Towards an Understanding of Microglia and Border-Associated Macrophages
Abstract
Simple Summary
Abstract
1. Introduction
2. The State of Microglia in the Brain Parenchyma
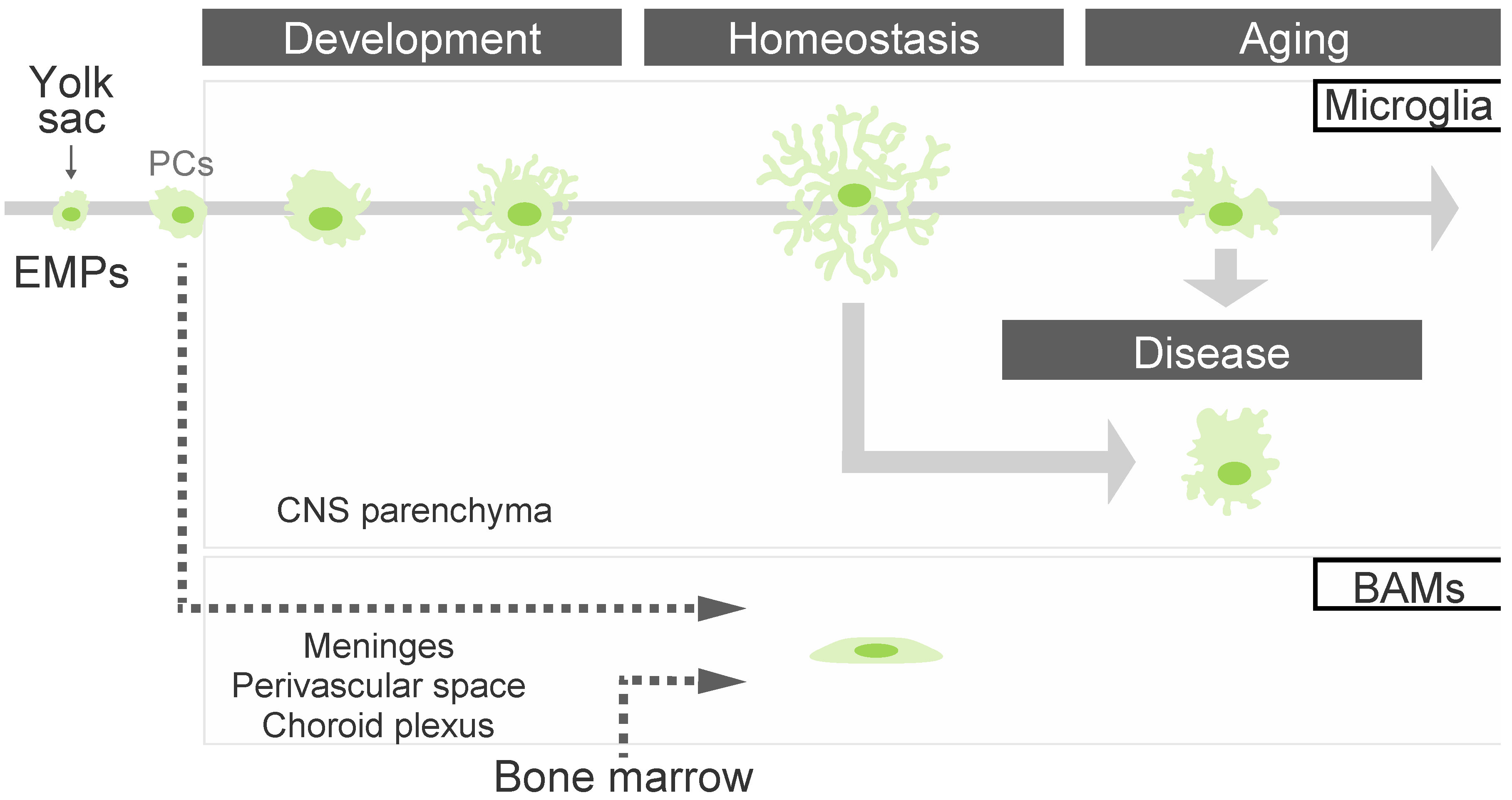
2.1. Microglial Differentiation and Maturation
2.2. The Microglial State in Aging and Diseases
2.3. Microglial Functions in the Homeostatic State
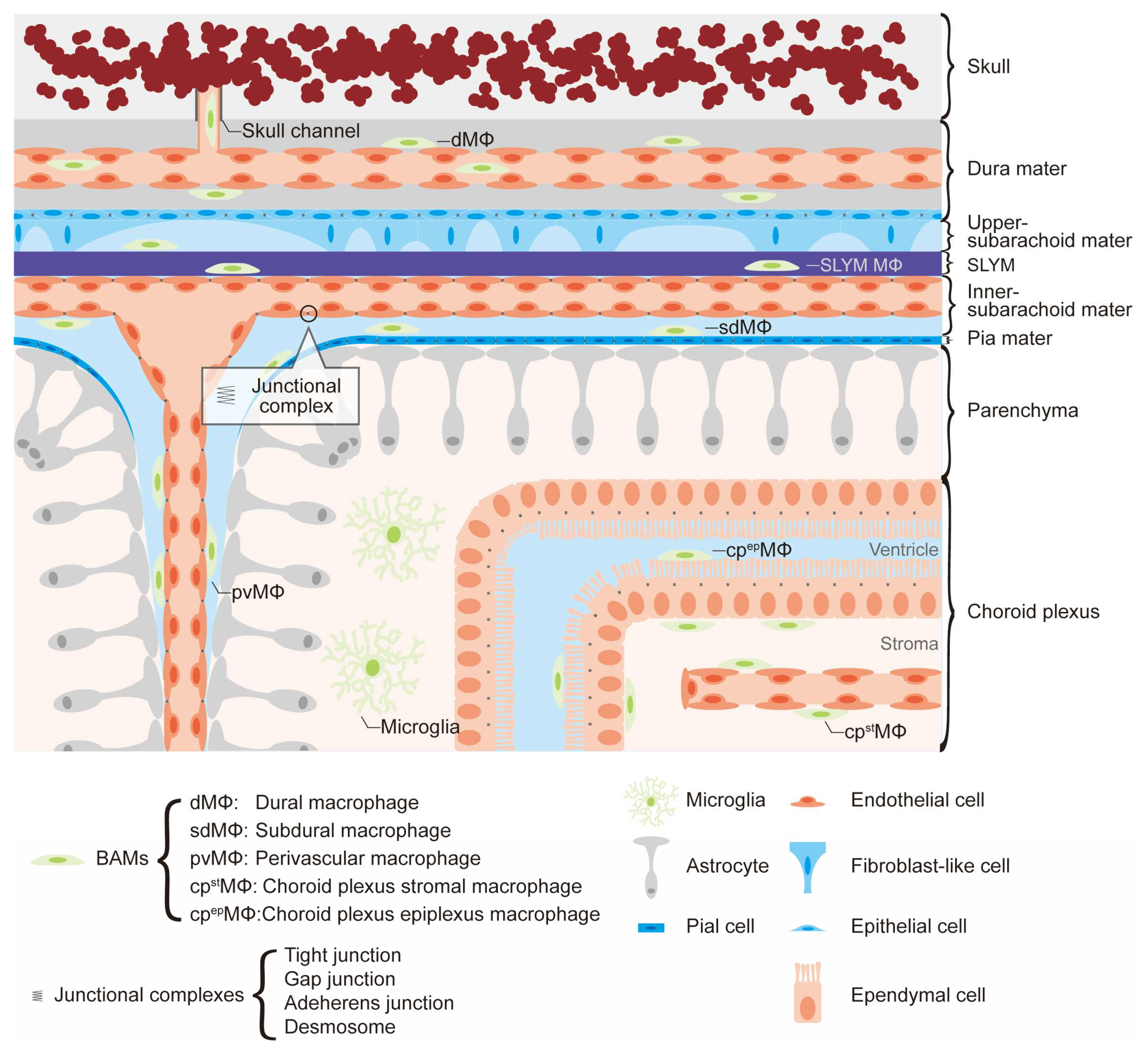
3. The Roles of Border-Associated Macrophages
3.1. Meningeal Macrophages
3.2. Perivascular Macrophages
3.3. Choroid Plexus Macrophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hill, R.A.; Damisah, E.C.; Chen, F.; Kwan, A.C.; Grutzendler, J. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat. Commun. 2017, 8, 15837. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; Nabekura, J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Aarum, J.; Sandberg, K.; Haeberlein, S.L.; Persson, M.A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl. Acad. Sci. USA 2003, 100, 15983–15988. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e315. [Google Scholar] [CrossRef]
- Tansley, S.; Gu, N.; Guzman, A.U.; Cai, W.; Wong, C.; Lister, K.C.; Munoz-Pino, E.; Yousefpour, N.; Roome, R.B.; Heal, J.; et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science 2022, 377, 80–86. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.Y.; Chen, H.R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Kracht, L.; Borggrewe, M.; Eskandar, S.; Brouwer, N.; Chuva de Sousa Lopes, S.M.; Laman, J.D.; Scherjon, S.A.; Prins, J.R.; Kooistra, S.M.; Eggen, B.J.L. Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 2020, 369, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Safaiyan, S.; Besson-Girard, S.; Kaya, T.; Cantuti-Castelvetri, L.; Liu, L.; Ji, H.; Schifferer, M.; Gouna, G.; Usifo, F.; Kannaiyan, N.; et al. White matter aging drives microglial diversity. Neuron 2021, 109, 1100–1117. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Friedman, B.A.; Etxeberria, A.; Huntley, M.A.; van der Brug, M.P.; Foreman, O.; Paw, J.S.; Modrusan, Z.; Beach, T.G.; Serrano, G.E.; et al. Alzheimer’s Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep. 2020, 31, 107843. [Google Scholar] [CrossRef]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.P.; et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef]
