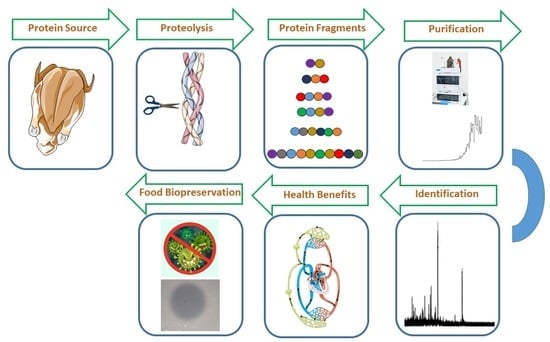
Identification of a Novel Bioactive Peptide Derived from Frozen Chicken Breast Hydrolysate and the Utilization of Hydrolysates as Biopreservatives
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Protein by Enzymatic Hydrolysis
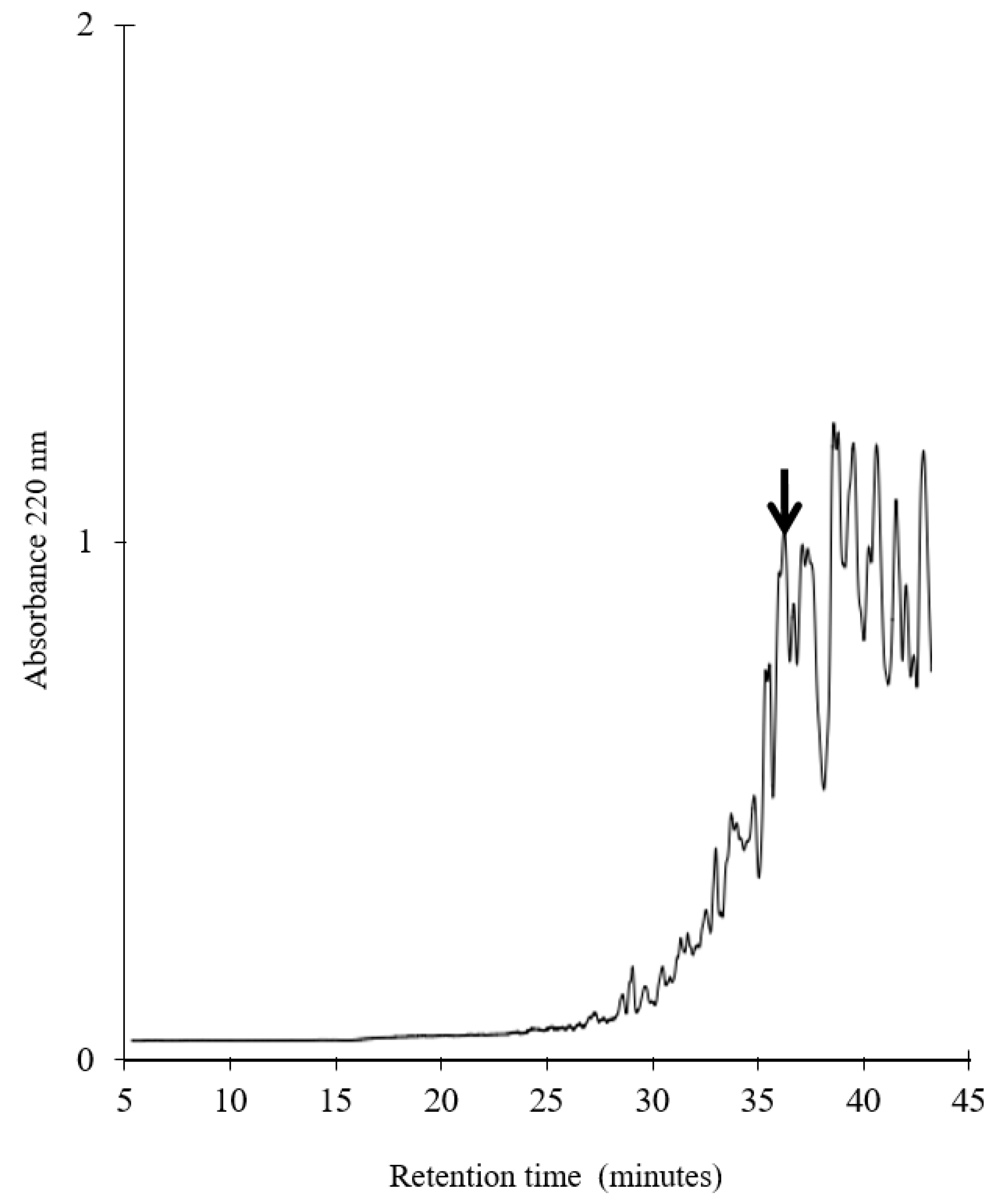
2.2. Purification of Chicken Breast Hydrolysate
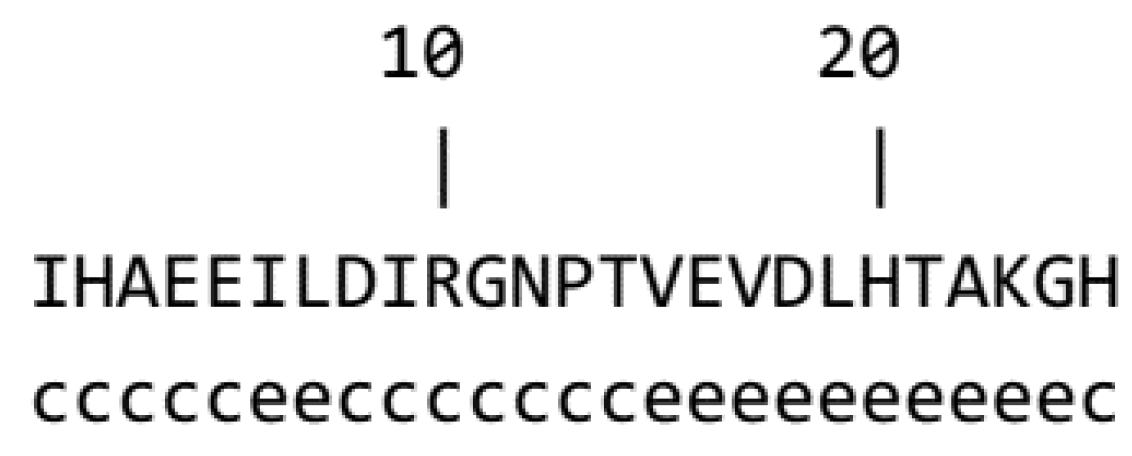
2.3. Mass Spectrometry and Amino Acid Sequencing
2.4. Sequences Analysis
2.5. ACE Inhibition Activity Assay
2.6. Antioxidant Activity Assay
2.7. Determination of Antimicrobial Activity
2.8. Preservative Effects of Beef, Fish and Chicken Hydrolysates
2.9. Evaluation of Antibacterial Activity of Hydrolysates on Bacterial Flora of Chicken Breast
2.10. Lipid Oxidation
2.11. Statistical Analysis
3. Results
3.1. Generation, Purification and Structural Analysis of the Chicken Peptide
3.2. ACE Inhibition and Antioxidant Activities
3.3. Antimicrobial Spectra
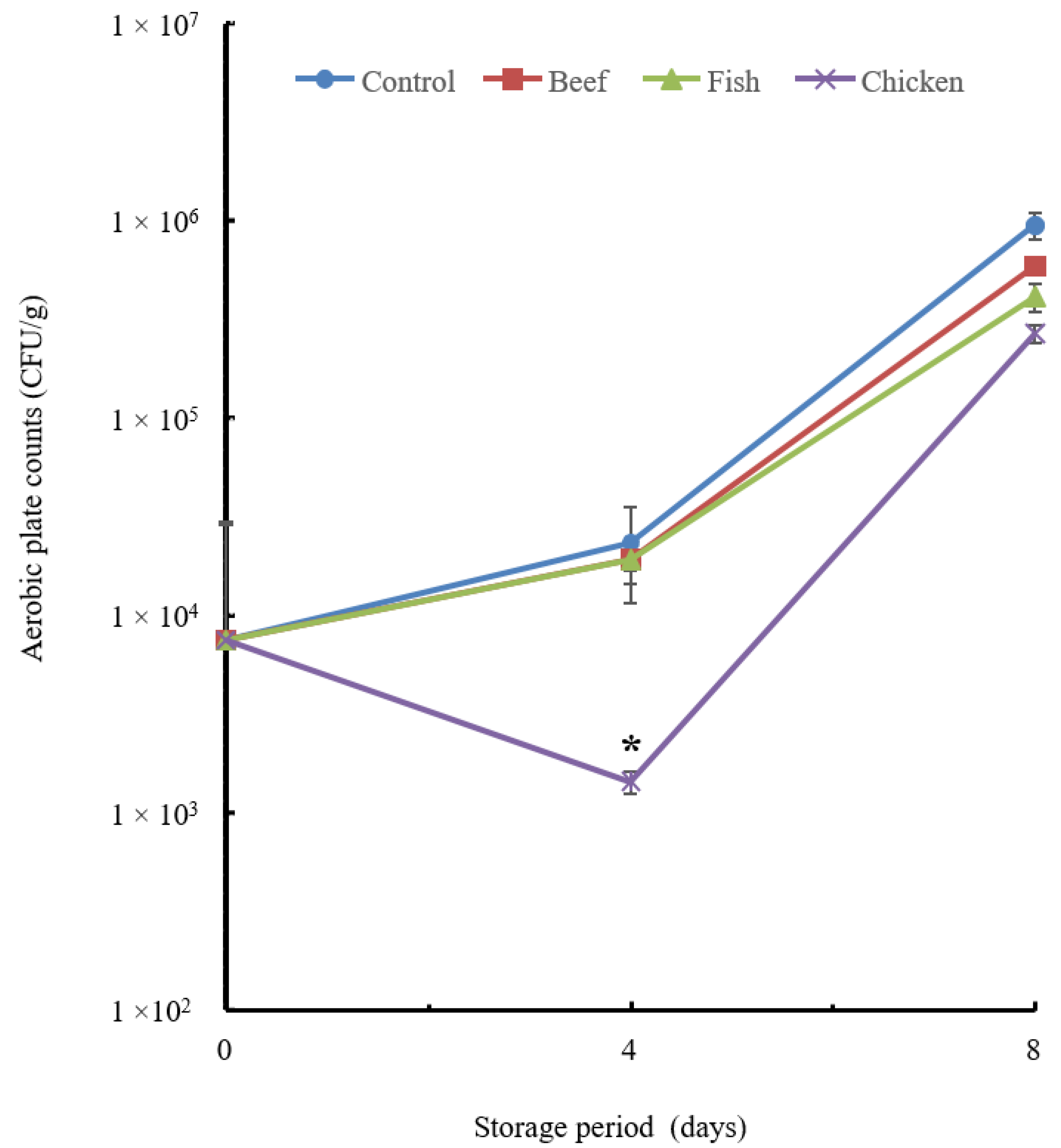
3.4. Preservative Effect of Beef, Fish and Chicken Hydrolysates
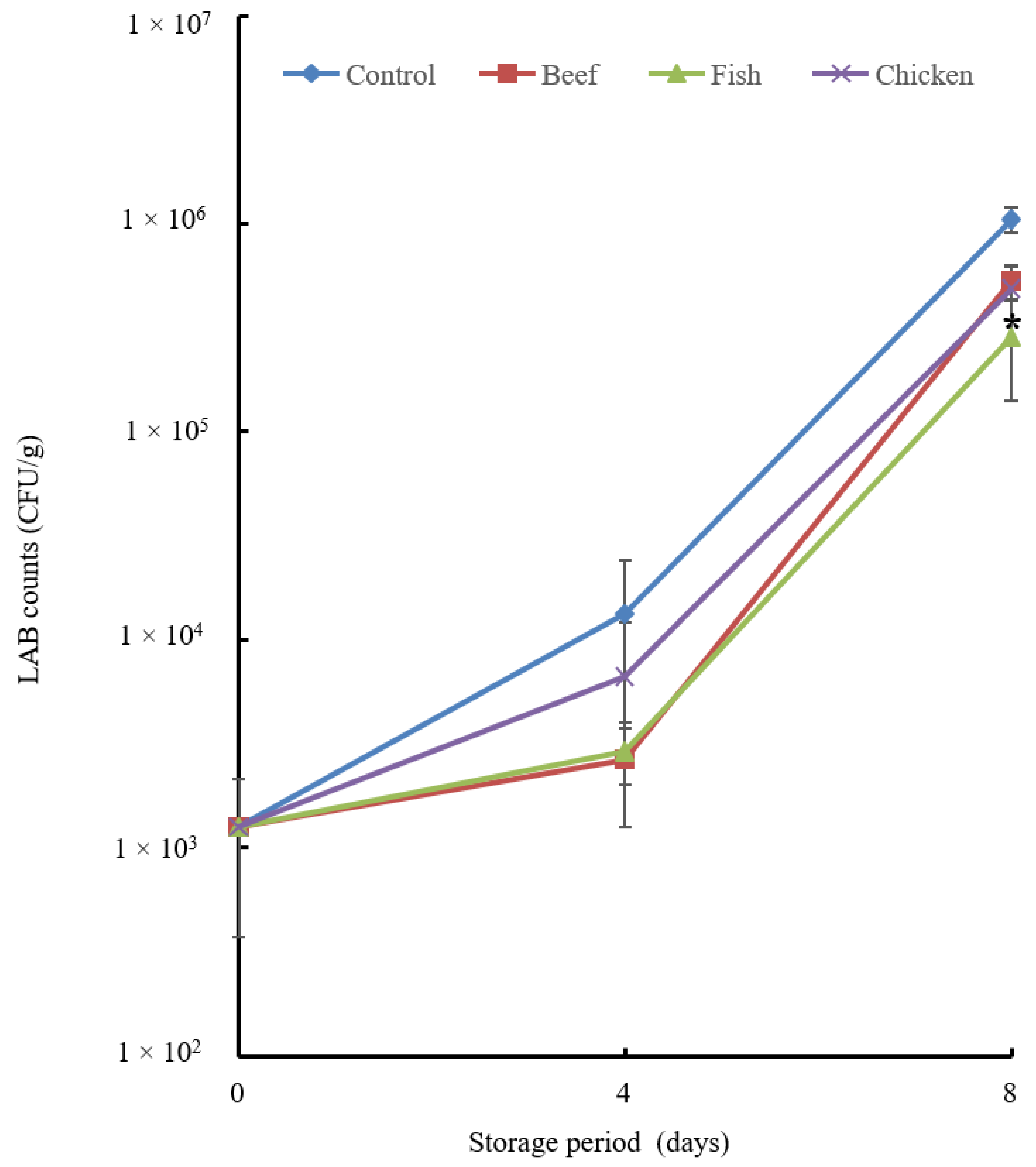
3.4.1. Microbial Analysis
3.4.2. Lipid Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, T.; He, T.; Li, H.; Tang, H.; Xia, E. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Gallego, M.; Toldrá, F. ACEI-inhibitory peptides naturally generated in meat and meat products and their health relevance. Nutrients 2018, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Ngo, D.H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Jang, A.; Lee, M. Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Sci. 2005, 69, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 15, 205–222. [Google Scholar] [CrossRef]
