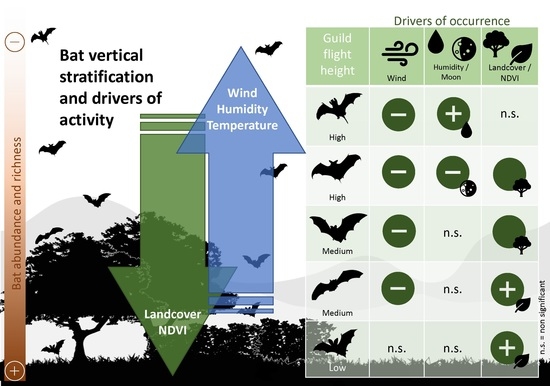
Guild Vertical Stratification and Drivers of Bat Foraging in a Semi-Arid Tropical Region, Kenya
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
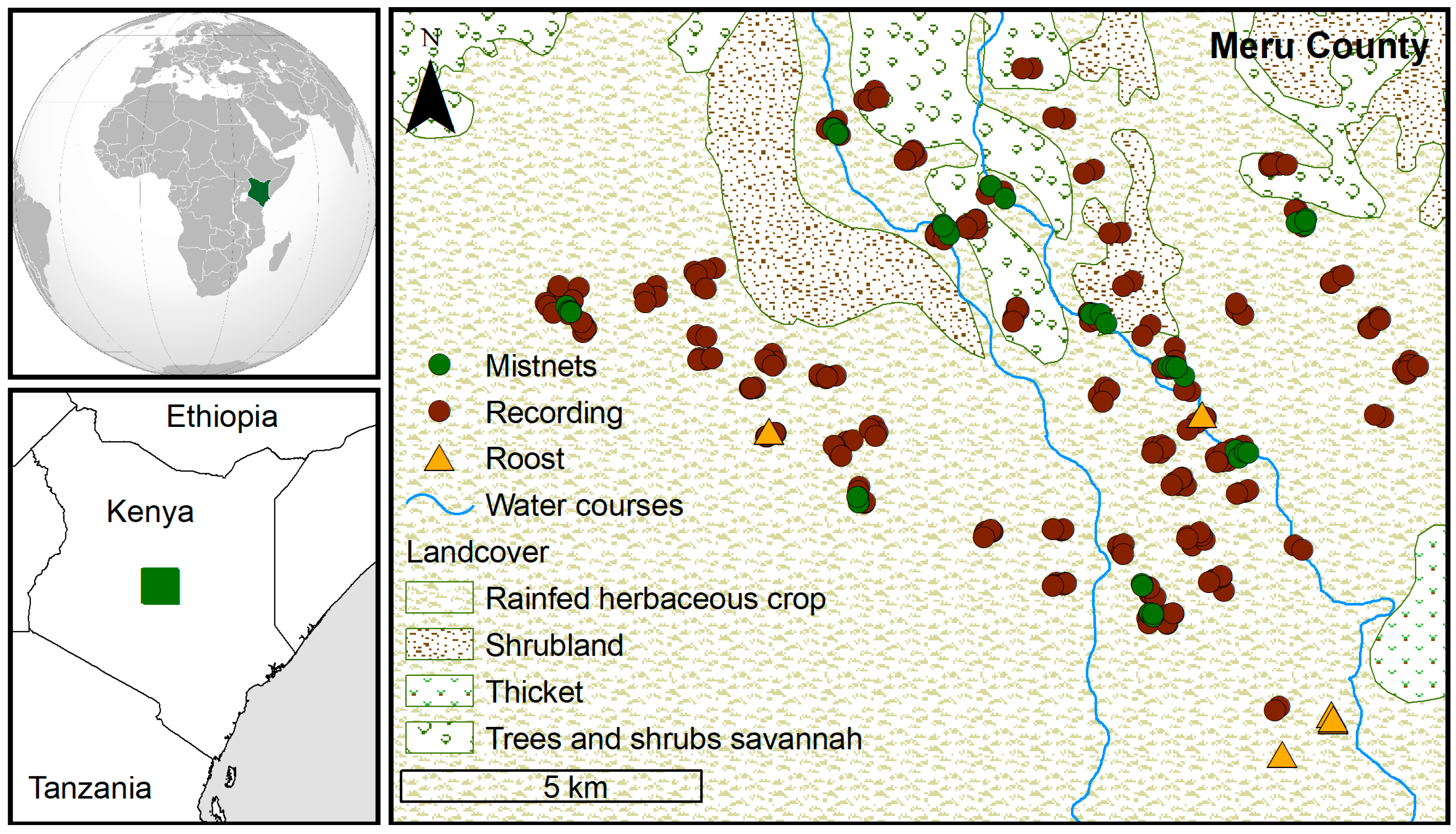
2.1. Study Area
2.2. Bat Sampling
2.3. Environmental and Landscape Descriptors
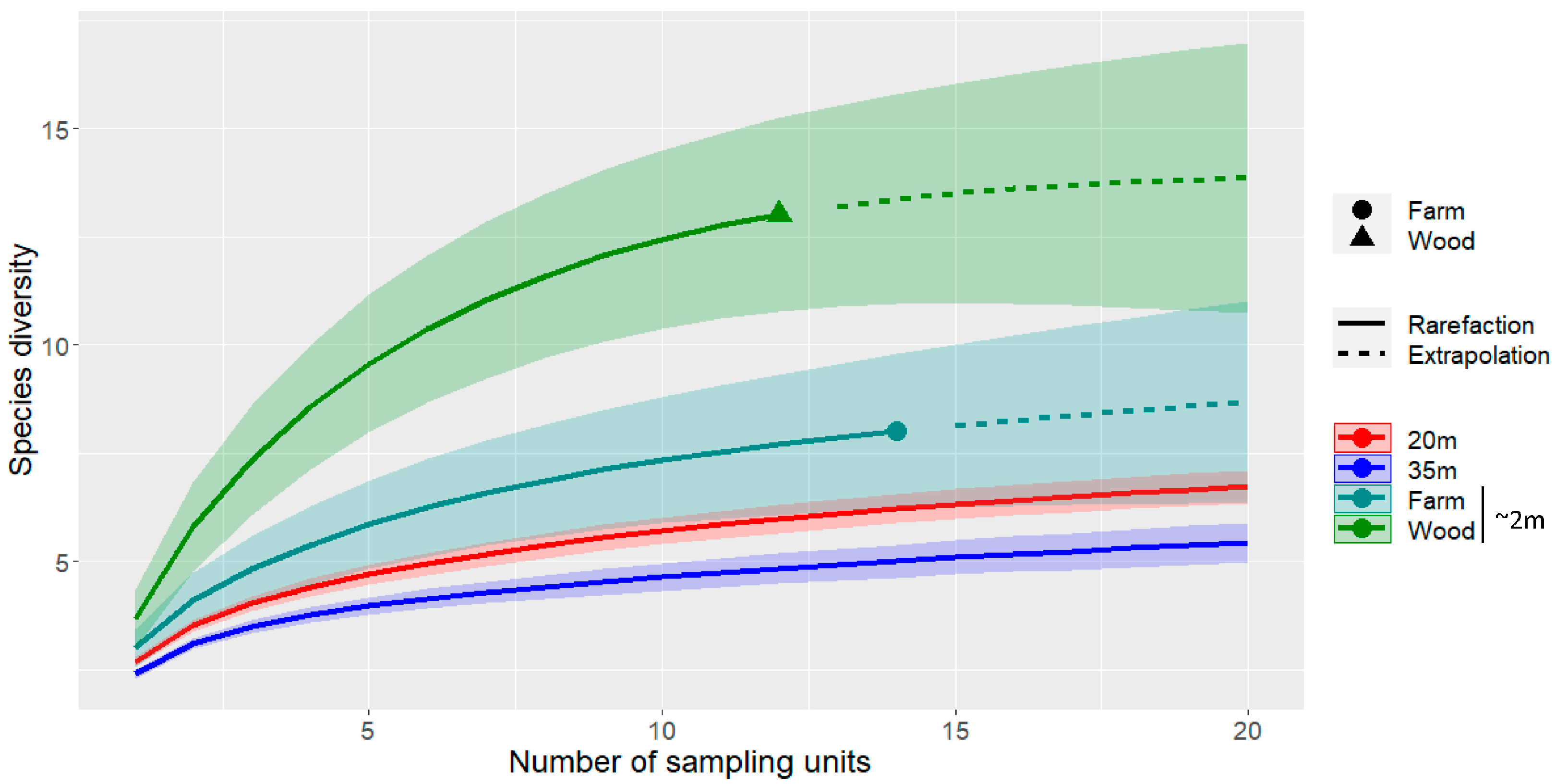
2.4. Data Analysis
2.4.1. Ground-Level Bat Occurrence
| Guild | Taxa | Approx. Flying Height (m) | Mean Recording Distance (m) | |
|---|---|---|---|---|
| High-flying bats | High_1 | Monospecific guild, including only Otomops harrisoni | 15–550 | N/A |
| High_2 | All species of the family Molossidae, other than O. harrisoni | 5–100 | M. pumilus: 12.0 ± 2.00 M. condylurus: 15.0 ± 2.67 | |
| Medium- to high-flying bats | Medium_3 | Genera Scotophilus and Scotoecus | 3–15 | [S. dinganii: 17.5] [S.viridis: 12.5] |
| Medium_4 | Genera Pipistrellus, Afronycteris, Neoromicia, Nycticeinops and Miniopterus | 2–10 | A. nanus: 5.8 ± 1.07 N. schlieffeni: 15.0 [M. natalensis: 5.0] | |
| Low-flying bats | Low_5 | Genera Lavia, Cardioderma and Nycteris | <3.5 | N/A |
| Low_6 | Genera Rhinolophus, Hipposideros and Triaenops | <1.5 | H. caffer: 0.2 ± 0.07 [R. darlingi: 2.0] |
2.4.2. High-Flying Bat Activity
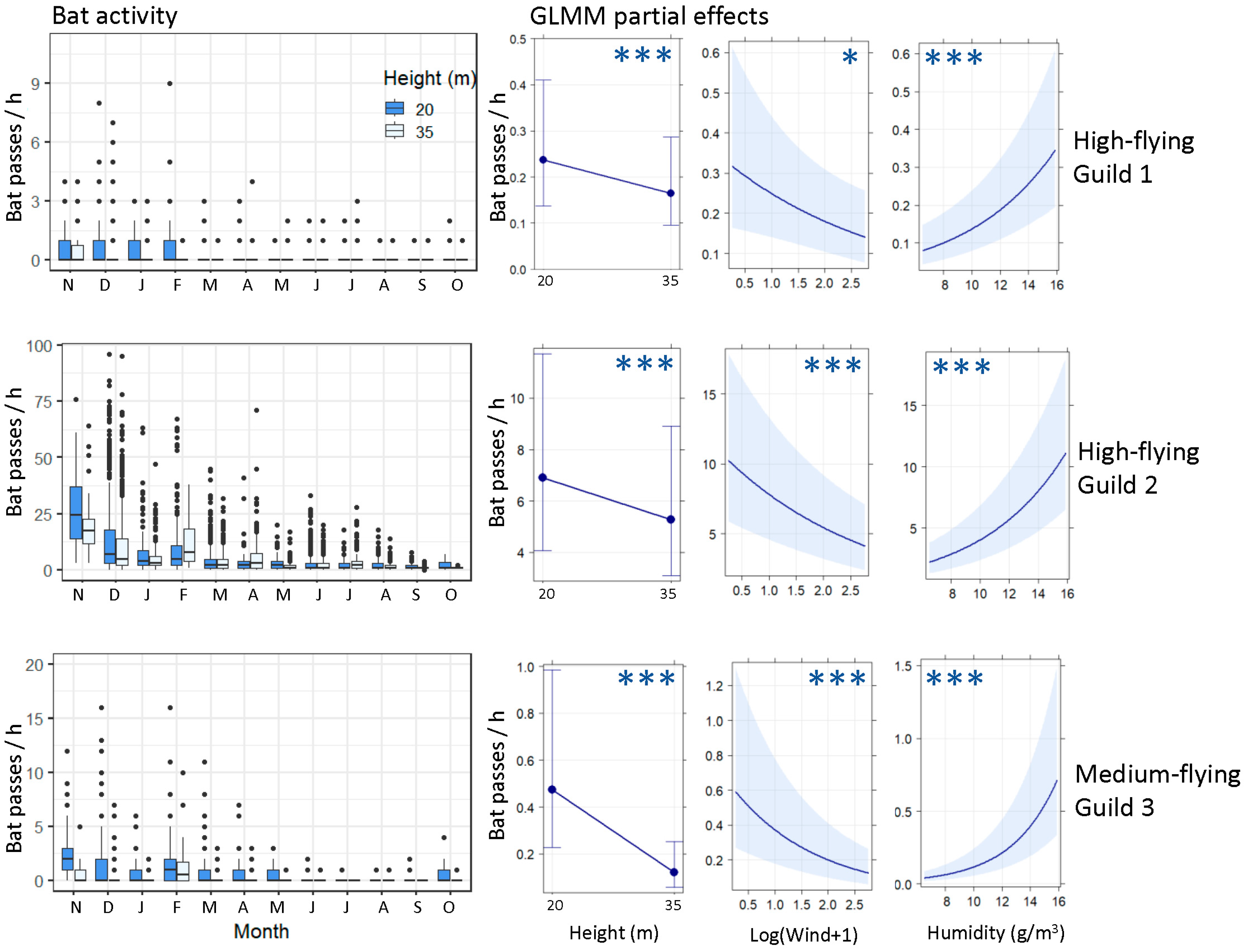
3. Results
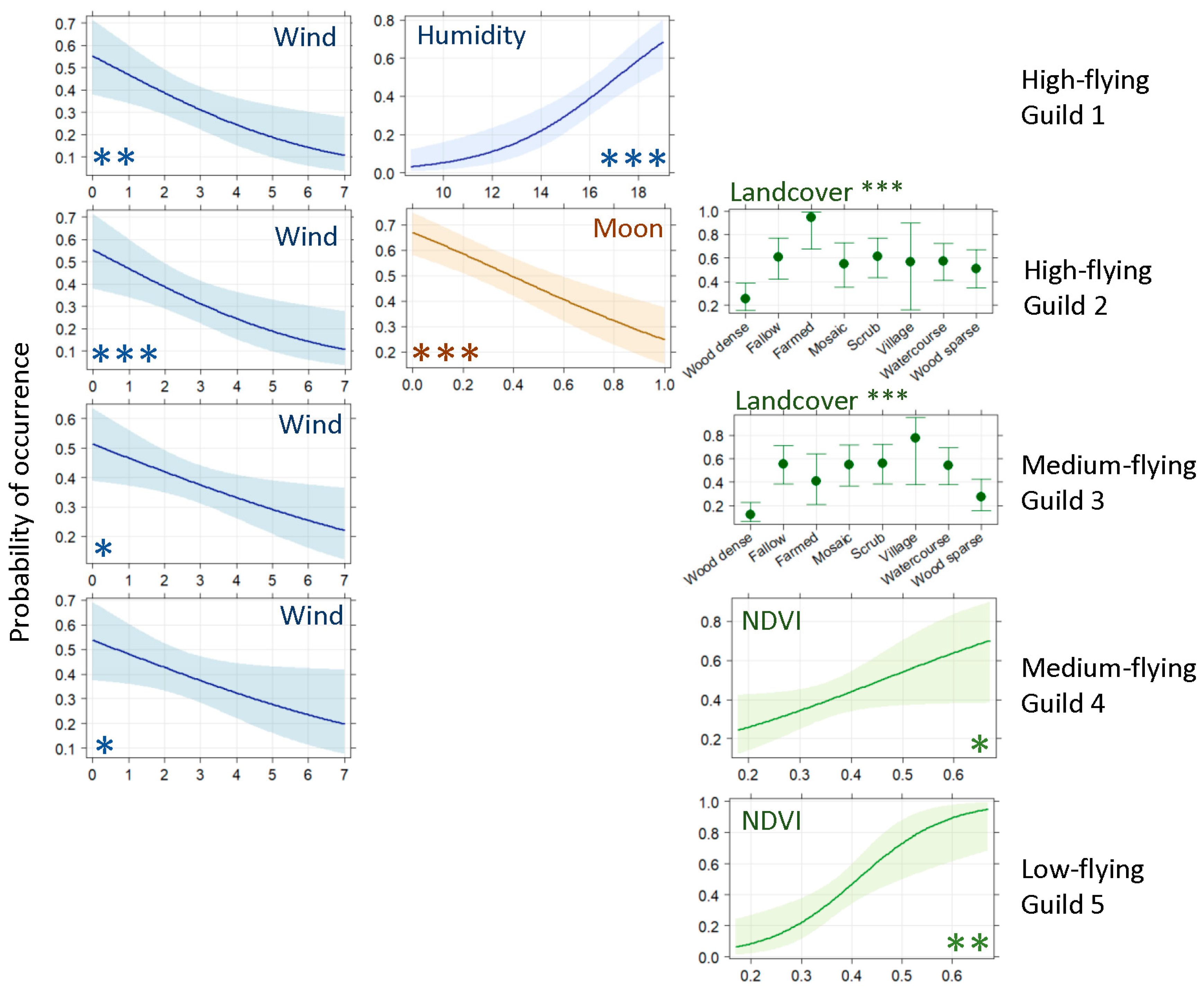
3.1. Ground-Level Bat Activity
3.2. High-Flying Bat Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oakleaf, J.R.; Kennedy, C.M.; Baruch-Mordo, S.; West, P.C.; Gerber, J.S.; Jarvis, L.; Kiesecker, J. A World at risk: Aggregating development trends to forecast global habitat conversion. PLoS ONE 2015, 10, e0138334. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jellason, N.P.; Robinson, E.J.Z.; Chapman, A.S.A.; Neina, D.; Devenish, A.J.M.; Po, J.Y.T.; Adolph, B. A Systematic review of drivers and constraints on agricultural expansion in Sub-Saharan Africa. Land 2021, 10, 332. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Ruesch, A.S.; Achard, F.; Clayton, M.K.; Holmgren, P.; Ramankutty, N.; Foley, J.A. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef]
- Weng, L.; Boedhihartono, A.K.; Dirks, P.H.G.M.; Dixon, J.; Lubis, M.I.; Sayer, J.A. Mineral industries, growth corridors and agricultural development in Africa. Glob. Food Secur. 2013, 2, 195–202. [Google Scholar] [CrossRef]
- Maitima, J.M.; Mugatha, S.M.; Reid, R.S.; Gachimbi, L.N.; Majule, A.; Lyaruu, H.; Pomery, D.; Mathai, S.; Mugisha, S. The linkages between land use change, land degradation and biodiversity across East Africa. Afr. J. Environ. Sci. Technol. 2009, 3, 310–325. [Google Scholar]
- Scholes, R.J.; Biggs, R. A biodiversity intactness index. Nature 2005, 434, 45–49. [Google Scholar] [CrossRef]
