Hyperglycemia and Hyperlipidemia with Kidney or Liver Transplantation: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Mammalian Target of Rapamycin in Diabetes Mellitus: Nephropathy and Kidney Transplantation
3. Hyperglycemia Associated with Medications for Transplant Immunosuppression
4. Lipogenesis before/after Transplantation
4.1. Kidney Allografts
4.2. Liver Allografts
5. Management of Post-Transplant Hyperglycemia
6. Management of Post-Transplant Hyperlipidemia
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach, M.C.; Adler, J.L.; Breman, J.; P’eng, F.K.; Sahyoun, A.; Schlesinger, R.M.; Madras, P.; Monaco, A.P. Influence of rejection therapy on fungal and nocardial infections in renal-transplant recipients. Lancet 1973, 1, 180–184. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.; Wang, Z.; Benoit, S.; Burros, N.R.; Imperatore, G.; Albright, A.L.; Patzer, R. Long-term mortality among kidney transplant recipients with and without diabetes: A nationwide cohort study in the USA. BMJ Open Diabetes Res. Care 2021, 9, e001962. [Google Scholar] [CrossRef]
- Weinrauch, L.A.; Segal, A.R.; Bayliss, G.; Liu, J.; Wisniewski, E.; D’Elia, J.A. Changes in treatment of hyperglycemia in a hypertensive type 2 diabetes population as renal function declines. Clin. Kidney J. 2017, 10, 661–665. [Google Scholar] [CrossRef][Green Version]
- Weinrauch, L.A.; D’Elia, J.A.; Weir, M.R.; Bunnapradist, S.; Finn, P.; Liu, J.; Claggett, B.; Monaco, A.P. Does diabetes impact therapeutic immune modulation therapy decisions for kidney transplant recipients? Data from the Folic Acid for Vascular Outcome Reduction in Transplant (FAVORIT) trial. Int. J. Nephrol. Renov. Dis. 2017, 10, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, L.A.; D’Elia, J.A.; Weir, M.R.; Bunnapradist, S.; Finn, P.V.; Liu, J.; Claggett, B.; Monaco, A.P. Infection and malignancy outweigh cardiovascular mortality in kidney transplant recipients: Post hoc analysis of the FAVORIT trial. Am. J. Med. 2018, 131, 165–172. [Google Scholar] [CrossRef]
- Yamagishi, S.; Takeuchi, M.; Inagaki, Y.; Imaizumi, T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int. J. Clin. Pharm. Res. 2003, 23, 129–134. [Google Scholar]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycated end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Prac. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fantus, D.; Rogers, N.M.; Grahammer, F.; Huber, T.B.; Thomson, A.W. Roles of mTOR complexes in the kidney: Implication for kidney disease and transplantation. Nat. Rev. Nephrol. 2016, 12, 587–609. [Google Scholar] [CrossRef]
- Viana, S.D.; Reis, F.; Alves, R. Therapeutic use of m-TOR inhibitors in renal diseases: Advances, drawbacks, and challenges. Oxid. Med. Cell Longev. 2018, 2018, 3693625. [Google Scholar] [CrossRef]
- Wolf, S.; Hoffmann Vs Habicht, A.; Kauke, T.; Bucher, J.; Schoenberg, M.; Werner, J.; Guba, M.; Andrassy, J. Effect of small mTOR-is on malignancy and survival following renal transplantation: A systematic review and meta-analysis of randomized trials with a minimum follow up of 24 months. PLoS ONE 2018, 13, e0194975. [Google Scholar] [CrossRef]
- Zeng, J.; Zhong, G.; Feng, X.; Li, I.; Fang, S.; Fan, Y.; Song, T.; Huang, Z.; Wang, X.; Lin, T. Conversion from calcineurin inhibitors to mammalian target of rapamycin kidney transplant recipients: A systematic and meta-analysis of randomized controlled trials. Front. Immunol. 2021, 12, 663602. [Google Scholar] [CrossRef] [PubMed]