- Yu, G.; Li, J.; He, H.; Huang, W.; Zhang, W. Ultrafiltration preparation of potent bioactive corn peptide as alcohol metabolism stimulator in vivo and study on its mechanism of action. J. Food Biochem. 2013, 37, 161–167. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive Peptides: A Promising Alternative to Chemical Preservatives for Food Preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant properties of casein calcium peptides and their effects on lipid oxidation in beef homogenates. J. Agric. Food Chem. 2005, 53, 464–468. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological activities and peptidomic profile of in vitro-digested cow, camel, goat and sheep milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef]
- Ryan, J.; Ross, R.; Bolton, D.; Fitzgerald, G.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Castellano, P.; Aristoy, M.; Sentandreu, M.; Vignolo, G.; Toldrá, F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J. Proteom. 2013, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. LWT 2020, 125, 109215. [Google Scholar] [CrossRef]
- Bashir, K.; Sohn, J.; Kim, J.; Choi, J. Identification and characterization of novel antioxidant peptides from mackerel (Scomber japonicus) muscle protein hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef]
- Maky, M.A.; Zendo, T. Generation and characterization of novel bioactive peptides from fish and beef hydrolysates. Appl. Sci. 2021, 11, 10452. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. 2012, 50, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Guo, X.; Li, R.; Huang, L. Effect of crude peptide extract from mutton ham on antioxidant properties and quality of mutton patties. J. Food Process. Preserv. 