- Ahrends, A.; Burgess, N.D.; Milledge, S.A.; Bulling, M.T.; Fisher, B.; Smart, J.C.; Clarke, G.P.; Mhoro, B.E.; Lewis, S.L. Predictable waves of sequential forest degradation and biodiversity loss spreading from an African city. Proc. Natl. Acad. Sci. USA 2010, 107, 14556–14561. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galetti, M.; Smith, F.A.; Svenning, J.-C.; Terborgh, J.W. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. USA 2016, 113, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Hempson, G.P.; Archibald, S.; Bond, W.J. The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 2017, 7, 17196. [Google Scholar] [CrossRef]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- Arnett, E.B.; Baerwald, E.F.; Mathews, F.; Rodrigues, L.; Rodríguez-Durán, A.; Rydell, J.; Villegas-Patraca, R.; Voigt, C.C. Impacts of wind energy development on bats: A global perspective. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016; pp. 295–323. [Google Scholar]
- Bull, J.W.; Suttle, K.B.; Gordon, A.; Singh, N.J.; Milner-Gulland, E.J. Biodiversity offsets in theory and practice. Oryx 2013, 47, 369–380. [Google Scholar] [CrossRef]
- Suding, K.N. Toward an era of restoration in ecology: Successes, failures, and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 465–487. [Google Scholar] [CrossRef]
- Ibebuchi, C.C. Revisiting the 1992 severe drought episode in South Africa: The role of El Niño in the anomalies of atmospheric circulation types in Africa south of the equator. Theor. Appl. Climatol. 2021, 146, 723–740. [Google Scholar] [CrossRef]
- Nguyen, P.-L.; Min, S.-K.; Kim, Y.-H. Combined impacts of the El Niño-Southern Oscillation and Pacific Decadal Oscillation on global droughts assessed using the standardized precipitation evapotranspiration index. Int. J. Climatol. 2021, 41, E1645–E1662. [Google Scholar] [CrossRef]
- Prăvălie, R.; Bandoc, G.; Patriche, C.; Sternberg, T. Recent changes in global drylands: Evidences from two major aridity databases. Catena 2019, 178, 209–231. [Google Scholar] [CrossRef]
- Peng, Y.; Fu, B.; Zhang, L.; Yu, X.; Fu, C.; Diop, S.; Hirwa, H.; Guisse, A.; Li, F. Global Dryland Ecosystem Programme (G-DEP): Africa consultative meeting report. J. Arid Land 2020, 12, 538–544. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hochard, J.P. Land degradation and poverty. Nat. Sustain. 2018, 1, 623–631. [Google Scholar] [CrossRef]
- Burke, M.B.; Miguel, E.; Satyanath, S.; Dykema, J.A.; Lobell, D.B. Warming increases the risk of civil war in Africa. Proc. Natl. Acad. Sci. USA 2009, 106, 20670–20674. [Google Scholar] [CrossRef]
- Zhang, Y.; Tariq, A.; Hughes, A.C.; Hong, D.; Wei, F.; Sun, H.; Sardans, J.; Peñuelas, J.; Perry, G.; Qiao, J.; et al. Challenges and solutions to biodiversity conservation in arid lands. Sci. Total Environ. 2023, 857, 159695. [Google Scholar] [CrossRef]
- Wilson, D.E.; Mittermeier, R.A. Handbook of the Mammals of the World: Hoofed Mammals; Lynx Ediciones: Barcelona, Spain, 2011; Volume 2. [Google Scholar]
- Lison, F.; Jiménez-Franco, M.V.; Altamirano, A.; Haz, A.; Calvo, J.F.; Jones, G. Bat ecology and conservation in semi-arid and arid landscapes: A global systematic review. Mammal Rev. 2020, 50, 52–67. [Google Scholar] [CrossRef]
- Naranjo, M.E.; Rengifo, C.; Soriano, P.J. Effect of ingestion by bats and birds on seed germination of Stenocereus griseus and Subpilocereus repandus (Cactaceae). J. Trop. Ecol. 2003, 19, 19–25. [Google Scholar] [CrossRef]