- Cucchiari, D.; Rios, J.; Molina-Andujar, A.; Montagud-Marrahi, E.; Revuelta, J.; Ventura-Aguiar, P.; Pineiro, G.P.; De Souza-Amorin, E.; Esforzado, N.; Cofan, F.; et al. Combination of calcineurin-mTor inhibitor in kidney transplantation: A propensity score analysis. J. Nephrol. 2020, 33, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Verges, B.; Cariou, B. mTor inhibitors and diabetes. Diabetes Res. Clin. Prac. 2015, 110, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Gesualdo, L. Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16 (Suppl. S1), s30–s33. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Inoki, K.; Masutani, K.; Wakabayashi, Y.; Komai, K.; Nakagawa, R.; Guan, K.L.; Yoshimura, A. The mTOR pathway is highly activated I diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophys. Res. Commun. 2009, 384, 471–475. [Google Scholar] [CrossRef]
- Flechner, S.M. mTor inhibition and clinical transplantation. Transplantation 2018, 102, 517–518. [Google Scholar] [CrossRef]
- Li, J.X.; Cummins, C.L. Fresh insights into glucocorticoid induced diabetes mellitus and new therapeutic directions, Nat. Rev. Endocrinol. 2022, 18, 540–555. [Google Scholar]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar]
- Midtvedt, K.; Hjelmesaeth, J.; Hartmann, A.; Lund, K.; Paulsen, D.; Egeland, T.; Jenssen, T. Insulin resistance after renal transplantation: The effect of steroid dose reduction and withdrawal. J. Am. Soc. Nephrol. 2004, 15, 3233–3239. [Google Scholar] [CrossRef]
- Akman, B.; Uyar, M.; Afsar, B.; Sezer, S.; Ozdemir, F.N.; Haberal, M. Lipid profile during azathioprine or mycophenolate mofetil combination with cyclosporine and steroids. Transplant. Proc. 2007, 39, 135–137. [Google Scholar] [CrossRef]
- Romero, F.; Rodriguez-Iturbe, B.; Pong, H.; Parra, G.; Rincon, J.; Gonzalez, I. Mycophenolate mofetil treatment reduces cholesterol-induced atherosclerosis in the rabbit. Atherosclerosis 2000, 152, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Munshi, V.N.; Saghafian, S.; Cook, C.B.; Werner, K.T.; Chakkera, H.A. Comparison of post- transplantation diabetes mellitus and risk factors between kidney and liver transplantation patients. PLoS ONE 2020, 15, e0226873. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Secchi, A. Post-transplantation diabetes in kidney transplant recipients: An update on management and prevention. Acta Diabetol. 2018, 55, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Speranza Henneicke, H.; Gasparini, S.J.; Blankenstein, K.I.; Heinevetter, U.; Cogger, V.C.; Svistounov, D.; Zhang, V.; Cooney, G.J.; Buttgereit, F.; Dunstan, C.R.; et al. Osteoblasts mediate the adverse effect of glucocorticoids on fuel metabolism. J. Clin. Invest. 2012, 112, 4172–4189. [Google Scholar] [CrossRef]
- Ferris, H.A.; Kahn, C.R. New mechanisms of glucocorticoid-induced insulin resistance: Make no bones about it. Clin. Invest. 2012, 112, 3854–3857. [Google Scholar] [CrossRef]
- Verzola, D.; Bertolotto, M.B.; Villaggio, B.; Ottonello, L.; Dallegri, F.; Salvatore, F.; Berruti, V.; Gandolfo, M.T.; Garibotto, G.; Deferrari, G. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J. Am. Soc. Nephrol. 2004, 15, 585–587. [Google Scholar] [CrossRef]