- Smajic, S.; Prada-Medina, C.A.; Landoulsi, Z.; Ghelfi, J.; Delcambre, S.; Dietrich, C.; Jarazo, J.; Henck, J.; Balachandran, S.; Pachchek, S.; et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain 2022, 145, 964–978. [Google Scholar] [CrossRef]
- De Andrade Costa, A.; Chatterjee, J.; Cobb, O.; Sanapala, S.; Scheaffer, S.; Guo, X.; Dahiya, S.; Gutmann, D.H. RNA sequence analysis reveals ITGAL/CD11A as a stromal regulator of murine low-grade glioma growth. Neuro. Oncol. 2022, 24, 14–26. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Jordao, M.J.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D.; et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 2018, 48, 380–395. [Google Scholar] [CrossRef]
- Van Hove, H.; Martens, L.; Scheyltjens, I.; De Vlaminck, K.; Pombo Antunes, A.R.; De Prijck, S.; Vandamme, N.; De Schepper, S.; Van Isterdael, G.; Scott, C.L.; et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Bottcher, C.; Amann, L.; Sagar, N.; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Utz, S.G.; See, P.; Mildenberger, W.; Thion, M.S.; Silvin, A.; Lutz, M.; Ingelfinger, F.; Rayan, N.A.; Lelios, I.; Buttgereit, A.; et al. Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell 2020, 181, 557–573. [Google Scholar] [CrossRef]
- Masuda, T.; Amann, L.; Monaco, G.; Sankowski, R.; Staszewski, O.; Krueger, M.; Del Gaudio, F.; He, L.; Paterson, N.; Nent, E.; et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 2022, 604, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kato, D.; Murayama, F.; Koike, S.; Asai, H.; Yamasaki, A.; Naito, Y.; Kawaguchi, A.; Konishi, H.; Prinz, M.; et al. CD206(+) macrophages transventricularly infiltrate the early embryonic cerebral wall to differentiate into microglia. Cell Rep. 2023, 42, 112092. [Google Scholar] [CrossRef]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuda, M.; Yoshinaga, R.; Tozaki-Saitoh, H.; Ozato, K.; Tamura, T.; Inoue, K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012, 1, 334–340. [Google Scholar] [CrossRef]
- Brioschi, S.; Belk, J.A.; Peng, V.; Molgora, M.; Rodrigues, P.F.; Nguyen, K.M.; Wang, S.; Du, S.; Wang, W.L.; Grajales-Reyes, G.E.; et al. A Cre-deleter specific for embryo-derived brain macrophages reveals distinct features of microglia and border macrophages. Immunity 2023, 56, 1027–1045. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Zoller, T.; Schneider, A.; Kleimeyer, C.; Masuda, T.; Potru, P.S.; Pfeifer, D.; Blank, T.; Prinz, M.; Spittau, B. Silencing of TGFbeta signalling in microglia results in impaired homeostasis. Nat. Commun. 