The Effect of the Pyrethroid Pesticide Fenpropathrin on the Cardiac Performance of Zebrafish and the Potential Mechanism of Toxicity
Abstract
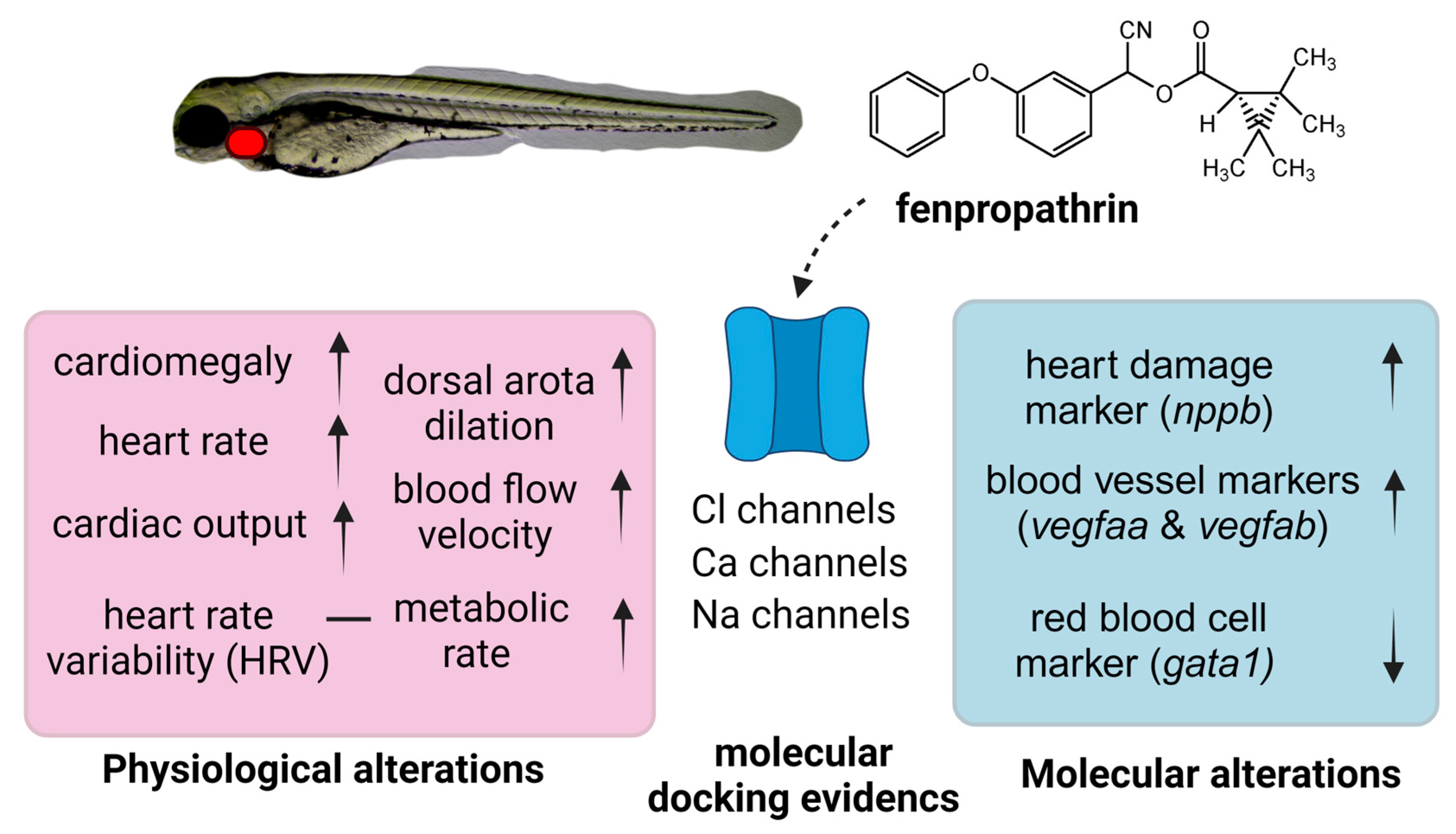
Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Animal Husbandry and Exposure
2.2. Chemical Preparation
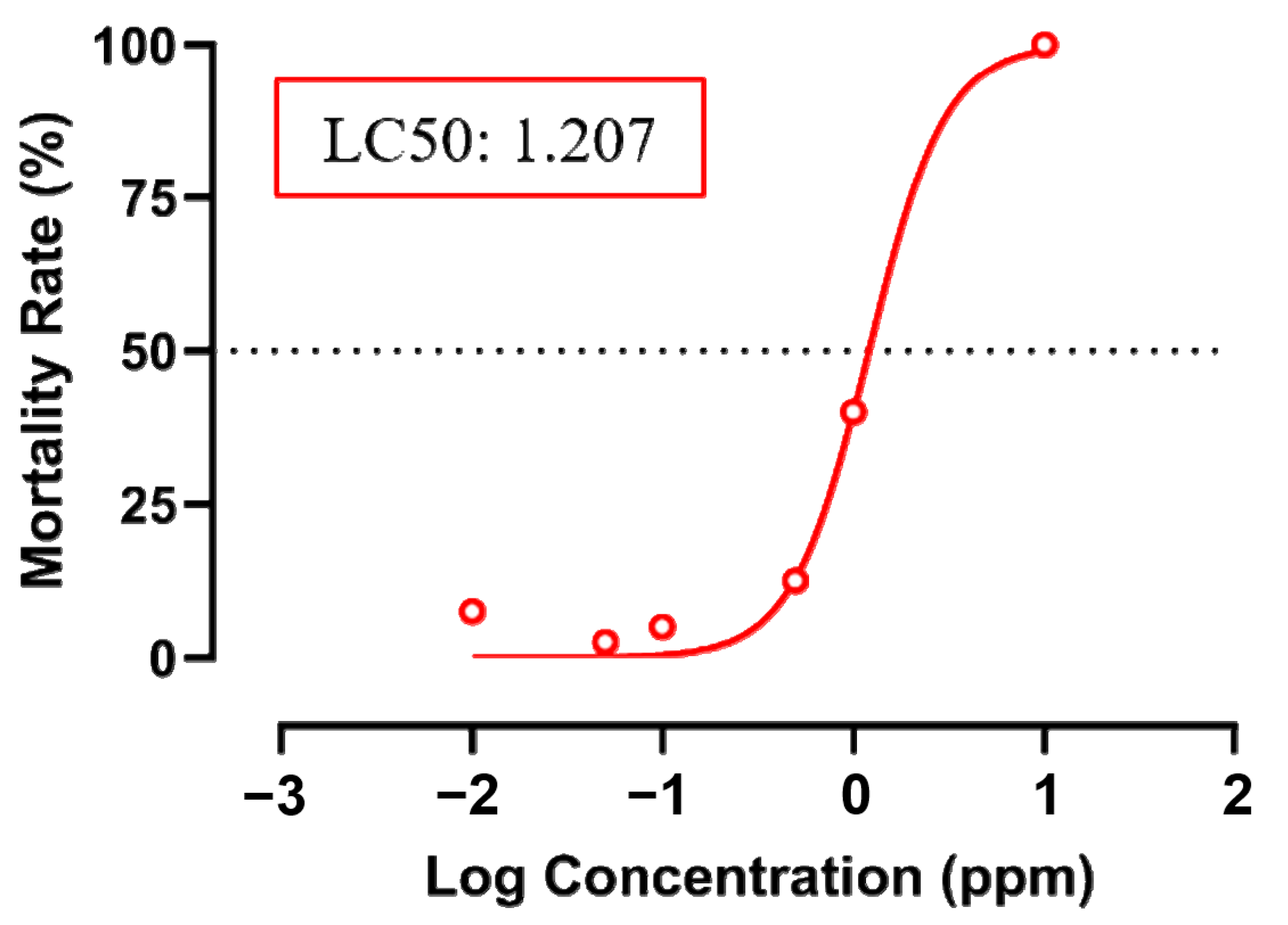
2.3. Acute Toxicity Test
2.4. Cardiac Performance Analysis
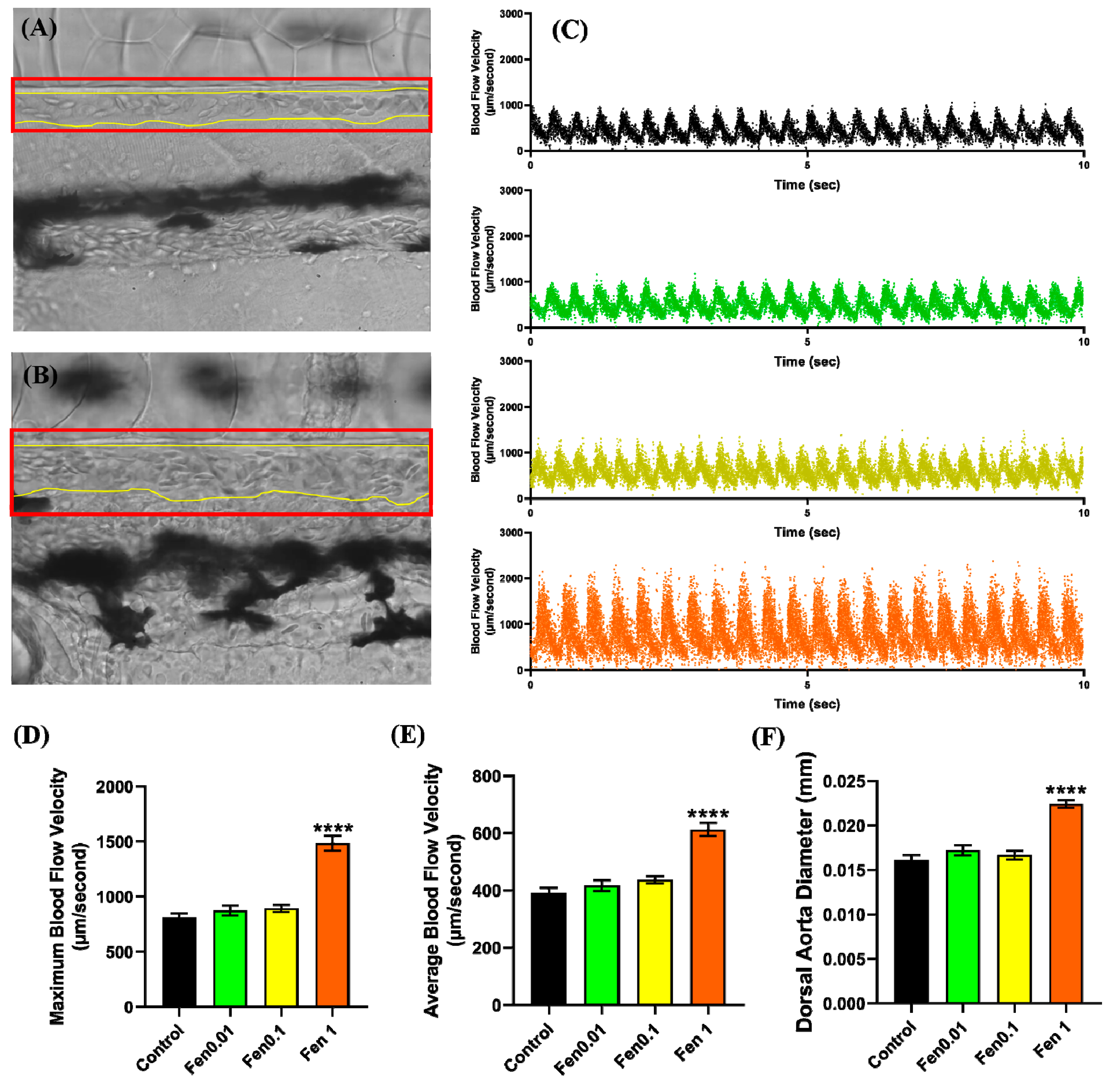
2.5. Vascular Performance Analysis
2.6. Oxygen Consumption Analysis
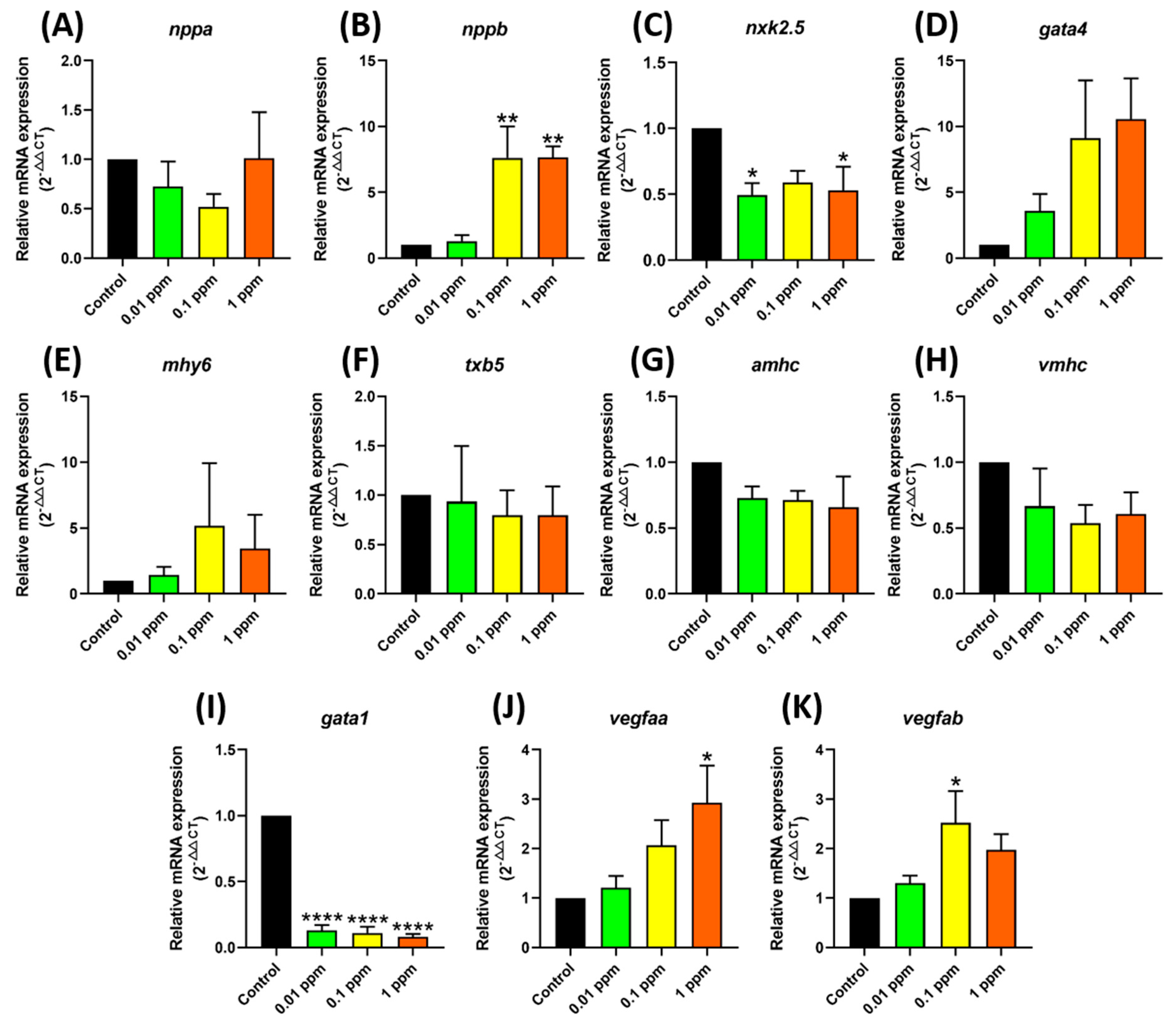
2.7. RT-PCR for Genes Related to Cardiovascular Performance