- Allen, D.A.; Harwood, S.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003, 17, 908–910. [Google Scholar] [CrossRef]
- De Graav, G.N.; van der Zwan, M.; Baan, C.C.; Janssen, J.A.M.J.L.; Hesselink, D.A. Improved glucose tolerance in a kidney transplant recipient with type 2 diabetes mellitus from tacrolimus to belatacept: A case report and review of mechanisms. Transplant. Direct 2018, 4, e350. [Google Scholar] [CrossRef]
- Kukla, A.; Hill, J.; Merzkani, A.; Lorenz, E.C.; Park, E.D.; D’Costa, M.; Kudva, Y.C.; Stengel, M.D.; Shah, P. The use of GLP 1R agonists for treatment of type 2 diabetes mellitus in kidney transplant recipients. Transplant. Direct 2020, 6, e524. [Google Scholar] [CrossRef]
- Pavlakis, M.; Goldfarb-Rumyantzev, A.S. Diabetes after transplantation and sirolimus: What’s the connection? J. Am. Soc. Nephrol. 2008, 19, 1255–1256. [Google Scholar] [CrossRef]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 56 kinase pathway: A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 27, 38052–38060. [Google Scholar] [CrossRef]
- Koya, D.; Jirousek, M.R.; Lin, Y.W.; Ishii, H.; Kuboki, K.; King, G.L. Characterization of Protein Kinase C beta isoform activation on the gene expression of transforming growth factor beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J. Clin. Invest. 1997, 100, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Koya, D.; King, G.L. Protein Kinase C activation in the development of diabetes mellitus complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef]
- Lang, G.E. Pharmacological treatment of diabetic retinopathy. Ophthalmologica 2007, 221, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Bo, H.; Vilani, V.; Spencer, P.H.; Fu, P. Inhibitory effect of rapamycin on toll-like receptor 4 and interleukin 17 in the early—Stage of diabetic nephropathy. Kidney Blood Press. Res. 2016, 41, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, M.; Bhandari, B.S.; Abboud-Werner, S.; Simone, S.; Abboud, H.E.; Habib, S.L. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J. Am. Soc. Nephrol. 2011, 22, 262–273. [Google Scholar] [CrossRef]
- Rosner, M.; Freilinger, A.; Hengstschlager, M. Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene 2007, 26, 521–531. [Google Scholar] [CrossRef]
- Rosales, I.A.; Mahowald, G.K.; Tomaszewski, K.; Hotta, K.; Iwahara, N.; Otsuka, T.; Tsuji, T.; Takada, Y.; Acheampong, E.; Araujo-Medina, M.; et al. Peritubular capillaritis in chronic active antibody-mediated rejection. J. Am. Soc. Nephrol. 2022, 33, 2306–2319. [Google Scholar] [CrossRef]
- Bissonnette, M.L.Z.; Riazy, M.; Cunningham, A.M.; Gill, J.S. A step toward understanding the story behind the pictures: Molecular diagnostics and the Banff Classification of renal allograft pathology. J. Am. Soc. Nephrol. 2022, 33, 2131–2132. [Google Scholar] [CrossRef]
- D’Elia, J.A.; Bayliss, G.; Weinrauch, L.A. The diabetic cardio renal nexus. Int. J. Mol. Sci. 2022, 23, 7351. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor-Beta (TGF)-B signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Hayashida, T.; Hubchak, C.B.; Hanna, C.; Platanias, L.C.; Schnaper, H.W. TGF-B/Smad3 activates mammalian target of rapamycin comples-1 o promote collagen produced by increasing HIF 1a expression. Am. J. Physiol. Renal Physiol. 2013, 305, F485–F494. [Google Scholar] [CrossRef]
