Microalgae to Bioenergy: Optimization of Aurantiochytrium sp. Saccharification
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Studies: Definition of Variable Ranges
2.2. Experimental Design
2.3. Sugars Quantification
3. Results and Discussion
3.1. Preliminary Results
3.2. Model Fitting
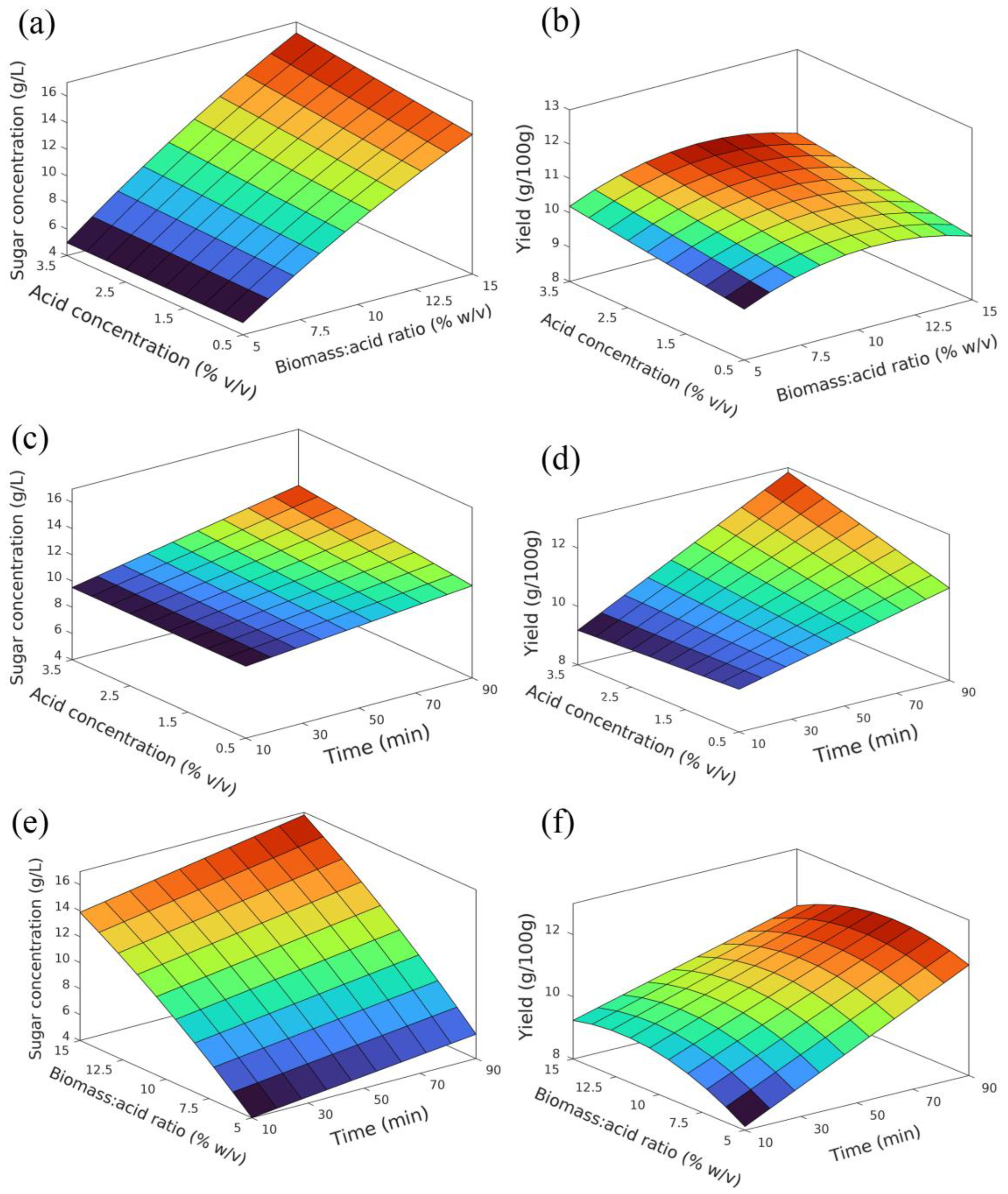
3.3. Surface Plots and Respective Analysis
3.3.1. Sugar Concentration Model
3.3.2. Yield Model
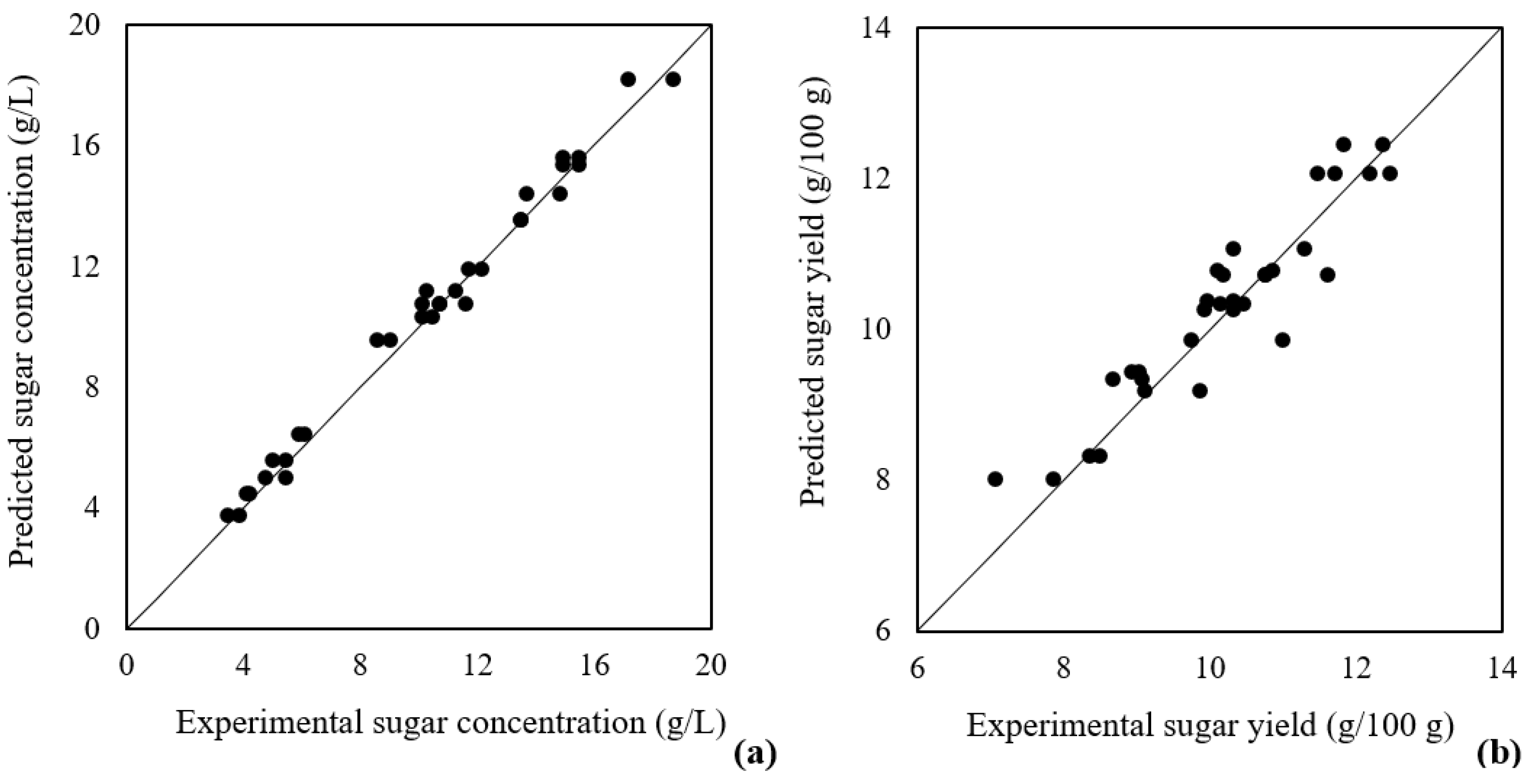
3.3.3. Model Validation
3.4. Critical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agustini, N.W.S.; Hidhayati, N.; Wibisono, S.A. Effect of hydrolysis time and acid concentration on bioethanol production of microalga Scenedesmus sp. IOP Conf. Ser. Earth Environ. Sci. 2019, 308, 012029. [Google Scholar] [CrossRef]
- Ho, S.-H.; Li, P.-J.; Liu, C.-C.; Chang, J.-S. Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresour. Technol. 2013, 145, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef] [PubMed]
- Kusmiyati, K.; Hadiyanto, H.; Fudholi, A. Treatment updates of microalgae biomass for bioethanol production: A comparative study. J. Clean. Prod. 2023, 383, 135236. [Google Scholar] [CrossRef]
- Minh Thu, N.; Seung Phill, C.; Jinwon, L.; Jae Hwa, L. Hydrothermal Acid Pretreatment of Chlamydomonas reinhardtii Biomass for Ethanol Production. J. Microbiol. Biotechnol. 2009, 19, 161–166. [Google Scholar] [CrossRef]
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Lee, O.K.; Oh, Y.-K.; Lee, E.Y. Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour. Technol. 2015, 196, 22–27. [Google Scholar] [CrossRef]
- Michael, D.; Boyin, L.; Razif, H. Analysis of process configurations for bioethanol production from microalgal biomass. In Progress in Biomass and Bioenergy Production; Syed Shahid, S., Ed.; IntechOpen: Rijeka, Italy, 2011; pp. Ch. 20, 395–408. [Google Scholar]
- Heo, S.-W.; Oh, Y.T.; Kim, Z.H.; Chang, Y.K.; Lee, B. Application of Jerusalem artichoke and lipid-extracted algae hydrolysate for docosahexaenoic acid production by Aurantiochytrium sp. KRS101. J. Appl. Phycol. 2020, 32, 3655–3666. [Google Scholar] [CrossRef]