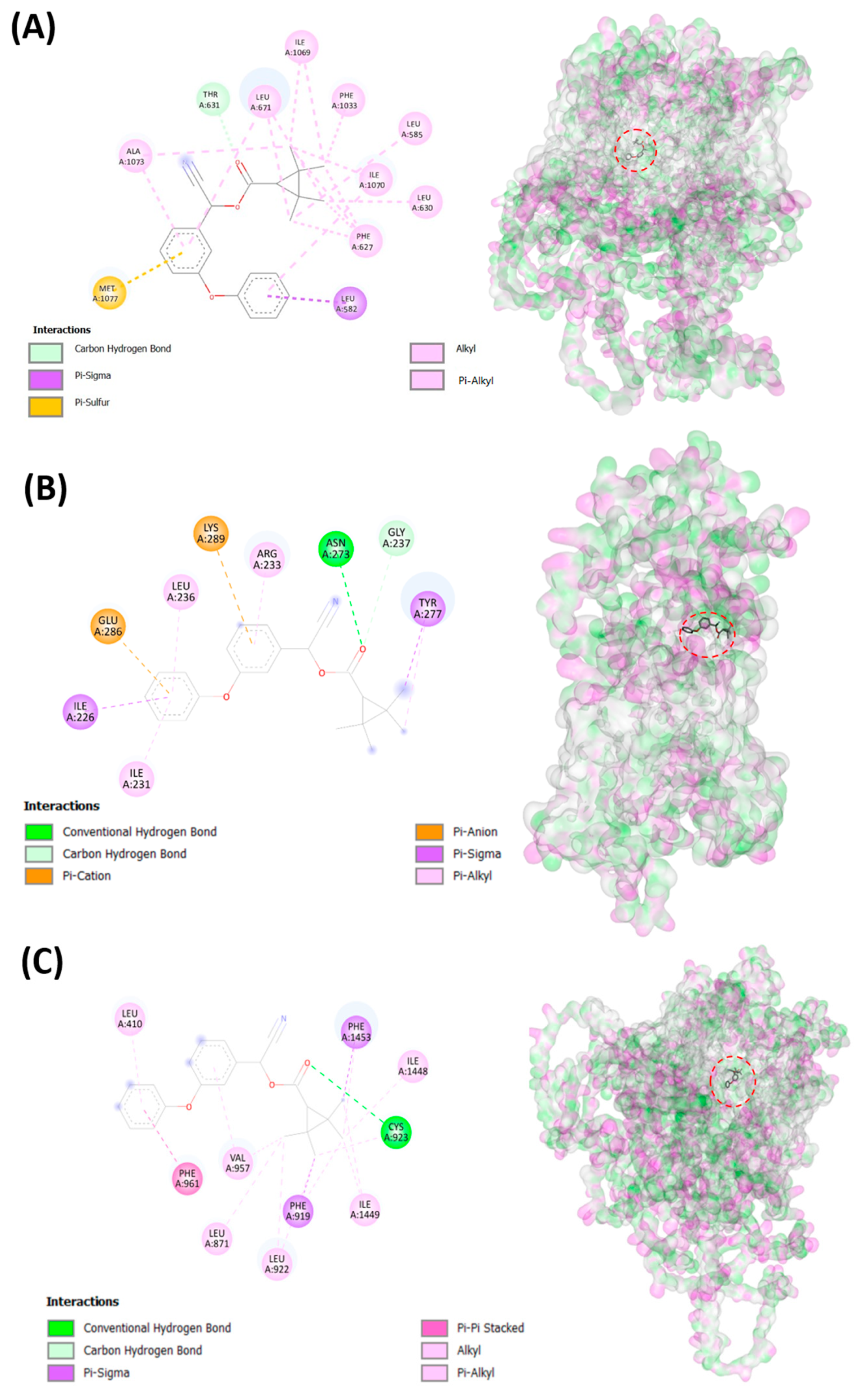
2.8. Molecular Docking Analysis
2.9. Statistical Analysis
3. Result
3.1. Testing Cardiovascular and Metabolic Performance Alterations after Fenpropathrin Exposure
3.2. Testing Fenpropathrin and Target Protein Interactions by Molecular Docking
3.3. Testing Marker Gene Expression after Fenpropathrin Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimdahl, R.L. A History of Weed Science in the United States; Elsevier Science: London, UK, 2010. [Google Scholar]
- Rao, V.S. Principles of Weed Science; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Pfeil, R. Pesticide Residues: Pyrethroids. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 31–34. [Google Scholar] [CrossRef]
- Al-Makkawy, H.; Madbouly, M.D. Persistence and accumulation of some organic insecticides in Nile water and fish. Resour. Conserv. Recycl. 1999, 27, 105–115. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Zohiery, F.; Hagi, B.N.; Garanzini, D.S. Alterations in biochemical parameters of the freshwater fish, Alburnus mossulensis, exposed to sub-lethal concentrations of Fenpropathrin. Int. J. Aquat. Biol. 2014, 2, 58–68. [Google Scholar]
- Kanawi, E.; Budd, R.; Tjeerdema, R.S. Environmental fate and ecotoxicology of fenpropathrin. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2013; pp. 77–93. [Google Scholar]
- Hideo, K. Pyrethroid Chemistry and Metabolism. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Robert, K., Ed.; Academic Press: London, UK, 2010; pp. 1635–1663. [Google Scholar]
- Ministry of Health and Welfare. Standards for Pesticide Residue Limits in Foods. Available online: https://law.moj.gov.tw/ENG/LawClass/LawGetFile.ashx?FileId=0000007831&lan=E (accessed on 16 March 2023).