- Tastemur, S.; Olcucuoglu, E.; Karaaslan, M. High perirenal fat volume affects negatively renal function in living renal transplantation. Transplant. Proc. 2022, 54, 1768–1772. [Google Scholar] [CrossRef]
- Bobulescu, I. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Feldcamp, T.; Kribben, A.; Roeser, N.; Senter, R.A.; Weinberg, J.M. Accumulation of fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-re-oxygenation. Am. J. Physiol. Renal Physiol. 2006, 290, F465–F477. [Google Scholar] [CrossRef] [PubMed]
- Feldcamp, T.; Weinberg, J.; Horbelt, M.; Von Kropff, C.; Witzke, O.; Nurnberger, J.; Kribben, A. Evidence for involvement of non-esterified fatty acid-induced protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and re-oxygenation. Nephrol. Dial. Transplant. 2009, 24, 43–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Biesenbach, G.; Margreiter, R.; Konigsrainer, A.; Bosmuller, C.; Janko, O.; Brucke, P.; Gross, C.; Zazgornik, J. Comparison of progression of macrovascular diseases after kidney or pancreas and kidney transplantation in patients with end-stage renal disease. Diabetologia 2000, 43, 231–234. [Google Scholar] [CrossRef]
- Biesenbach, G.; Konigsrainer, A.; Gross, C.; Margreiter, R. Progression of macrovascular disease is reduced in type 1 diabetic patients after more than five years successful combined pancreas-kidney transplantation in comparison to kidney transplantation alone. Transpl. Int. 2005, 18, 1054–1060. [Google Scholar] [CrossRef]
- Lombardo, C.; Perrone, V.G.; Amorese, G.; Vistoli, F.; Baronti, W.; Marchetti, P.; Boggi, V. Update on Pancreatic Transplantation in the Management of Diabetes in Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Fiorina, P.; LaRocca, E.; Venturini, M.; Minicucci, F.; Fermo, I.; Paroni, R.; D’Angelo, A.; Sblendido, M.; DiCarlo, V.; Cris-tallo, M.; et al. Effects of kidney-pancreas transplantation on atherosclerotic risk factors and endothelial function in patients with uremia and type 1 diabetes mellitus. Diabetes 2001, 50, 496–501. [Google Scholar] [CrossRef]
- Larsen, J.L.; Ratanasuwan, T.; Burkman, T.; Lynch, T.; Erickson, J.; Colling, C.; Lane, J.; Mack-Shipman, L.; Lyden, E.; Lo-seke, M.; et al. Carotid intima media thickness is decreased after pancreas transplantation. Transplantation 2002, 73, 936–940. [Google Scholar] [CrossRef]
- Larssen, J.S.; Bragadottir, G.; Redfors, B.; Ricksten, S.-E. Renal function and oxygenation are impaired early after liver transplantation despite hyperdynamic systemic circulation. Crit. Care 2017, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fan, H.; He, O. Pretransplant diabetes mellitus predicts worse outcome of liver transplantation: Evidence from meta-analysis. J. Endocrinol. Invest. 2018, 41, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Orsi, E.; Grancini, V.; Menini, S.; Aghemo, A.; Pugliese, G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2017, 37, 950–962. [Google Scholar] [CrossRef]
- Kim, M.G.; Cho, W.C. Differential diagnosis of diabetes caused by liver cirrhosis and other type 2 diabetes mellitus. Korean J. Hepatol. 2006, 12, 524–529. [Google Scholar] [PubMed]
- Liangpunsakul, S.; Chalasani, N. Lipid mediators of liver injury in nonalcoholic fatty liver disease. Am. J. Physiol. Gastroenterol. Liver Physiol. 2019, 316, 675–681.52. [Google Scholar] [CrossRef]