- Trovão, M.; Pereira, H.; Costa, M.; Machado, A.; Barros, A.; Soares, M.; Carvalho, B.; Silva, J.T.; Varela, J.; Silva, J. Lab-Scale Optimization of Aurantiochytrium sp. Culture Medium for Improved Growth and DHA Production. Appl. Sci. 2020, 10, 2500. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol. 2012, 104, 342–348. [Google Scholar] [CrossRef]
- Castro, Y.A.; Ellis, J.T.; Miller, C.D.; Sims, R.C. Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone, butanol, and ethanol fermentation. Appl. Energy 2015, 140, 14–19. [Google Scholar] [CrossRef]
- Onay, M. Bioethanol production via different saccharification strategies from H. tetrachotoma ME03 grown at various concentrations of municipal wastewater in a flat-photobioreactor. Fuel 2019, 239, 1315–1323. [Google Scholar] [CrossRef]
- Favier, L.; Andrei-Ionuț, S.; Hlihor, R.M.; Fekete-Kertész, I.; Molnár, M.; Harja, M.; Vial, C. Intensification of the photodegradation efficiency of an emergent water pollutant through process conditions optimization by means of response surface methodology. J. Environ. Manag. 2023, 328, 116928. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Wang, G. Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J. Integr. Plant Biol. 2011, 53, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley: Hoboken, NJ, USA, 2016; p. 705. [Google Scholar]
- Lawson, J. Design and Analysis of Experiments with R; Chapman and Hall/CRC: New York, NY, USA, 2015; p. 620. [Google Scholar]
- Czyrski, A.; Jarzębski, H. Response Surface Methodology as a Useful Tool for Evaluation of the Recovery of the Fluoroquinolones from Plasma—The Study on Applicability of Box-Behnken Design, Central Composite Design and Doehlert Design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J.B. Comparative study of Box–Behnken, central composite, and Doehlert matrix for multivariate optimization of Pb (II) adsorption onto Robinia tree leaves. J. Chemom. 2013, 27, 12–20. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Natarajan, P.; Periyasamy, M.; Jamuna, R.; Sakunthala, K.; Mohanraj, M. Optimization of electrodeposition parameters to maximize the microhardness of Ni-SiC nanocomposite coatings using RSM. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Raj, R.; Tirkey, J.V.; Singh, D.K.; Jena, P. Co-gasification of waste triple feed-material blends using downdraft gasifier integrated with dual fuel diesel engine: An RSM-based comparative parametric optimization. J. Energy Inst. 2023, 109, 101271. [Google Scholar] [CrossRef]
- Connan, S. Spectrophotometric Assays of Major Compounds Extracted from Algae. Methods Mol. Biol. 2015, 1308, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Quinn, M.; Van Wychen, S.; Hyman, D.; Laurens, L.M.L. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Nerantzis, E.; Georgakakis, D. Bioethanol Production by Carbohydrate-Enriched Biomass of Arthrospira (Spirulina) platensis. Energis 2013, 6, 3937–3950. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Raethong, N.; Nazir, Y.; Halim, H.; Yang, W.; Vongsangnak, W.; Abdul Hamid, A.; Song, Y. Whole genome analysis and elucidation of docosahexaenoic acid (DHA) biosynthetic pathway in Aurantiochytrium sp. SW1. Gene 2022, 846, 146850. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gindaba, G.T.; Gebeyehu, K.B.; Periyasamy, S.; Jabesa, A.; Baskar, G.; John, B.I.; Pugazhendhi, A. Bioethanol production from agricultural residues as lignocellulosic biomass feedstock’s waste valorization approach: A comprehensive review. Sci. Total Environ. 2023, 879, 163158. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Laohakunjit, N.; Kerdchoechuen, O.; Kyu, K.L.; Ratanakhanokchai, K. Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol. 2013, 58, 163–176. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Range and Level | |||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| x1 | Time | min | 10 | 50 | 90 |
| x2 | Biomass/acid ratio | % (w/v) | 5 | 10 | 15 |
| x3 | Acid concentration | % (v/v) | 0.5 | 2 | 3.5 |
| Temperature (°C) | [H2SO4] (% (v/v)) | Time (min) | Biomass/Acid Ratio (% (w/v)) | Yield (g/100 g) | Sugar Concentration (g/L) |