2020, 44, 14436. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Assessment of physicochemical, antioxidant and antimicrobial activity of porcine blood proteinhydrolysate in pork emulsion stored under aerobic packaging condition at 4 ± 1 C. LWT 2018, 88, 71–79. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Zendo, T.; Nakayama, J.; Fujita, K.; Sonomoto, K. Bacteriocin detection by liquid chromatography/mass spectrometry for rapid identification. J. Appl. Microbiol. 2008, 104, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Edman, P. Method for determination of the amino acid sequence in peptides. Acta Chem. Scand. 1950, 4, 283–293. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Maky, M.A.; Ishibashi, N.; Nakayama, J.; Zendo, T. Characterization of the biosynthetic gene cluster of enterocin F4-9, a glycosylated bacteriocin. Microorganisms 2021, 9, 2276. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC; Association of Official Analytical Chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Khan, M.; Parrish, C.; Shahidi, F. Effects of mechanical handling, storage on ice and ascorbic acid treatment on lipid oxidation in cultured Newfoundland blue mussel (Mytilus edulis). Food Chem. 2006, 99, 605–614. [Google Scholar] [CrossRef]
- Leitao, H.G.; Diedericks, G.; Baeckens, S.; Svardal, H. Chromosome-level genome assembly of the cape cliff lizard (Hemicordylus capensis). Genome Biol. Evol. 2023, 15, evad001. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Loje, H.; Risbo, J.; Van den Berg, F.W.; Christensen, M. Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Sci. 2013, 93, 787–795. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Malva, A.; Marino, R. Bioactive peptides in animal food products. Foods 2017, 6, 35. [Google Scholar] [CrossRef]