- Mordier, S.; Iynedjian, P.B. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem. Biophys. Res. Commun. 2007, 362, 206–211. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Non-alcoholic fatty liver disease: Basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Farrell, G.C.; Haczeyni, F.; Chitturi, S. Pathogenesis of NASH: How metabolic complications of over nutrition favors lipo-toxicity and proinflammatory fatty liver disease. Adv. Exp. Med. Biol. 2018, 1001, 19–44. [Google Scholar]
- Minehira, K.; Novel-Chate, V.; Schwarz, J.M.; Gillet, M.; Darioli, R.; Chiolero, R.; Tappy, L. Hepatic de novo lipogenesis after liver transplantation. J. Parenter. Enteral Nutr. 2001, 25, 229–235. [Google Scholar] [CrossRef]
- Stegall, M.D.; Everson, G.T.; Schroter, G.; Karrer, F.; Bilir, B.; Sternberg, T.; Shrestha, R.; Wachs, M.; Kam, I. Prednisone withdrawal late after liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology 1997, 25, 173–177. [Google Scholar] [CrossRef]
- Wang, Y.; Voy, B.J.; Urs, S.; Kim, S.; Soltani–Bejnood, M.; Quigley, M.; Heo, Y.-R.; Standridge, M.; Andersen, B.; Dhar, M.; et al. The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr. 2004, 134, 1032–1038. [Google Scholar] [CrossRef]
- Berdanier, C.D. Role of glucocorticoids in the regulation of lipogenesis. FASEB J. 1989, 3, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Perez, M.; Gonzalez-Grande, R.; Guzman, E.O.; Trillo, V.A.; Rodrigo-Lopez, J.M. Metabolic complications in liver transplantation recipients. World J. Gastroenterol. 2016, 22, 6416–6423. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kneteman, N.; Lilly, L.; Marotta, P.; Peltekian, K.; Scudamore, C.; Tchervenkov, J. Tacrolimus as intervention in the treatment of hyperlipidemia after liver transplantation. Transplantation 2006, 82, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, L.A.; Smidt, K.; Mortensen, D.M.; Carstens, J.; Jorgensen, J.A.; Rungby, J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1Ebeta-cells. Br. J. Pharmacol. 2011, 162, 136–146. [Google Scholar] [CrossRef]
- Nordheim, E.; Birkeland, K.I.; Asberg, A.; Hartmann, A.; Horneland, R.; Jenssen, T. Preserved insulin secretion and kidney function in recipients with functional pancreas grafts 1 year after transplantation: A single-center prospective observation study. Eur. J. Endocrinol. 2018, 179, 251–259. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Fasting and rapamycin: Diabetes versus benevolent glucose intolerance. Cell Death Dis. 2019, 10, 607. [Google Scholar] [CrossRef]
- Iqbal, A.; Zhou, K.; Kashyap, S.R.; Lansang, M.B. Early renal transplant hyperglycemia. J. Clin. Endocrinol. Metab. 2022, 107, 549–562. [Google Scholar] [CrossRef]
- Hoang, K.; Chen, I.; Reaven, G.; Zhang, L.; Ross, H.; Billingham, M.; Valentine, H. Diabetes and dyslipidemia: A new model for transplant coronary artery disease. Circulation 1998, 97, 2160–2168. [Google Scholar] [CrossRef]
- Radu, R.G.; Fujimoto, S.; Mukai, E.; Takehiro, M.; Shimono, D.; Nabe, K.; Shimodahira, M.; Kominato, R.; Aramaki, Y.; Nishi, Y.; et al. Tacrolimus suppresses glucose-induced insulin from pancreatic islets by reducing glucokinase activity. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E365–E371. [Google Scholar] [CrossRef][Green Version]
