Blasio, A.; Di Liberto, D.; Giuliano, M. P62: Friend or Foe? Evidences for OncoJanus and NeuroJanus Roles. Int. J. Mol. Sci. 2020, 21, 5029. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin–Proteasome System upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor P62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Garcia-Canton, C.; Anadón, A.; Meredith, C. ΓH2AX as a Novel Endpoint to Detect DNA Damage: Applications for the Assessment of the in Vitro Genotoxicity of Cigarette Smoke. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2012, 26, 1075–1086. [Google Scholar] [CrossRef]
- Valente, D.; Gentileschi, M.P.; Guerrisi, A.; Bruzzaniti, V.; Morrone, A.; Soddu, S.; Verdina, A. Factors to Consider for the Correct Use of ΓH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation. Cancers 2022, 14, 6204. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; de Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Canales, J.J. Adult Neurogenesis and the Memories of Drug Addiction. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 261–270. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo. Transl. Psychiatry 2020, 10, 331. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. Clifton NJ 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Darbinian, N. Cultured Cell Line Models of Neuronal Differentiation: NT2, PC12. Methods Mol. Biol. Clifton NJ 2013, 1078, 23–33. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Ricci, J.-E.; Gottlieb, R.A.; Green, D.R. Caspase-Mediated Loss of Mitochondrial Function and Generation of Reactive Oxygen Species during Apoptosis. J. Cell Biol. 2003, 160, 65–75. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; de la Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Stoll, E.A.; Cheung, W.; Mikheev, A.M.; Sweet, I.R.; Bielas, J.H.; Zhang, J.; Rostomily, R.C.; Horner, P.J. Aging Neural Progenitor Cells Have Decreased Mitochondrial Content and Lower Oxidative Metabolism. J. Biol. Chem. 2011, 286, 38592–38601. [Google Scholar] [CrossRef] [PubMed]
- Cabon, L.; Galán-Malo, P.; Bouharrour, A.; Delavallée, L.; Brunelle-Navas, M.-N.; Lorenzo, H.K.; Gross, A.; Susin, S.A. BID Regulates AIF-Mediated Caspase-Independent Necroptosis by Promoting BAX Activation. Cell Death Differ. 2012, 19, 245–256. [Google Scholar] [CrossRef]
- Correia, B.; Fernandes, J.; Botica, M.J.; Ferreira, C.; Quintas, A. Novel Psychoactive Substances: The Razor’s Edge between Therapeutical Potential and Psychoactive Recreational Misuse. Medicines 2022, 9, 19. [Google Scholar] [CrossRef]
- Costa, L.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G.; Fonseca, B.M. Cannabinoid-Induced Autophagy: Protective or Death Role? Prostaglandins Other Lipid Mediat. 2016, 122, 54–63. [Google Scholar] [CrossRef]
- Niklaus, M.; Adams, O.; Berezowska, S.; Zlobec, I.; Graber, F.; Slotta-Huspenina, J.; Nitsche, U.; Rosenberg, R.; Tschan, M.P.; Langer, R. Expression Analysis of LC3B and P62 Indicates Intact Activated Autophagy Is Associated with an Unfavorable Prognosis in Colon Cancer. Oncotarget 2017, 8, 54604–54615. [Google Scholar] [CrossRef]
- Dias da Silva, D.; Ferreira, B.; Roque Bravo, R.; Rebelo, R.; Duarte de Almeida, T.; Valente, M.J.; Silva, J.P.; Carvalho, F.; Bastos, M.d.L.; Carmo, H. The New Psychoactive Substance 3-Methylmethcathinone (3-MMC or Metaphedrone) Induces Oxidative Stress, Apoptosis, and Autophagy in Primary Rat Hepatocytes at Human-Relevant Concentrations. Arch. Toxicol. 2019, 93, 2617–2634. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, Y.; Bai, C.; Zhang, X.; Fang, M.; Ju, Z.; Liu, B. Membrane Dynamics of ATG4B and LC3 in Autophagosome Formation. J. Mol. Cell Biol. 2022, 13, 853–863. [Google Scholar] [CrossRef]