2018, 9, 4011. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Abeta Plaques. Cell Rep. 2019, 27, 1293–1306. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Itoh, H.; Tsugawa, Y.; Nishida, Y.; Kurata, K.; Uemura, A.; Miyata, T. Embryonic Pericytes Promote Microglial Homeostasis and Their Effects on Neural Progenitors in the Developing Cerebral Cortex. J. Neurosci. 2022, 42, 362–376. [Google Scholar] [CrossRef]
- McNamara, N.B.; Munro, D.A.D.; Bestard-Cuche, N.; Uyeda, A.; Bogie, J.F.J.; Hoffmann, A.; Holloway, R.K.; Molina-Gonzalez, I.; Askew, K.E.; Mitchell, S.; et al. Microglia regulate central nervous system myelin growth and integrity. Nature 2023, 613, 120–129. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Kohno, K.; Shirasaka, R.; Yoshihara, K.; Mikuriya, S.; Tanaka, K.; Takanami, K.; Inoue, K.; Sakamoto, H.; Ohkawa, Y.; Masuda, T.; et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 2022, 376, 86–90. [Google Scholar] [CrossRef]
- Jordao, M.J.C.; Sankowski, R.; Brendecke, S.M.; Sagar; Locatelli, G.; Tai, Y.H.; Tay, T.L.; Schramm, E.; Armbruster, S.; Hagemeyer, N.; et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019, 363, eaat7554. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Sidorov, S.; Ravussin, E.; Artyomov, M.; Iwasaki, A.; Wang, A.; Dixit, V.D. The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity 2022, 55, 1609–1626. [Google Scholar] [CrossRef] [PubMed]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef]
- Alves de Lima, K.; Rustenhoven, J.; Kipnis, J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu. Rev. Immunol. 2020, 38, 597–620. [Google Scholar] [CrossRef]
- Adeeb, N.; Mortazavi, M.M.; Deep, A.; Griessenauer, C.J.; Watanabe, K.; Shoja, M.M.; Loukas, M.; Tubbs, R.S. The pia mater: A comprehensive review of literature. Childs Nerv. Syst. 2013, 29, 1803–1810. [Google Scholar] [CrossRef]
- Alcolado, R.; Weller, R.O.; Parrish, E.P.; Garrod, D. The cranial arachnoid and pia mater in man: Anatomical and ultrastructural observations. Neuropathol. Appl. Neurobiol. 1988, 14, 1–17. [Google Scholar] [CrossRef]