- Tong, L.; Li, W.; Huang, Y.; Zhou, F.; Zhou, Y.; Zhan, L.; Li, J.; Song, Z.; Yu, M.; Zhou, C.; et al. Tacrolimus inhibits insulin release and promotes apoptosis of Mon6 cells through the inhibition of the P13k/Akt/mTOR pathway. Mol. Med. Rep. 2021, 24, 658. [Google Scholar] [CrossRef]
- D’Elia, J.A.; Mulla, C.; Weinrauch, L.A. Variations in Glucose/C-Peptide in persons with diabetes mellitus associated with renal function. Diabetes Res. Clin. Prac. 2019, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, J.A.; Kaldany, A.; Miller, D.G.; Rolla, A.; Weinrauch, L.A. Elimination for requirement of exogenous insulin therapy in diabetic renal failure. Ren. Fail. 1982, 6, 75–82. [Google Scholar] [CrossRef]
- Chmielnicka, K.; Heleniak, Z.; Debska-Slizien, A. Dyslipidemia in renal transplant recipients. Transplantology 2022, 3, 188–199. [Google Scholar] [CrossRef]
- Rahmani, M.; Cruz, R.P.; Granville, D.J.; McManus, B.M. Allograft Vasculopathy Versus Atherosclerosis. Circ. Res. 2006, 99, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.A. Statins and cardiac allograft vasculopathy after heart transplantation. Semin. Vasc. Med. 2004, 4, 401–406. [Google Scholar] [CrossRef]
- Valantine, H.J. Cardiac allograft vasculopathy after heart transplantation: Risk factors and management. Heart Lung Transpl. 2004, 23 (Suppl. 5), S187–S193. [Google Scholar] [CrossRef]
- Bagley, J.; Williams, L.; Hyde, M.; Birriel, C.R.; Iacomini, J. Hyperlipidemia and Allograft Rejection. Curr. Transplant. Rep. 2019, 6, 90–98. [Google Scholar] [CrossRef]
- Opazo-Rios, L.; Mas, S.; Marin-Royo, G.; Mezzano, S.; Gomez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and Diabetic Nephropathy: Novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Perkovic, V.; Johnson, D.W.; Nigwekar, S.U.; Hegbrant, J.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2014, CD005019. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Duell, P.B. Management of dyslipidemia in adult solid organ transplant recipients. J. Clin. Lipidol. 2019, 13, 231–245. [Google Scholar] [CrossRef]
- Cardarelli, F.; Saidman, S.; Theruvath, T.; Tolkoff-Rubin, N.; Cosimi, A.B.; Pascual, M. The problem of late allograft loss in kidney transplantation Minerva. Urol. Nefrol. 2003, 55, 1–11. [Google Scholar]
- Agarwal, A.; Prasad, G.V. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 6, 125–134. [Google Scholar] [CrossRef]
- Chowdhury, T.A. Post-transplant diabetes mellitus. Clin. Med. 2019, 19, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Escasany, E.; Izquierdo-Lahuerta, A.; Medina-Gomez, G. Underlying mechanisms of renal lipotoxicity in obesity. Nephron 2019, 143, 28–32. [Google Scholar] [CrossRef]
- Hecking, M.; Sharif, A.; Eller, K.; Jenssen, T. Management of post-transplant diabetes: Immunosuppression, early prevention, and antidiabetics. Transpl. Int. 2021, 34, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Crowther, C.A.; Samuel, D.; McCowan, L.M.E.; Edlin, R.; Tran, T.; McKinlay, C.J. for the GEMS Trial Group. Lower versus higher glycemic criteria for diagnosis of gestational diabetes. N. Engl. J. Med. 2022, 387, 587–598. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Kota, K. Bariatric surgery and diabetes remission: Who would have thought it? Indian. J. Diabetes Metab. 2015, 19, 563–576. [Google Scholar] [CrossRef]