- Schäckermann, J.; Morris, E.J.; Alberdi, A.; Razgour, O.; Korine, C. The contribution of desert-dwelling bats to pest control in hyper-arid date agriculture. Diversity 2022, 14, 1034. [Google Scholar] [CrossRef]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Monadjem, A.; Conenna, I.; Taylor, P.J.; Schoeman, M.C. Species richness patterns and functional traits of the bat fauna of arid southern Africa. Hystrix Ital. J. Mammal. 2018, 29, 19–24. [Google Scholar] [CrossRef]
- Russo, D.; Salinas-Ramos, V.B.; Cistrone, L.; Smeraldo, S.; Bosso, L.; Ancillotto, L. Do we need to use bats as bioindicators? Biology 2021, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Rainho, A.; Palmeirim, J.M. Understanding the long term consequences of fragmentation: Lessons from the bats of Bijagós (Guinea-Bissau, West Africa). Hystrix 2017, 28, 173–179. [Google Scholar] [CrossRef]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Schwarz, C.J.; Fahr, J. Activity patterns and habitat preferences of insectivorous bats in a West African forest–savanna mosaic. J. Trop. Ecol. 2004, 20, 397–407. [Google Scholar] [CrossRef]
- Karp, D.S.; Daily, G.C. Cascading effects of insectivorous birds and bats in tropical coffee plantations. Ecology 2014, 95, 1065–1074. [Google Scholar] [CrossRef]
- Ramírez-Fráncel, L.A.; García-Herrera, L.V.; Losada-Prado, S.; Reinoso-Flórez, G.; Sánchez-Hernández, A.; Estrada-Villegas, S.; Lim, B.K.; Guevara, G. Bats and their vital ecosystem services: A global review. Integr. Zool. 2022, 17, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.T.; Monadjem, A.; Röder, T.; McCleery, R.A. Response of bat activity to land cover and land use in savannas is scale-, season-, and guild-specific. Biol. Conserv. 2020, 241, 108245. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Vaughan, T.A. Ecological observations on an East African bat community. Mammalia 1980, 44, 485–496. [Google Scholar] [CrossRef]
- Roemer, C.; Coulon, A.; Disca, T.; Bas, Y. Bat sonar and wing morphology predict species vertical niche. J. Acoust. Soc. Am. 2019, 145, 3242–3251. [Google Scholar] [CrossRef]
- Erasmy, M.; Leuschner, C.; Balkenhol, N.; Dietz, M. Three-dimensional stratification pattern in an old-growth lowland forest: How does height in canopy and season influence temperate bat activity? Ecol. Evol. 2021, 11, 17273–17288. [Google Scholar] [CrossRef]
- Yoh, N.; Clarke, J.A.; López-Baucells, A.; Mas, M.; Bobrowiec, P.E.; Rocha, R.; Meyer, C.F. Edge effects and vertical stratification of aerial insectivorous bats across the interface of primary-secondary Amazonian rainforest. PLoS ONE 2022, 17, e0274637. [Google Scholar] [CrossRef]
- Marques, J.T.; Ramos Pereira, M.J.; Palmeirim, J.M. Patterns in the use of rainforest vertical space by Neotropical aerial insectivorous bats: All the action is up in the canopy. Ecography 2016, 39, 476–486. [Google Scholar] [CrossRef]
- Henry, M.; Barriere, P.; Gautier-Hion, A.; Colyn, M. Species composition, abundance and vertical stratification of a bat community (Megachiroptera: Pteropodidae) in a West African rain forest. J. Trop. Ecol. 2004, 20, 21–29. [Google Scholar] [CrossRef]
- Musila, S.; Gichuki, N.; Castro-Arellano, I.; Rainho, A. Composition and diversity of bat assemblages at Arabuko-Sokoke Forest and the adjacent farmlands, Kenya. Mammalia 2020, 84, 121–135. [Google Scholar] [CrossRef]