- Møllgård, K.; Beinlich, F.R.M.; Kusk, P.; Miyakoshi, L.M.; Delle, C.; Plá, V.; Hauglund, N.L.; Esmail, T.; Rasmussen, M.K.; Gomolka, R.S.; et al. A mesothelium divides the subarachnoid space into functional compartments. Science 2023, 379, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Rebejac, J.; Eme-Scolan, E.; Arnaud Paroutaud, L.; Kharbouche, S.; Teleman, M.; Spinelli, L.; Gallo, E.; Roussel-Queval, A.; Zarubica, A.; Sansoni, A.; et al. Meningeal macrophages protect against viral neuroinfection. Immunity 2022, 55, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kolesnikov, M.; Peled-Hajaj, S.; Scheyltjens, I.; Xia, Y.; Trzebanski, S.; Haimon, Z.; Shemer, A.; Lubart, A.; Van Hove, H.; et al. A Binary Cre Transgenic Approach Dissects Microglia and CNS Border-Associated Macrophages. Immunity 2021, 54, 176–190. [Google Scholar] [CrossRef]
- Qin, J.; Lovelace, M.D.; Mitchell, A.J.; de Koning-Ward, T.; Grau, G.E.; Pai, S. Perivascular macrophages create an intravascular niche for CD8+ T cell localisation prior to the onset of fatal experimental cerebral malaria. Clin. Transl. Immunol. 2021, 10, e1273. [Google Scholar] [CrossRef]
- Schonhoff, A.M.; Figge, D.A.; Williams, G.P.; Jurkuvenaite, A.; Gallups, N.J.; Childers, G.M.; Webster, J.M.; Standaert, D.G.; Goldman, J.E.; Harms, A.S. Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nat. Commun. 2023, 14, 3754. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Firulyova, M.; Manis, M.; Herz, J.; Smirnov, I.; Aladyeva, E.; Wang, C.; Bao, X.; Finn, M.B.; Hu, H.; et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 2023, 615, 668–677. [Google Scholar] [CrossRef]
- Greter, M.; Heppner, F.L.; Lemos, M.P.; Odermatt, B.M.; Goebels, N.; Laufer, T.; Noelle, R.J.; Becher, B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005, 11, 328–334. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, M.; Fridberger, A.; Hassan, A.; Degagne, J.; Neng, L.; Zhang, F.; He, W.; Ren, T.; Trune, D.; et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. USA 2012, 109, 10388–10393. [Google Scholar] [CrossRef]
- He, H.; Mack, J.J.; Güç, E.; Warren, C.M.; Squadrito, M.L.; Kilarski, W.W.; Baer, C.; Freshman, R.D.; McDonald, A.I.; Ziyad, S.; et al. Perivascular Macrophages Limit Permeability. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2203–2212. [Google Scholar] [CrossRef]
- Faraco, G.; Sugiyama, Y.; Lane, D.; Garcia-Bonilla, L.; Chang, H.; Santisteban, M.M.; Racchumi, G.; Murphy, M.; Van Rooijen, N.; Anrather, J.; et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Investig. 2016, 126, 4674–4689. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Haj-Yasein, N.N.; Vindedal, G.F.; Eilert-Olsen, M.; Gundersen, G.A.; Skare, Ø.; Laake, P.; Klungland, A.; Thorén, A.E.; Burkhardt, J.M.; Ottersen, O.P.; et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood–brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. USA 2011, 108, 17815–17820. [Google Scholar] [CrossRef] [PubMed]