- Vaz, I.; Carvalho, T.; Valente, M.J.; Castro, A.; Araújo, A.M.; Bastos, M.L.; Carvalho, M. The Interplay between Autophagy and Apoptosis Mediates Toxicity Triggered by Synthetic Cathinones in Human Kidney Cells. Toxicol. Lett. 2020, 331, 42–52. [Google Scholar] [CrossRef]
- Lee, H.L.; Pike, R.; Chong, M.H.A.; Vossenkamper, A.; Warnes, G. Simultaneous Flow Cytometric Immunophenotyping of Necroptosis, Apoptosis and RIP1-Dependent Apoptosis. Methods San Diego Calif 2018, 134–135, 56–66. [Google Scholar] [CrossRef]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. GammaH2AX: A Sensitive Molecular Marker of DNA Damage and Repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Koedrith, P.; Kim, H.S.; Yu, W.J.; Kim, J.C.; Seo, Y.R. Comparative Genotoxicity Investigation Using Comet and GammaH2AX Assays for Screening of Genotoxicants in HepG2 Human Hepatoma Cells. Toxicol. Environ. Health Sci. 2016, 8, 68–78. [Google Scholar] [CrossRef]
- Nguyen, L.; Malgrange, B.; Belachew, S.; Rogister, B.; Rocher, V.; Moonen, G.; Rigo, J.-M. Functional Glycine Receptors Are Expressed by Postnatal Nestin-Positive Neural Stem/Progenitor Cells. Eur. J. Neurosci. 2002, 15, 1299–1305. [Google Scholar] [CrossRef]
- Cai, J.; Cheng, A.; Luo, Y.; Lu, C.; Mattson, M.P.; Rao, M.S.; Furukawa, K. Membrane Properties of Rat Embryonic Multipotent Neural Stem Cells. J. Neurochem. 2004, 88, 212–226. [Google Scholar] [CrossRef]
- Smith, D.O.; Rosenheimer, J.L.; Kalil, R.E. Delayed Rectifier and A-Type Potassium Channels Associated with Kv 2.1 and Kv 4.3 Expression in Embryonic Rat Neural Progenitor Cells. PLoS ONE 2008, 3, e1604. [Google Scholar] [CrossRef]
- Franco, R.; Bortner, C.D.; Cidlowski, J.A. Potential Roles of Electrogenic Ion Transport and Plasma Membrane Depolarization in Apoptosis. J. Membr. Biol. 2006, 209, 43–58. [Google Scholar] [CrossRef]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anticancer Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; Feng, S.; Cheng, W.; Tang, B.; Shi, Y.-L.; Hua, Z.-C. Plasma Membrane Depolarization and Na,K-ATPase Impairment Induced by Mitochondrial Toxins Augment Leukemia Cell Apoptosis via a Novel Mitochondrial Amplification Mechanism. Biochem. Pharmacol. 2009, 78, 191–202. [Google Scholar] [CrossRef]
- Düssmann, H.; Rehm, M.; Kögel, D.; Prehn, J.H.M. Outer Mitochondrial Membrane Permeabilization during Apoptosis Triggers Caspase-Independent Mitochondrial and Caspase-Dependent Plasma Membrane Potential Depolarization: A Single-Cell Analysis. J. Cell Sci. 2003, 116, 525–536. [Google Scholar] [CrossRef]



| Antigen | Immunogen | Manufacturer, Species, Mono-Polyclonal, Cat./Lot. No., RRID | Dilution | |
|---|---|---|---|---|
| Primary antibodies | Anti-β-tubulin (H-235): | Purified antibody raised against amino acids 210–444 mapping at the C-terminus of β-Tubulin of human origin. | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-9104, RRID: AB_2241191 | 1:200 |
| Anti-apoptosis-inducing factor (E-1) | Purified antibody raised against amino acids 1–300 of AIF of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Mouse monoclonal IgG, Cat# sc-13116, RRID: AB_626654 | 1:100 | |