- Gorman, K.M.; Barr, E.L.; Ries, L.; Nocera, T.; Ford, W.M. Bat activity patterns relative to temporal and weather effects in a temperate coastal environment. Glob. Ecol. Conserv. 2021, 30, e01769. [Google Scholar] [CrossRef]
- Voigt, C.C.; Currie, S.E.; Fritze, M.; Roeleke, M.; Lindecke, O. Conservation strategies for bats flying at high altitudes. BioScience 2018, 68, 427–435. [Google Scholar] [CrossRef]
- Collins, J. Bat Surveys for Professional Ecologists: Good Practice Guidelines, 3rd ed.; The Bat Conservation Trust: London, UK, 2016. [Google Scholar]
- Kunz, T.H.; Betke, M.; Hristov, N.I.; Vonhof, M.J. Methods for assessing colony size, population size, and relative abundance of bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 133–157. [Google Scholar]
- Patterson, B.D.; Webala, P.W. Keys to the bats (Mammalia: Chiroptera) of East Africa. Fieldiana Life Earth Sci. 2012, 2012, 1–60. [Google Scholar] [CrossRef]
- Happold, M.; Happold, D.C.D. (Eds.) Mammals of Africa: Hedgehogs, Shrews and Bats; Bloomsbury Publishing: London, UK, 2013; Volume IV, p. 800. [Google Scholar]
- Claessen, C.; De Vree, F. Systematic and taxonomic notes on the Epomophorus anurus-labiatus-minor complex with the description of a new species (Mammalia: Chiroptera: Pteropodidae). Senckenberg. Biol. 1991, 71, 209–238. [Google Scholar]
- Musila, S.; Monadjem, A.; Webala, P.W.; Patterson, B.D.; Hutterer, R.; Jong, Y.A.D.; Butynski, T.M.; Mwangi, G.; Chen, Z.-Z.; Jiang, X.-L. An annotated checklist of mammals of Kenya. Zool. Res. 2019, 40, 3–52. [Google Scholar] [CrossRef]
- Monadjem, A.; Demos, T.C.; Dalton, D.L.; Webala, P.W.; Musila, S.; Kerbis Peterhans, J.C.; Patterson, B.D. A revision of pipistrelle-like bats (Mammalia: Chiroptera: Vespertilionidae) in East Africa with the description of new genera and species. Zool. J. Linn. Soc. 2021, 191, 1114–1146. [Google Scholar] [CrossRef]
- Demos, T.C.; Webala, P.W.; Bartonjo, M.; Patterson, B.D. Hidden diversity of African yellow house bats (Vespertilionidae, Scotophilus): Insights from multilocus phylogenetics and lineage delimitation. Front. Ecol. Evol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Ralph, T.; Richards, L.; Taylor, P.; Napier, M.; Lamb, J. Revision of Afro-Malagasy Otomops (Chiroptera: Molossidae) with the description of a new Afro-Arabion species. Zootaxa 2015, 4057, 1–49. [Google Scholar] [CrossRef][Green Version]
- Taylor, P. Echolocation calls of twenty southern African bat species. S. Afr. J. Zool. 1999, 34, 114–124. [Google Scholar] [CrossRef]
- Monadjem, A.; Taylor, P.J.; Cotterill, W.; Schoeman, M. Bats of Southern and Central Africa: A Biogeographic and Taxonomic Synthesis; Wits University Press: Johannesburg, South Africa, 2010. [Google Scholar]
- Monadjem, A.; Rasmussen, M.; Van der Mewe, D.C. Echolocation calls and wing morphology of selected bats in western Uganda. Durb. Nat. Sci. Mus. Novit. 2011, 34, 29–44. [Google Scholar]
- Collen, A. The Evolution of Echolocation in Bats: A Comparative Approach; UCL (University College London): London, UK, 2012. [Google Scholar]