- Drieu, A.; Du, S.; Storck, S.E.; Rustenhoven, J.; Papadopoulos, Z.; Dykstra, T.; Zhong, F.; Kim, K.; Blackburn, S.; Mamuladze, T.; et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 2022, 611, 585–593. [Google Scholar] [CrossRef]
- Siret, C.; van Lessen, M.; Bavais, J.; Jeong, H.W.; Reddy Samawar, S.K.; Kapupara, K.; Wang, S.; Simic, M.; de Fabritus, L.; Tchoghandjian, A.; et al. Deciphering the heterogeneity of the Lyve1+ perivascular macrophages in the mouse brain. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2009, 106, 1261–1266. [Google Scholar] [CrossRef]
- Cui, J.; Xu, H.; Lehtinen, M.K. Macrophages on the margin: Choroid plexus immune responses. Trends Neurosci. 2021, 44, 864–875. [Google Scholar] [CrossRef]
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.-J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.M.; Pletikos, M.; et al. Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef]
- Shipley, F.B.; Dani, N.; Xu, H.; Deister, C.; Cui, J.; Head, J.P.; Sadegh, C.; Fame, R.M.; Shannon, M.L.; Flores, V.I.; et al. Tracking Calcium Dynamics and Immune Surveillance at the Choroid Plexus Blood-Cerebrospinal Fluid Interface. Neuron 2020, 108, 623–639. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.-H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Robert, S.M.; Reeves, B.C.; Kiziltug, E.; Duy, P.Q.; Karimy, J.K.; Mansuri, M.S.; Marlier, A.; Allington, G.; Greenberg, A.B.W.; DeSpenza, T., Jr.; et al. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell 2023, 186, 764–785. [Google Scholar] [CrossRef] [PubMed]
| Name/Description | Abbr. | Subjects | State | Analyzed Stages | Characteristics of Typical Gene Signature | Signatures and Functions | Ref. |
|---|---|---|---|---|---|---|---|
| Proliferative-region-associated microglia | PAM | Mice | Development | Early postnatal stage (P7) Embryonic stage (E14.5) Adult (P60) | Gpnmb, Spp1, Clec7a, Itgax, Lilrb4 | PAM appear in the developing corpus callosum and white matter around P7. PAM share a gene signature with DAM. PAM engulf excess oligodendrocytes. PAM appearance does not depend on a TREM2-APOE axis. | [16] |