| Anti-Bcl-2-associated X protein (P-19) | Purified antibody raised against a peptide mapping at the N-terminus of BAX of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-526, RRID: AB_2064668 | 1:100 | |
| Anti- caspase-3 (31A1067) | Purified antibody raised against amino acids 50–86 of caspase-3 of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Mouse monoclonal IgG, Cat# sc-56053, RRID: AB_781826 | 1:100 | |
| Anti-Histone H2A.X (6L16) | Purified antibody raised against KLH-conjugated linear peptide corresponding to 9 amino acids surrounding serine 139 of human Histone H2AX | Merck KGaA (Darmstadt, Germany), Rabbit Monoclonal Antibody, Cat# ZRB05636, RRID: AB_309864. | 1:100 | |
| Anti-p62/SQSTM1 | Recombinant full-length protein corresponding to Human SQSTM1/p62 aa 1–440 | Abcam (Cambridge, United States), Mouse monoclonal, Cat# ab56416, RRID: AB_945626 | 1:100 | |
| Anti-Mitochondria serum | Human autoimmune serum recognizing the 70 kDa E2 subunit of pyruvate dehydrogenase complex (kindly given by IRCCS San Matteo Pavia, Italy) a | 1:300 | ||
| Anti-LC3B | Rabbit polyclonal anti-LC3B (Cell Signaling Technology, Danvers, United States) | Cell Signaling Technology, (Danvers, United States), Rabbit polyclonal, Cat# 2775S, RRID: AB_915950 | 1:200 | |
| Anti-Lysosomes serum | Human autoimmune serum (kindly given by IRCCS San Matteo Pavia, Italy) b | 1:500 | ||
| Anti-Vimentin (V-9) | Purified vimentin from pig eye lens | Thermo Fisher Scientific (Monza, Italy), Mouse monoclonal, Cat# MA5-11883, RRID: AB_10985392 | 1:200 | |
| Secondary antibodies | Alexa Fluor™ 488 goat anti-rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 |
| Alexa Fluor™ 488 goat anti-mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 | |
| Alexa Fluor™ 594 goat anti-rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 | |
| Alexa Fluor™ 594 goat anti-mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 | |
| Alexa Fluor™ 594 goat anti-human IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 | |
| Alexa Fluor™ 647 goat anti-human IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma Immunoglobins Heavy and Light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, E.; De Luca, F.; Priori, E.C.; Ratto, D.; Pinelli, S.; Corradini, E.; Mozzoni, P.; Poli, D.; Mazzini, G.; Bottone, M.G.; et al. The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells. Biology 2023, 12, 1225. https://doi.org/10.3390/biology12091225
Roda E, De Luca F, Priori EC, Ratto D, Pinelli S, Corradini E, Mozzoni P, Poli D, Mazzini G, Bottone MG, et al. The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells. Biology. 2023; 12(9):1225. https://doi.org/10.3390/biology12091225
Chicago/Turabian StyleRoda, Elisa, Fabrizio De Luca, Erica Cecilia Priori, Daniela Ratto, Silvana Pinelli, Emilia Corradini, Paola Mozzoni, Diana Poli, Giuliano Mazzini, Maria Grazia Bottone, and et al. 2023. "The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells" Biology 12, no. 9: 1225. https://doi.org/10.3390/biology12091225
APA StyleRoda, E., De Luca, F., Priori, E. C., Ratto, D., Pinelli, S., Corradini, E., Mozzoni, P., Poli, D., Mazzini, G., Bottone, M. G., Gatti, A. M., Marti, M., Locatelli, C. A., Rossi, P., & Bottai, D. (2023). The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells. Biology, 12(9), 1225. https://doi.org/10.3390/biology12091225