- Szewczak, J. SonoBat: Bat Call Analysis Software; 3.2; SonoBat: Arcata, CA, USA, 2013. [Google Scholar]
- Jacobs, D.S.; Barclay, R.M.R.; Walker, M.H. The allometry of echolocation call frequencies of insectivorous bats: Why do some species deviate from the pattern? Oecologia 2007, 152, 583–594. [Google Scholar] [CrossRef]
- Norberg, U.M.; Rayner, J.M. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1987, 316, 335–427. [Google Scholar] [CrossRef]
- Fenton, M.; Griffin, D. High-altitude pursuit of insects by echolocating bats. J. Mammal. 1997, 78, 247–250. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: www.R-project.org (accessed on 8 September 2022).
- Cryan, P.M.; Barclay, R.M.R. Causes of bat fatalities at wind turbines: Hypotheses and predictions. J. Mammal. 2009, 90, 1330–1340. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Newcastle, UK, 2018. [Google Scholar]
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef]
- Chao, A.; Henderson, P.A.; Chiu, C.H.; Moyes, F.; Hu, K.H.; Dornelas, M.; Magurran, A.E. Measuring temporal change in alpha diversity: A framework integrating taxonomic, phylogenetic and functional diversity and the iNEXT. 3D standardization. Methods Ecol. Evol. 2021, 12, 1926–1940. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics, 3rd ed.; HarperCollins Publishers: New York, NY, USA, 1996; p. 880. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference; Springer: New York, NY, USA, 2004; p. 488. [Google Scholar]
- Roscioni, F.; Rebelo, H.; Russo, D.; Carranza, M.L.; Di Febbraro, M.; Loy, A. A modelling approach to infer the effects of wind farms on landscape connectivity for bats. Landsc. Ecol. 2014, 29, 891–903. [Google Scholar] [CrossRef]
- Monadjem, A.; Shapiro, J.T.; Mtsetfwa, F.; Reside, A.E.; McCleery, R.A. Acoustic call library and detection distances for bats of Swaziland. Acta Chiropterologica 2017, 19, 175–187. [Google Scholar] [CrossRef]
- Fenton, M.B. A technique for monitoring bat activity with results obtained from different environments in southern Ontario. Can. J. Zool. 1970, 48, 847–851. [Google Scholar] [CrossRef]
- Richards, L.R. Otomops harrisoni. The IUCN Red List of Threatened Species 2017: E.T95558305A95558309; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Taylor, P.J.; Monadjem, A.; Steyn, J.N. Seasonal patterns of habitat use by insectivorous bats in a subtropical African agro-ecosystem dominated by macadamia orchards. Afr. J. Ecol. 2013, 51, 552–561. [Google Scholar] [CrossRef]
- Mushabati, L.M.; Eiseb, S.J.; Benda, P.; Laverty, T.M. Effects of lunar phase and temperature on bat activity and species richness at varying altitudes in the Kunene Region, Namibia. Afr. J. Ecol. 2022, 60, 467–480. [Google Scholar] [CrossRef]
- McCracken, G.F. Bats aloft: A study of high-altitude feeding. Bats 1996, 14, 7–10. [Google Scholar]
- Voigt, C.C.; Holderied, M.W. High manoeuvring costs force narrow-winged molossid bats to forage in open space. J. Comp. Physiol. B 2012, 182, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.S.; Bernard, R.T.F. Rainfall, food abundance and timing of parturition in African bats. Oecologia 1997, 111, 309–317. [Google Scholar] [CrossRef]