| Axon tract-associated microglia Injury-responsive microglia | ATM IRM | Mice | Development Disease | Embryonic stage (E14.5) Early postnatal stage (P4, P5) Adult (P30, P100 ) Aging (P540) Injury (lysolecithine stimulation) | ARM; Spp1, Igf1, Gpnmb, Lgals1, Lgals3,Lamp1, Cd68, Fabp5 IRM; Birc5, Cxcl10, Ccl4, Apoe, Ifi27l2a, Ifi204 | ATM appear in axon tracts of the corpus callosum and cerebellum around P4/P5 ATM disappear before myelination occurs. IRM appear after injection of lysolecithin. Gene expression signature in IRM is partially common to DAM and ATM. | [17] |
| Human fetal brain microglia | _ | Human | Development | Embryonic stage (GW 9–18) | Csf1r, Cx3cr1, P2ry12, P2ry13, Tmem119, Axl, Apoe, Cd68, Mrpl23, Parp4, Mtx1, Hba/Hbg, Zp3, Nampt | The developmental human microglia are similar to DAM/MGnD in mice. Microglia transform to a more mature, immune-responsive phenotype during embryonic periods. | [18] |
| White matter-associated microglia | WAM | Mice | Aging | 2, 6, 12, 18, 20 and 24 months old | ApoE, Cst7, Bm2, Lyz2, Cd63, Clec7a, Ctsb, Ctss, Ctsz, H2-D1, H2-K1 | WAM phagocytose damaged myelin in aging white matter. WAM share a gene signature with DAM. WAM appearance depends on a TREM2-dependent but ApoE-independent manner. | [19] |
| Lipid-droplet accumulating microglia | LDAM | Human Mice | Aging | Human; <35 years old, >60 years old Mice; 18–20 month old | Slc33a1, Snx17, Vps35, Cln3, Npc2, Grn | LDAM are aged microglia with lipid droplets LDAM are defective in phagocytosis, LDAM produce reactive oxygen species and secrete pro-inflammatory cytokines. | [20] |
| Disease-associated microglia | DAM | Mice | Disease | 5x FAD and mSOD G93A mice Approximately 6 months old | Tyrobp, Ctsb, Ctsd, Apoe, B2m, Fth1, Lyz2, Trem2, Axl, Ctsl, Lpl, Cd9, Csf1, Ccl6, Itgax, Clec7a, Lilrb4, Timp2 | DAM appear in the disease model mice (5x FAD and mSOD G93A) DAM appearance occurs sequentially in a Trem2-independent and Trem2-dependent manner. DAM activation is required for downregulation of checkpoint genes (Cx3cr1, P2ry12, P2ry13). | [21] |
| Microglial neurodegenerative phenotype | MGnD | Mice | Disease | APP-PS, mSOD1 G93A, and EAE mice 2, 3, 4, 9, 20 and 24 months old | Spp1, Itgax, Axl, Lilrb4, Clec7a, Csf1, Apoe | MGnD are induced by neuritic Aβ plaque and apoptotic neurons. Trem2-ApoE pathway promotes a swith from homeostatic microglia to MGnD. | [22] |