- Authority, E.E.F.S.; Bellisai, G.; Bernasconi, G.; Binaglia, M.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F. Targeted review of maximum residue levels (MRLs) for bifenthrin. EFSA J. 2023, 21, e07864. [Google Scholar]
- Schuttens, T.; Heudorf, U.; Drexler, H.; Angerer, J. Pyrethroid exposure of the general population—Is this due to diet. Toxicol. Lett. 2002, 134, 141–145. [Google Scholar]
- Whyatt, R.M.; Camann, D.E.; Kinney, P.L.; Reyes, A.; Ramirez, J.; Dietrich, J.; Diaz, D.; Holmes, D.; Perera, F.P. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect. 2002, 110, 507–514. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, X.; Huang, J.; Chen, C.; Chen, Z.; Liu, L.; Zhang, G.; Yang, J.; Zhang, Z.; Zhang, Z. Fenpropathrin, a widely used pesticide, causes dopaminergic degeneration. Mol. Neurobiol. 2016, 53, 995–1008. [Google Scholar]
- Jiao, Z.; Wu, Y.; Qu, S. Fenpropathrin induces degeneration of dopaminergic neurons via disruption of the mitochondrial quality control system. Cell Death Discov. 2020, 6, 78. [Google Scholar] [CrossRef]
- Yu, T.; Wan, F.; Liu, C.; Zhang, X.; Liu, Z.; Zhang, J.; Xiong, J.; Wang, T.; Zhang, Z. Asparagine endopeptidase inhibitor protects against fenpropathrin-induced neurodegeneration via suppressing α-synuclein aggregation and neuroinflammation. Eur. J. Pharmacol. 2020, 888, 173586. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Borzęcki, A. Effect of 28-day exposure to fenpropathrin on the activities of serum alanine transaminase and liver antioxidant enzymes in mice. J. Vet. Res. 2015, 59, 165–169. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Borzęcki, A. The 28-day exposure to fenpropathrin decreases locomotor activity and reduces activity of antioxidant enzymes in mice brains. Pharmacol. Rep. 2016, 68, 495–501. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish embryos and larvae: A new generation of disease models and drug screens. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 115–133. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar] [PubMed]
- Yu, T.; Xu, X.; Mao, H.; Han, X.; Liu, Y.; Zhang, H.; Lai, J.; Gu, J.; Xia, M.; Hu, C. Fenpropathrin exposure induces neurotoxicity in zebrafish embryos. Fish Physiol. Biochem. 2022, 48, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M. Zebrafish models of cardiac disease: From fortuitous mutants to precision medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.-B.; He, K.-J.; Wang, F.; Liu, C.-F. Advances of zebrafish in neurodegenerative disease: From models to drug discovery. Front. Pharmacol. 2021, 12, 713963. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Santoso, F.; Farhan, A.; Castillo, A.L.; Malhotra, N.; Saputra, F.; Kurnia, K.A.; Chen, K.H.-C.; Huang, J.-C.; Chen, J.-R.; Hsiao, C.-D. An Overview of Methods for Cardiac Rhythm Detection in Zebrafish. Biomedicines 2020, 8, 329. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- DeMarco, K.; Clancy, C. Cardiac Na channels: Structure to function. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2016; Volume 78, pp. 287–311. [Google Scholar]
- Organisation for Economic Cooperation and Development. Test No. 203: Fish, Acute Toxicity Test. Available online: https://www.oecd-ilibrary.org/environment/test-no-203-fish-acute-toxicity-test_9789264069961-en (accessed on 30 August 2023).
- Hoage, T.; Ding, Y.; Xu, X. Quantifying cardiac functions in embryonic and adult zebrafish. Cardiovasc. Dev. Methods Protoc. 2012, 83, 11–20. [Google Scholar]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A simple imagej-based method to measure cardiac rhythm in zebrafish embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Santoso, F.; Krylov, V.V.; Castillo, A.L.; Saputra, F.; Chen, H.-M.; Lai, H.-T.; Hsiao, C.-D. Cardiovascular performance measurement in water fleas by utilizing high-speed videography and ImageJ software and its application for pesticide toxicity assessment. Animals 2020, 10, 1587. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Chem. Biol. Methods Protoc. 2015, 1263, 243–250. [Google Scholar]
- EPA (United States Environmental Protection Agency). Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0 (accessed on 27 March 2023).