- Musila, S.; Bogdanowicz, W.; Syingi, R.; Zuhura, A.; Chylarecki, P.; Rydell, J. No lunar phobia in insectivorous bats in Kenya. Mamm. Biol. 2019, 95, 77–84. [Google Scholar] [CrossRef]
- Fenton, M.; Boyle, N.H.; Harrison, T.; Oxley, D. Activity patterns, habitat use, and prey selection by some African insectivorous bats. Biotropica 1977, 9, 73–85. [Google Scholar] [CrossRef]
- Monadjem, A.; Reside, A. The influence of riparian vegetation on the distribution and abundance of bats in an African savanna. Acta Chiropterologica 2008, 10, 339–348. [Google Scholar] [CrossRef]
- Herkt, K.M.B.; Barnikel, G.; Skidmore, A.K.; Fahr, J. A high-resolution model of bat diversity and endemism for continental Africa. Ecol. Model. 2016, 320, 9–28. [Google Scholar] [CrossRef]
- Marsden, G.E.; Vosloo, D.; Schoeman, M.C. Urban tolerance is phylogenetically constrained and mediated by pre-adaptations in African bats. Ecol. Evol. 2023, 13, e9840. [Google Scholar] [CrossRef]
- Hackett, T.D.; Korine, C.; Holderied, M.W. The Importance of Acacia Trees for Insectivorous Bats and Arthropods in the Arava Desert. PLoS ONE 2013, 8, e52999. [Google Scholar] [CrossRef]
- Kolkert, H.; Andrew, R.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. 2020, 10, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- ESMAP. Global Solar Atlas 2.0 Technical Report; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Arnett, E.B.; May, R.F. Mitigating wind energy impacts on wildlife: Approaches for multiple taxa. Hum.–Wildl. Interact. 2016, 10, 5. [Google Scholar] [CrossRef]
- Turney, D.; Fthenakis, V. Environmental impacts from the installation and operation of large-scale solar power plants. Renew. Sustain. Energy Rev. 2011, 15, 3261–3270. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, Z. Wind farms dry surface soil in temporal and spatial variation. Sci. Total Environ. 2023, 857, 159293. [Google Scholar] [CrossRef] [PubMed]
- Stechert, C.; Kolb, M.; Bahadir, M.; Djossa, B.A.; Fahr, J. Insecticide residues in bats along a land use-gradient dominated by cotton cultivation in northern Benin, West Africa. Environ. Sci. Pollut. Res. 2014, 21, 8812–8821. [Google Scholar] [CrossRef]
- Weier, S.M.; Linden, V.M.G.; Hammer, A.; Grass, I.; Tscharntke, T.; Taylor, P.J. Bat guilds respond differently to habitat loss and fragmentation at different scales in macadamia orchards in South Africa. Agric. Ecosyst. Environ. 2021, 320, 107588. [Google Scholar] [CrossRef]
- van der Zijpp, A.; Wilke, P.; Carsan, S. Sustainable livestock intensification. In The Role of Livestock in Developing Communities: Enhancing Multifunctionality; Swanepoel, F., Stroebel, A., Moyo, S., Eds.; Sun Media and The Technical Centre for Agricultural and Rural Cooperation: Bloemfontein, South Africa, 2010; Volume 123, pp. 123–150. [Google Scholar]
- Schurch, M.P.; McManus, J.; Goets, S.; Pardo, L.E.; Gaynor, D.; Samuels, I.; Cupido, C.; Couldridge, V.; Smuts, B. Wildlife-friendly livestock management promotes mammalian biodiversity recovery on a semi-arid Karoo farm in South Africa. Front. Conserv. Sci. 2021, 2, 652415. [Google Scholar] [CrossRef]