| Human AD microglia | HAM | Human Mice | Disease | Human; 64 ± 16 years, 77 ± 17 years Mice; 4, 12 and 22 months | Apoe, Abca7, Gpr141, Ptk2b, Spl1, Zyx | HAM were identified from frozen cerebrocortical tissues from human AD brain. HAM profile is entirely distinct from the DAM profile defined in mouse models. | [23] |
| Microglia inflamed in MS | MIMS | Human | Disease | 30–60 years | Trem2, Apoe, Lpl, Cd68, Cd9, Cd74, Grn, Tyrobp, Timp2, Spp1, Ctsz, Ctsb, Fth1, C1qa, C1qb, C1qc | MIMS appears in demyelinated white matter lesions. MIMS share a gene signature with other neurodegenerative diseases. C1q acts as a critical mediator of MIMS activation. | [24] |
| Human PD microglia | _ | Human | Disease | >60 years old | Il1b, Gpnmb, Hsp90aa1 | The nigral microglia in PD show a pro-inflammatory state. | [25] |
| Glioma-associated microglia | GAM | Human | Disease | Approximately 10 years old | Itgal/Cd11a | Itgal/CD11a is a novel marker for GAM. Itgal/CD11a ablation inhibits the growth of NF1 LGG. | [26] |
| Activated-response microglia Transiting response microglia Interferon response microglia Cycling/proliferating microglia | ARM TRM IRM CPM | Mice | Disease | APP NL-G-F mice APP/PS1-Apoe null mice 3, 6, 12, and 21 months old | ARM; Cst7, Clec7a, Itgax, MHC class II, Cd74, H2-Ab1, H2-Aa, Ctsb, Ctsd, Spp1, Gpnmb, Dkk2 TRM; similar to ARM IRM; Ifit2, Ifit3, Ifitm3, Irf7, Oasl2 CPM; Top2a, Mcm2, Tubb5, Mki67, Cdk1 | ARM are enriched with AD risk genes. IRM show a high expression of genes involved in innate immune response. CPM enriched in genes involved in DNA replication, chromatin rearrangement, and cell cycle. | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taketomi, T.; Tsuruta, F. Towards an Understanding of Microglia and Border-Associated Macrophages. Biology 2023, 12, 1091. https://doi.org/10.3390/biology12081091
Taketomi T, Tsuruta F. Towards an Understanding of Microglia and Border-Associated Macrophages. Biology. 2023; 12(8):1091. https://doi.org/10.3390/biology12081091
Chicago/Turabian StyleTaketomi, Takumi, and Fuminori Tsuruta. 2023. "Towards an Understanding of Microglia and Border-Associated Macrophages" Biology 12, no. 8: 1091. https://doi.org/10.3390/biology12081091
APA StyleTaketomi, T., & Tsuruta, F. (2023). Towards an Understanding of Microglia and Border-Associated Macrophages. Biology, 12(8), 1091. https://doi.org/10.3390/biology12081091