- Gupta, R.C.; Miller Mukherjee, I.R.; Malik, J.K.; Doss, R.B.; Dettbarn, W.-D.; Milatovic, D. Chapter 26—Insecticides. In Biomarkers in Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 455–475. [Google Scholar] [CrossRef]
- Cao, Z.; Shafer, T.J.; Murray, T.F. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. J. Pharmacol. Exp. Ther. 2011, 336, 197–205. [Google Scholar] [CrossRef]
- Borg, J.J.; Hancox, J.C.; Zhang, H.; Spencer, C.I.; Li, H.; Kozlowski, R.Z. Differential pharmacology of the cardiac anionic background current IAB. Eur. J. Pharmacol. 2007, 569, 163–170. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G.; Guazzi, M.; Palermo, P.; Bussotti, M.; Marenzi, G. Cardiomegaly as a possible cause of lung dysfunction in patients with heart failure. Am. Heart J. 2000, 140, A17–A21. [Google Scholar] [CrossRef]
- Amin, H.; Siddiqui, W.J. Cardiomegaly. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Olson, T.P.; Beck, K.C.; Johnson, B.D. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J. Card. Fail. 2007, 13, 100–107. [Google Scholar] [CrossRef]
- Tavora, F.; Zhang, Y.; Zhang, M.; Li, L.; Ripple, M.; Fowler, D.; Burke, A. Cardiomegaly is a common arrhythmogenic substrate in adult sudden cardiac deaths, and is associated with obesity. Pathology 2012, 44, 187–191. [Google Scholar] [CrossRef]
- Othman, A.I.; Mahmoud, H.S. Chronic Toxicity of Ectomethrin: A Biochemical and Histological Study. Egypt. J. Histol. 2019, 42, 526–533. [Google Scholar] [CrossRef]
- Ghazouani, L.; Feriani, A.; Mufti, A.; Tir, M.; Baaziz, I.; Mansour, H.B.; Mnafgui, K. Toxic effect of alpha cypermethrin, an environmental pollutant, on myocardial tissue in male wistar rats. Environ. Sci. Pollut. Res. 2020, 27, 5709–5717. [Google Scholar] [CrossRef] [PubMed]
- Ramanlal, R.; Gupta, V. Physiology, vasodilation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Krčmová, I.; Novosad, J. Anaphylactic symptoms and anaphylactic shock. Vnitr. Lek. 2019, 65, 149–156. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Vallabhajosyula, S.; Khanna, A.K.; Chawla, L.S.; Busse, L.W.; Kashani, K.B. Management of refractory vasodilatory shock. Chest 2018, 154, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T. Calcium antagonists and vasodilation. Pharmacol. Ther. 1994, 64, 37–75. [Google Scholar] [CrossRef]
- Alison, M.G.; Lucie, H.C. Calcium Channels and Vasodilation. Adv. Mol. Cell Biol. 1994, 8, 21–41. [Google Scholar] [CrossRef]
- Fernandes, C.E.; da Silveira, A.W.; do Nascimento Silva, A.L.; de Souza, A.I.; Povh, J.A.; dos Santos Jaques, J.A.; Dos Santos, E.D.A.; Yonekawa, M.K.A.; de Barros Penteadoall, B.; Franco-Belussi, L. Osmoregulatory profiles and gill histological changes in Nile tilapia (Oreochromis niloticus) exposed to lambda-cyhalothrin. Aquat. Toxicol. 2020, 227, 105612. [Google Scholar] [CrossRef] [PubMed]
- Sudan, S.; Kaur, N.; Taneja, R.; Mehmi, P. Management of Lambda-Cyhalothrin Poisoning in a North Indian Healthcare Setup: A Rare Case. Cureus 2022, 14, e32746. [Google Scholar] [CrossRef]
- Eells, J.T.; Dubocovich, M. Pyrethroid insecticides evoke neurotransmitter release from rabbit striatal slices. J. Pharmacol. Exp. Ther. 1988, 246, 514–521. [Google Scholar]
- Kirby, M.L.; Castagnoli, K.; Bloomquist, J.R. In vivo effects of deltamethrin on dopamine neurochemistry and the role of augmented neurotransmitter release. Pestic. Biochem. Physiol. 1999, 65, 160–168. [Google Scholar] [CrossRef]
- Reitsamer, H.A.; Zawinka, C.; Branka, M. Dopaminergic vasodilation in the choroidal circulation by d1/d5 receptor activation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, D.L.; Bean, B.P. Voltage-dependent calcium channels in rat midbrain dopamine neurons: Modulation by dopamine and GABAB receptors. J. Neurophysiol. 1995, 74, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, E.; Schiemann, J.; Liss, B. Dopamine midbrain neurons in health and Parkinson’s disease: Emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 2015, 284, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T.; Frey, J.; Ginsburg, K.S.; Roy, M. Sodium and GABA-activated channels as the targets of pyrethroids and cyclodienes. Toxicol. Lett. 1992, 64, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M. Pyrethroids, knockdown resistance and sodium channels. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 610–616. [Google Scholar] [CrossRef]