- Chapman, S.; Birch, C.E.; Galdos, M.V.; Pope, E.; Davie, J.; Bradshaw, C.; Eze, S.; Marsham, J.H. Assessing the impact of climate change on soil erosion in East Africa using a convection-permitting climate model. Environ. Res. Lett. 2021, 16, 084006. [Google Scholar] [CrossRef]
- Conenna, I.; López-Baucells, A.; Rocha, R.; Ripperger, S.; Cabeza, M. Movement seasonality in a desert-dwelling bat revealed by miniature GPS loggers. Mov. Ecol. 2019, 7, 27. [Google Scholar] [CrossRef]
- Korine, C.; Adams, R.; Russo, D.; Fisher-Phelps, M.; Jacobs, D. Bats and water: Anthropogenic alterations threaten global bat populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016; pp. 215–241. [Google Scholar] [CrossRef]
- Fuller, A.; Mitchell, D.; Maloney, S.K.; Hetem, R.S.; Fonsêca, V.F.C.; Meyer, L.C.R.; van de Ven, T.M.F.N.; Snelling, E.P. How dryland mammals will respond to climate change: The effects of body size, heat load and a lack of food and water. J. Exp. Biol. 2021, 224 (Suppl. S1), jeb238113. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal Rev. 2013, 43, 171–182. [Google Scholar] [CrossRef]
- Di Gregorio, C.; Iannella, M.; Biondi, M. Revealing the role of past and current climate in shaping the distribution of two parapatric European bats, Myotis daubentonii and M. capaccinii. Eur. Zool. J. 2021, 88, 669–683. [Google Scholar] [CrossRef]
- Ibáñez, C. Winter reproduction in the greater mouse-eared bat (Myotis myotis) in South Iberia. J. Zool. 1997, 243, 836–840. [Google Scholar] [CrossRef]
- Festa, F.; Ancillotto, L.; Santini, L.; Pacifici, M.; Rocha, R.; Toshkova, N.; Amorim, F.; Benítez-López, A.; Domer, A.; Hamidović, D.; et al. Bat responses to climate change: A systematic review. Biol. Rev. 2023, 98, 19–33. [Google Scholar] [CrossRef]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]

| Variables | Type, Units, and Classes | Source/Scale | |
|---|---|---|---|
| Landscape Variables | Landcover | Land cover containing all habitat types categorised during fieldwork. Categorical: fallow, farmed, mosaic, pond, scrub, village, watercourse, wood dense, and wood sparse. Wood dense was used as a reference. | Recorded in the field |
| NDVI | Normalised Difference Vegetation Index—a measure of green biomass for April. Continuous (−1 to 1). Available at: www.usgs.gov (accessed on 12 January 2018). | Derived from Landsat 8. 30 m | |
| DEM | Digital elevation model—a measure of altitude. Continuous, ranging from 1090 to 1288 m. Available at: http://www.cgiar-csi.org/data/srtm-90m-digital-elevation-database-v4-1 (accessed on 12 January 2018). | SRTM. 90 m DEM | |
| Distance to water | Distance to main watercourses in the study area (often maintained water in scattered puddles). Continuous, ranging from 0 to 4859 m. | Derived from AGID | |
| Environmental Variables | Temperature | Ambient temperature sampled at the meteorological mast at Kandebene. Continuous, ranging from 19.3 to 27 °C. | Sensor at 20 m height |
| RH | Relative air humidity was sampled at the meteorological mast at Kandebene. Continuous, ranging from 32.3 to 87.7%. | Sensor at 20 m height | |
| AH | Absolute air humidity was sampled at the meteorological mast at Kandebene. Continuous, ranging from 6.5 to 15.9 (g/m3) | Sensor at 20 m height | |