- Bao, C.; Chen, H.; Chen, L.; Cao, J.; Meng, J. Comparison of ACE inhibitory activity in skimmed goat and cow milk hydrolyzed by alcalase, flavourzyme, neutral protease and proteinase K. Acta Univ. Cibiniensis Ser. E Food Technol. 2016, 20, 77–84. [Google Scholar] [CrossRef]
- Arihara, K. Functional foods. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier: Oxford, UK, 2004; pp. 492–499. [Google Scholar]
- Pancholi, V. Multifunctional α-enolase: Its role in diseases. Cell. Mol. Life Sci. 2001, 58, 902–920. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Wan, J.; Zhou, Y.; Zhu, Q. Extraction and identification of bioactive peptides from Panxian dry-cured. LWT 2022, 160, 113326. [Google Scholar] [CrossRef]
- Terashima, M.; Baba, T.; Ikemoto, N.; Katayama, M. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef]
- Nakamura, Y. Studies on anti-hypertensive peptides in milk fermented with Lactobacillus helveticus. Biosci. Microflora 2004, 23, 131–138. [Google Scholar] [CrossRef][Green Version]
- Matsui, T.; Tanaka, M. Antihypertensive peptides and their underlying mechanisms. In Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 43–53. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE inhibitory peptides derived from simulated gastrointestinal digestion in vitro of Sesame (Sesamum indicum L.) protein and molecular docking study. Int. J. Mol. Sci. 2020, 21, 1059. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, H.; Li, X.; Lai, F.; Xiao, X. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochem. Biophys. Res. Commun. 2014, 452, 888–894. [Google Scholar] [CrossRef]