- Spencer, C.I.; Yuill, K.H.; Borg, J.J.; Hancox, J.C.; Kozlowski, R.Z. Actions of pyrethroid insecticides on sodium currents, action potentials, and contractile rhythm in isolated mammalian ventricular myocytes and perfused hearts. J. Pharmacol. Exp. Ther. 2001, 298, 1067–1082. [Google Scholar]
- Shafer, T.J.; Meyer, D.A. Effects of pyrethroids on voltage-sensitive calcium channels: A critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol. Appl. Pharmacol. 2004, 196, 303–318. [Google Scholar] [CrossRef]
- Clark, J.M.; Symington, S.B. Pyrethroid action on calcium channels: Neurotoxicological implications. Invertebr. Neurosci. 2007, 7, 3–16. [Google Scholar] [CrossRef]
- Quintavalle, A. Voltage-gated calcium channels in honey bees: Physiological roles and potential targets for insecticides. BioSci. Master Rev. 2013, 2013, 1–11. [Google Scholar]
- Supnet, C.; Bezprozvanny, I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and excitation-contraction coupling in the heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D. Calcium in the heart: From physiology to disease. Exp. Physiol. 2014, 99, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Chaya, G.; Erik, K. Chapter 1—The heart. In Biology of Cardiovascular and Metabolic Diseases; Fernandez, B., Ed.; Academic Press: London, OH, USA, 2022; pp. 1–33. [Google Scholar] [CrossRef]
- Duan, D. Phenomics of cardiac chloride channels: The systematic study of chloride channel function in the heart. J. Physiol. 2009, 587, 2163–2177. [Google Scholar] [CrossRef]
- Ponce, A.; Jimenez-Peña, L.; Tejeda-Guzman, C. The role of swelling-activated chloride currents (ICL, swell) in the regulatory volume decrease response of freshly dissociated rat articular chondrocytes. Cell. Physiol. Biochem. 2012, 30, 1254–1270. [Google Scholar] [CrossRef]
- Sardini, A.; Amey, J.S.; Weylandt, K.-H.; Nobles, M.; Valverde, M.A.; Higgins, C.F. Cell volume regulation and swelling-activated chloride channels. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1618, 153–162. [Google Scholar] [CrossRef]
- David, M.S.; John, M.C.; Larry, P.S.; Linda, S.M.; Vincent, J.P.; Dana, S.; James, T.S.; Myra, L.W. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Forshaw, P.; Lister, T.; Ray, D. The role of voltage-gated chloride channels in type II pyrethroid insecticide poisoning. Toxicol. Appl. Pharmacol. 2000, 163, 1–8. [Google Scholar] [CrossRef]
- Ray, D.; Sutharsan, S.; Forshaw, P. Actions of pyrethroid insecticides on voltage-gated chloride channels in neuroblastoma cells. Neurotoxicology 1997, 18, 755–760. [Google Scholar]
- Gutiérrez, L.; Caballero, N.; Fernández-Calleja, L.; Karkoulia, E.; Strouboulis, J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life 2020, 72, 89–105. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Tsukamoto, S.; Suzuki, M.; Yamamoto-Mukai, H.; Yamamoto, M.; Philipsen, S.; Ohneda, K. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood J. Am. Soc. Hematol. 2008, 111, 4375–4385. [Google Scholar] [CrossRef]
- Tos-Luty, S.; Haratym-Maj, A.; Latuszynska, J.; Obuchowska-Przebirowska, D.; Tokarska-Rodak, M. Oral toxicity of deltamethrin and fenvalerate in Swiss mice. Ann. Agric. Environ. Med. 2001, 8, 245–254. [Google Scholar]
- Khan, A.; Faridi, H.A.; Ali, M.; Khan, M.Z.; Siddique, M.; Hussain, I.; Ahmad, M. Effects of cypermethrin on some clinico-hemato-biochemical and pathological parameters in male dwarf goats (Capra hircus). Exp. Toxicol. Pathol. 2009, 61, 151–160. [Google Scholar] [CrossRef]
- Dar, S.A.; Kaur, R. Hematobiochemical evaluation of dermal subacute cypermethrin toxicity in buffalo calves. Toxicol. Int. 2014, 21, 283. [Google Scholar] [PubMed]
- Guo, S.; Wu, Y.; Li, W.; Xiao, P. Tralomethrin causes cardiovascular toxicity in zebrafish (Danio rerio) embryos. Environ. Toxicol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wolansky, M.; Gennings, C.; Crofton, K. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol. Sci. 2006, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′-3′) | Reversed Primer (5′-3′) |
|---|---|---|
| myh6 | F: CACCAGCAGACACTGGATG | R: GCTCCAAGTCCATTCTGAC |
| tbx5 | F: ATTCGCCGATAACAAATGG | R: CGCCTTGACGATGTGGAT |
| vmhc | F: GAAGAGGCAGAGGCATCACT | R: AATTGCGTTTGCTCTGCTCC |
| amhc | F: AAGCCACTACCGCCTCTCTA | R: TTTGAGGCAAGGTCGTCCAA |
| nxk2.5 | F: GTCCAGGCAACTCGAACTACTC | R: AACATCCCAGCCAAACCATA |
| gata4 | F: TCCAGGCGGGTGGGTTTATC | R: TGTCTGGTTCAGTCTTGATGGGTC |
| vegfaa | F: AAAAGAGTGCGTGCAAGACC | R: GACGTTTCGTGTCTCTGTCG |
| vegfab | F: TGTTGGTGGAAATTCAGCAG | R: CACCCTGATGACGAAGAGGT |
| nppa | F: AAGCAAAAGCTTGTCTGG | R: ACTGTATCCGCATATTGCAGC |