- Chumacova-Orin, M.; Vanetta, C.; Moris, D.P.; Guerron, A.D. Diabetes mellitus remission after bariatric surgery. World J. Diabetes 2021, 12, 1093–1158. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. mTOR inhibitors improve both humeral and cellular responses to SARS-CoV-2 mRNA BNT 16b2 vaccine in kidney transplantation recipients. Am. J. Transplant. 2022, 22, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips GL 2nd Robertson, R.P.; Rubino, F.; Kahn, R.; et al. How do we define cure of diabetes? Diabetes Care. 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus report: Definition and interpretation of remission in type 2 diabetes. Diabetologia. 2021, 64, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Gelfand, J.; D’Agati, V.; Tomaszewski, J.; Appel, G.; Hardy, M.; Pirani, C.L. De novo membranous glomerulopathy in renal allografts: A review of ten cases and review of the literature. Am. J. Kidney Dis. 1989, 14, 131–144. [Google Scholar] [CrossRef]
- Markowitz, G. Membranous glomerulopathy: Emphasis on secondary forms and disease variants. Adv. Anat. Pathol. 2001, 8, 119–125.63. [Google Scholar] [CrossRef]
- Bhadauria, D.; Chellappan, A.; Kaul, A.; Etta, P.; Badri, V.; Sharma RKPrasad, N.; Gupta, A.; Jain, M. Idiopathic membranous glomerulopathy in patients with diabetes mellitus: A diagnostic and therapeutic quandary. Clin. Kidney J. 2018, 11, 46–56. [Google Scholar] [CrossRef]
| Drug | Dysmetabolic Effect | Observation |
|---|---|---|
| Prednisone | Increased lipogenesis and gluconeogenesis | Rat liver study (rat model) |
| Azathioprine | Increased triglycerides | Transplant patients treated with azathioprine |
| Mycophenolate mofetil | Decreased atheroma size | Atheroma prone rabbit on atherogenic diet |
| Calcineurin inhibitors (cyclosporin, tacrolimus) | Decreased insulin secretion | Humans post-organ-transplant |
| mTOR * inhibitors (sirolimus, temsirolimus, everolimus) | Increased insulin resistance | Hepatocytes (rat), muscle cell cultures |
|
|
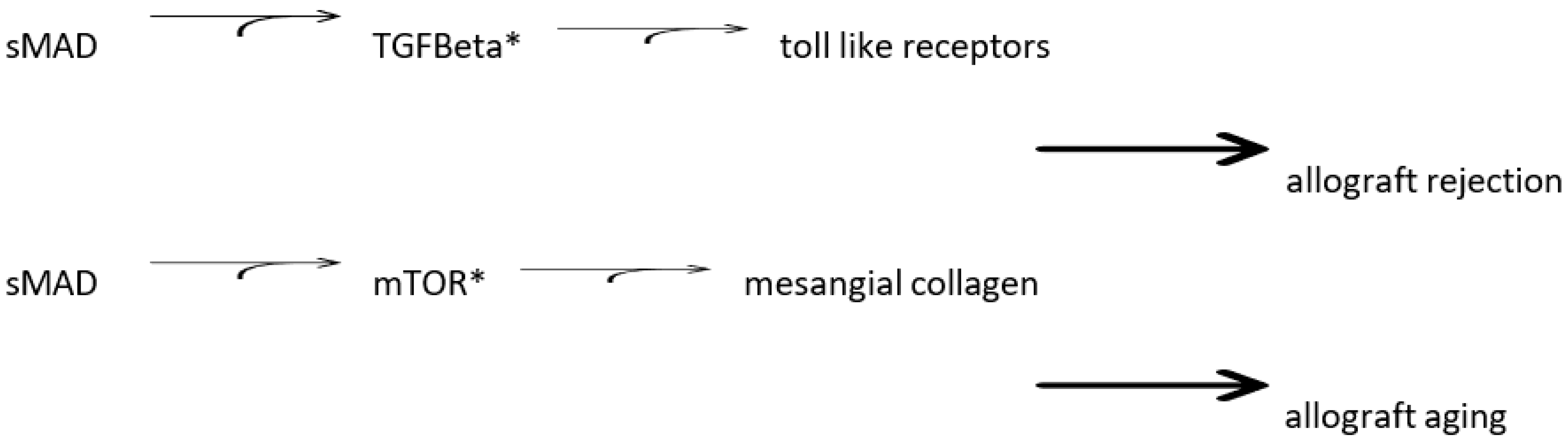
| Pathologic Anatomy | |||||
|---|---|---|---|---|---|
| Mechanism | |||||
| AGE * | sMAD ** | TGF-Beta *** | mTOR **** | ||
| Pericyte/Fibrosis | Hyperglycemia | + | + | - | - |
| - | Fibronectin | + | + | - | - |
| Collagen 4 | + | + | - | - | |
| Artery/Atheroma | |||||
| Hyperlipidemia | - | - | + | + | |
| Toll-like receptors | - | - | + | + | |
| Interleukin 17 | - | - | + | + | |
| Cardiac Muscle Hypertrophy | |||||
| Hypertension | - | - | - | - | |
| Fibrosis | + | + | - | - |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, J.A.; Weinrauch, L.A. Hyperglycemia and Hyperlipidemia with Kidney or Liver Transplantation: A Review. Biology 2023, 12, 1185. https://doi.org/10.3390/biology12091185
D’Elia JA, Weinrauch LA. Hyperglycemia and Hyperlipidemia with Kidney or Liver Transplantation: A Review. Biology. 2023; 12(9):1185. https://doi.org/10.3390/biology12091185
Chicago/Turabian StyleD’Elia, John A., and Larry A. Weinrauch. 2023. "Hyperglycemia and Hyperlipidemia with Kidney or Liver Transplantation: A Review" Biology 12, no. 9: 1185. https://doi.org/10.3390/biology12091185
APA StyleD’Elia, J. A., & Weinrauch, L. A. (2023). Hyperglycemia and Hyperlipidemia with Kidney or Liver Transplantation: A Review. Biology, 12(9), 1185. https://doi.org/10.3390/biology12091185