- Wang, B.; Gong, Y.D.; Li, Z.R.; Yu, D.; Chi, C.F.; Ma, J.Y. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods. 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Chen, L. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly proteins. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Ambigaipalan, P.S.; Al-Khalifa, A.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using alcalase, flavourzyme and thermolysin. J. Funct. Foods. 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Xiong, S.; Yao, X.; Li, A. Antioxidant properties of peptide from cowpea seed. Int. J. Food Prop. 2013, 16, 1245–1256. [Google Scholar] [CrossRef]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2014, 43, 101–109. [Google Scholar]
- Corrêa, J.A.F.; de Melo Nazareth, T.; Rocha, G.F.d.; Luciano, F.B. Bioactive Antimicrobial Peptides from Food Proteins: Perspectives and Challenges for Controlling Foodborne Pathogens. Pathogens 2023, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Chakka, A.K.; Elias, M.; Jini, R.; Sakhare, P.Z.; Bhaskar, N. In-vitro antioxidant and antibacterial properties of fermentatively and enzymatically prepared chicken liver protein hydrolysates. J. Food Sci. Technol. 2015, 52, 8059–8067. [Google Scholar] [CrossRef] [PubMed]
- Rezaharsamto, B.; Subroto, E. A review on bioactive peptides derived from various sources of meat and meat by-products. Int. J. Sci. Technol Res. 2019, 8, 3151–3156. [Google Scholar]
- Bhat, Z.; Kumar, S.; Bhat, H. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef]