| nppb | F: CATTCCCGTAGTCGGCCTTC | R: CTTCAATATTTGCCGCCTTTAC |
| Gene | Protein | UniProt | Coordinates and Dimension of the Grid Box |
|---|---|---|---|
| cacna1fa | Voltage-dependent L-type calcium channel subunit alpha | F1R736 | Center: x = −21.175, y = −1.963, z = 8.991 Dimensions (Å): x = 83.040, y = 64.471, z = 92.663 |
| cacna1g | Voltage-dependent T-type calcium channel subunit alpha | e7f9f6 | Center: x = −22.230, y = −6.492, z = 22.740 Dimensions (Å): x = 116.140, y = 81.918, z = 124.875 |
| cacna1ha | Calcium channel, voltage-dependent, T type, alpha 1H subunit a | A0A2R8PWF8 | Center: x = 3.168, y = 6.847, z = 12.372 Dimensions (Å): x = 86.560, y = 63.668, z = 60.626 |
| cacna1sb | Voltage-dependent L-type calcium channel subunit alpha | Q6RKB0 | Center: x = −23.067, y = −10.319, z = 10.135 Dimensions (Å): x = 106.084, y = 103.402, z = 72.540 |
| clcn1a | Chloride channel, voltage-sensitive 1a | A0A0R4ID92 | Center: x = 2.964, y = 8.362, z = 7.264 Dimensions (Å): x = 34.792, y = 33.053, z = 29.360 |
| clcn1b | Chloride channel, voltage-sensitive 1b | A0A2R8Q7B5 | Center: x = 1.488, y = −0.489, z = −0.657 Dimensions (Å): x = 44.666, y = 30.449, z = 36.670 |
| clcn2a | Chloride channel, voltage-sensitive 2a | A5PMN6 | Center: x = 0.671, y = 3.518, z = 15.953 Dimensions (Å): x = 66.344, y = 77.634, z = 108.854 |
| clcn2b | Chloride channel protein | A0A098DN12 | Center: x = −3.741, y = 6.447, z = −1.055 Dimensions (Å): x = 44.981, y = 31.420, z = 39.037 |
| clcn3 | Chloride channel protein | A0A140LGW7 | Center: x = 2.777, y = 1.185, z = 5.672 Dimensions (Å): x = 61.361, y = 42.659, z = 56.203 |
| scn1lab | Sodium channel protein | F1QXF5 | Center: x = −20.241, y = 2.816, z = −1.385 Dimensions (Å): x = 45.601, y = 38.333, z = 44.803 |
| scn4aa | Voltage-gated sodium channel type 4 subunit alpha A | Q2XVR3 | Center: x = −22.588, y = 3.698, z = 1.059 Dimensions (Å): x = 55.172, y = 49.391, z = 71.775 |
| scn4ab | Sodium channel protein type 4 subunit alpha B | Q20JQ7 | Center: x = −2.948, y = 8.484, z = 7.078 Dimensions (Å): x = 86.715, y = 130.031, z = 121.072 |
| scn5lab | Voltage-gated sodium channel type V-like, alpha B | A0A0G2KYI1 | Center: x = −24.519, y = 1.712, z = 18.384 Dimensions (Å): x = 64.739, y = 50.480, z = 71.871 |
| scn8aa | Sodium channel 8 | A0A2R8QLY1 | Center: x = −25.787, y = 12.332, z = −9.831 Dimensions (Å): x = 56.054, y = 47.937, z = 47.219 |
| bcl2a | Apoptosis regulator Bcl-2 | Q564A4 | Center: x = −0.361, y = 1.914, z = 5.890 Dimensions (Å): x = 36.912, y = 45.937, z = 37.158 |
| mdm2 | E3 ubiquitin-protein ligase Mdm2 | Q561Z0 | Center: x = −1.626, y = 2.424, z = 12.751 Dimensions (Å): x = 45.601, y = 38.333, z = 44.803 |
| p53 | Tumor protein p53 inducible protein 3 | A0A286YBA3 | Center: x = −20.241, y = 2.816, z = −1.385 Dimensions (Å): x = 45.601, y = 38.333, z = 44.803 |
| Gene | Binding Energy (kcal/mol) | Gene | Binding Energy (kcal/mol) |
|---|---|---|---|
| cacna1fa | −8.3 | scn1lab | −9.7 |
| cacna1g | −7.4 | scn4aa | −9.5 |
| cacna1ha | −7.5 | scn4ab | −9.4 |
| cacna1sb | −8.6 | scn5lab | −9.5 |
| clcn1a | −8.2 | scn8aa | −9.5 |
| clcn1b | −8.1 | bcl2a | −8.5 |
| clcn2a | −8.6 | p53 | −8.4 |
| clcn2b | −8.5 | mdm2 | −6.4 |
| clcn3 | −8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, F.; Lai, Y.-H.; Roldan, M.J.M.; Alos, H.C.; Aventurado, C.A.; Vasquez, R.D.; Hsiao, C.-D. The Effect of the Pyrethroid Pesticide Fenpropathrin on the Cardiac Performance of Zebrafish and the Potential Mechanism of Toxicity. Biology 2023, 12, 1214. https://doi.org/10.3390/biology12091214
Saputra F, Lai Y-H, Roldan MJM, Alos HC, Aventurado CA, Vasquez RD, Hsiao C-D. The Effect of the Pyrethroid Pesticide Fenpropathrin on the Cardiac Performance of Zebrafish and the Potential Mechanism of Toxicity. Biology. 2023; 12(9):1214. https://doi.org/10.3390/biology12091214
Chicago/Turabian StyleSaputra, Ferry, Yu-Heng Lai, Marri Jmelou M. Roldan, Honeymae C. Alos, Charlaine A. Aventurado, Ross D. Vasquez, and Chung-Der Hsiao. 2023. "The Effect of the Pyrethroid Pesticide Fenpropathrin on the Cardiac Performance of Zebrafish and the Potential Mechanism of Toxicity" Biology 12, no. 9: 1214. https://doi.org/10.3390/biology12091214
APA StyleSaputra, F., Lai, Y.-H., Roldan, M. J. M., Alos, H. C., Aventurado, C. A., Vasquez, R. D., & Hsiao, C.-D. (2023). The Effect of the Pyrethroid Pesticide Fenpropathrin on the Cardiac Performance of Zebrafish and the Potential Mechanism of Toxicity. Biology, 12(9), 1214. https://doi.org/10.3390/biology12091214