| WindClass | A measure of wind speed at ground level, using the Beauford scale (0—calm to 7—high wind speed). | Recorded in the field | |
| Wind speed | A measure of wind speed was sampled between November 2016 to October 2017. Continuous, ranging from 0.3 to 14.8 m/s. | Sensor at 20 m height. | |
| Moon | Fraction of the moon visible from November 2016 to October 2017. Continuous, ranging from 0 (new) to 1 (full moon). Available at: www.timeanddate.com/moon/kenya/isiolo (accessed on 12 January 2018). | Time and date website | |
| Sampling Method | ||||||
|---|---|---|---|---|---|---|
| Family/Species | Guild | Roost Search | Mist Net | Ground Acoustic | Height Acoustic | |
| 20 m | 35 m | |||||
| Pteropodidae | ||||||
| Epomophorus minimus | x | x | - | - | - | |
| Rhinolophidae | ||||||
| Rhinolophus fumigatus | Low_6 | x | ||||
| R. cf. landeri | Low_6 | x | ||||
| Hipposideridae | ||||||
| Hipposideros caffer | Low_6 | x | ||||
| Rhinonycteridae | ||||||
| Triaenops afer | Low_6 | x | x | |||
| Megadermatidae | ||||||
| Cardioderma cor | Low_5 | x | x | |||
| Lavia frons | Low_5 | x | x | |||
| Nycteridae | ||||||
| Nycteris sp. | Low_5 | x | x | |||
| Molossidae | ||||||
| Mops condylurus | High_2 | x | x | [x] | [x] | [x] |
| Mops pumilus | High_2 | x | x | [x] | [x] | [x] |
| Otomops harrisoni | High_1 | x | x | x | ||
| Miniopteridae | ||||||
| Miniopterus sp. | Medium_4 | x | x | x | ||
| Vespertilionidae | ||||||
| Afronycteris nana | Medium_4 | x | ||||
| Laephotis kirinyaga | Medium_4 | x | x | x | ||
| Neoromicia somalica | Medium_4 | x | x | |||
| Nycticeinops schlieffeni | Medium_4 | x | x | |||
| Pipistrellus hesperidus | Medium_4 | x | x | x | ||
| Scotoecus sp. | Medium_3 | x | x | x | x | |
| Scotophilus andrewreborii | Medium_3 | x | x | x | x | x |
| Vansonia rueppellii | Medium_4 | x | x | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rainho, A.; Ferreira, D.F.; Makori, B.; Bartonjo, M.; Repas-Gonçalves, M.; Kirakou, S.; Maghuwa, F.; Webala, P.W.; Tomé, R. Guild Vertical Stratification and Drivers of Bat Foraging in a Semi-Arid Tropical Region, Kenya. Biology 2023, 12, 1116. https://doi.org/10.3390/biology12081116
Rainho A, Ferreira DF, Makori B, Bartonjo M, Repas-Gonçalves M, Kirakou S, Maghuwa F, Webala PW, Tomé R. Guild Vertical Stratification and Drivers of Bat Foraging in a Semi-Arid Tropical Region, Kenya. Biology. 2023; 12(8):1116. https://doi.org/10.3390/biology12081116
Chicago/Turabian StyleRainho, Ana, Diogo F. Ferreira, Beryl Makori, Michael Bartonjo, Miguel Repas-Gonçalves, Stanley Kirakou, Florah Maghuwa, Paul W. Webala, and Ricardo Tomé. 2023. "Guild Vertical Stratification and Drivers of Bat Foraging in a Semi-Arid Tropical Region, Kenya" Biology 12, no. 8: 1116. https://doi.org/10.3390/biology12081116
APA StyleRainho, A., Ferreira, D. F., Makori, B., Bartonjo, M., Repas-Gonçalves, M., Kirakou, S., Maghuwa, F., Webala, P. W., & Tomé, R. (2023). Guild Vertical Stratification and Drivers of Bat Foraging in a Semi-Arid Tropical Region, Kenya. Biology, 12(8), 1116. https://doi.org/10.3390/biology12081116