- Przybylski, R.; Firdaous, L.; Chataigne, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse byproduct and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Catiau, L.; Traisnel, J.; Delval-Dubois, V.; Chihib, N.-E.; Guillochon, D.; Nedjar-Arroume, N. Minimal antimicrobial peptide sequence from hemoglobin alpha-chain: KYR. Peptides 2011, 32, 633–638. [Google Scholar] [CrossRef]



| Indicator Strain | MIC (μg/mL) | |
|---|---|---|
| Hydrolysate | C25 | |
| Enterococcus faecalis JCM 5803T | NA 1 | NA 2 |
| Listeria innocua ATCC 33090T | 400 | NA 2 |
| Escherichia coli JM109 | NA 1 | 24 |
| Weizmannia coagulans JCM 2257T | NA 1 | NA 2 |
| Pseudomonas putida ATCC 12633T | 800 | NA 2 |
| Salmonella enterica serovar Typhimurium NBRC 13245T | NA 1 | NA 2 |
| Proteus vulgaris F24-B | 100 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maky, M.A.; Zendo, T. Identification of a Novel Bioactive Peptide Derived from Frozen Chicken Breast Hydrolysate and the Utilization of Hydrolysates as Biopreservatives. Biology 2023, 12, 1218. https://doi.org/10.3390/biology12091218
Maky MA, Zendo T. Identification of a Novel Bioactive Peptide Derived from Frozen Chicken Breast Hydrolysate and the Utilization of Hydrolysates as Biopreservatives. Biology. 2023; 12(9):1218. https://doi.org/10.3390/biology12091218
Chicago/Turabian StyleMaky, Mohamed Abdelfattah, and Takeshi Zendo. 2023. "Identification of a Novel Bioactive Peptide Derived from Frozen Chicken Breast Hydrolysate and the Utilization of Hydrolysates as Biopreservatives" Biology 12, no. 9: 1218. https://doi.org/10.3390/biology12091218
APA StyleMaky, M. A., & Zendo, T. (2023). Identification of a Novel Bioactive Peptide Derived from Frozen Chicken Breast Hydrolysate and the Utilization of Hydrolysates as Biopreservatives. Biology, 12(9), 1218. https://doi.org/10.3390/biology12091218