|---|---|---|---|---|---|
| 90 | 2.5 | 30 | 2.5 | 13 ± 1 | 3.2 ± 0.3 |
| 2.5 | 60 | 2.5 | 13.2 ± 0.6 | 3.3 ± 0.1 | |
| 2.5 | 90 | 2.5 | 14 ± 1 | 3.6 ± 0.4 | |
| 2.5 | 90 | 1 | 24 ± 3 | 2.4 ± 0.3 | |
| 2.5 | 90 | 10 | 13.3 ± 0.7 | 13.3 ± 0.7 | |
| 3 | 90 | 2.5 | 13.7 ± 0.2 | 3.48 ± 0.05 | |
| 4 | 90 | 2.5 | 14 ± 1 | 3.7 ± 0.4 | |
| 121 | 2.5 | 90 | 2.5 | 18 ± 1 | 4.6 ± 0.3 |
| 100–148 * | 3 | 90 | 2.5 | 13.2 ± 0.7 | 3.3 ± 0.2 |
| Run. | Time (min) | Biomass/Acid Ratio (% (w/v)) | [H2SO4] (% (v/v)) | x1 | x2 | x3 | y1 (g/L) | y2 (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 10 | 0.5 | 0 | 0 | −1 | 10.55 | 10.52 |
| 2 | 50 | 5 | 2 | 0 | −1 | 0 | 5.54 | 11.05 |
| 3 | 50 | 15 | 2 | 0 | 1 | 0 | 15.57 | 10.38 |
| 4 | 10 | 5 | 0.5 | −1 | −1 | −1 | 4.32 | 8.57 |
| 5 | 10 | 10 | 2 | −1 | 0 | 0 | 9.13 | 9.12 |
| 6 | 10 | 15 | 3.5 | −1 | 1 | 1 | 14.92 | 9.95 |
| 7 | 90 | 5 | 0.5 | 1 | −1 | −1 | 5.11 | 10.16 |
| 8 | 10 | 10 | 2 | −1 | 0 | 0 | 8.72 | 8.72 |
| 9 | 10 | 5 | 3.5 | −1 | −1 | 1 | 3.98 | 7.92 |
| 10 | 90 | 5 | 0.5 | 1 | −1 | −1 | 5.52 | 10.92 |
| 11 | 10 | 15 | 0.5 | −1 | 1 | −1 | 13.63 | 9.07 |
| 12 | 10 | 5 | 0.5 | −1 | −1 | −1 | 4.24 | 8.44 |
| 13 | 90 | 10 | 2 | 1 | 0 | 0 | 11.78 | 11.76 |
| 14 | 50 | 5 | 2 | 0 | −1 | 0 | 4.91 | 9.80 |
| 15 | 90 | 15 | 3.5 | 1 | 1 | 1 | 18.89 | 12.54 |
| 16 | 50 | 10 | 2 | 0 | 0 | 0 | 10.84 | 10.82 |
| 17 | 90 | 15 | 3.5 | 1 | 1 | 1 | 17.32 | 11.53 |
| 18 | 10 | 5 | 3.5 | −1 | −1 | 1 | 3.59 | 7.14 |
| 19 | 50 | 10 | 0.5 | 0 | 0 | −1 | 10.23 | 10.22 |
| 20 | 90 | 15 | 0.5 | 1 | 1 | −1 | 15.08 | 10.05 |
| 21 | 50 | 10 | 2 | 0 | 0 | 0 | 10.85 | 10.83 |
| 22 | 90 | 5 | 3.5 | 1 | −1 | 1 | 5.98 | 11.92 |
| 23 | 90 | 5 | 3.5 | 1 | −1 | 1 | 6.23 | 12.43 |
| 24 | 10 | 15 | 3.5 | −1 | 1 | 1 | 13.77 | 9.18 |
| 25 | 50 | 10 | 2 | 0 | 0 | 0 | 10.28 | 10.26 |
| 26 | 10 | 15 | 0.5 | −1 | 1 | −1 | 13.54 | 8.98 |
| 27 | 50 | 10 | 2 | 0 | 0 | 0 | 11.70 | 11.68 |
| 28 | 50 | 15 | 2 | 0 | 1 | 0 | 15.02 | 10.00 |
| 29 | 50 | 10 | 3.5 | 0 | 0 | 1 | 10.41 | 10.39 |
| 30 | 50 | 10 | 3.5 | 0 | 0 | 1 | 11.39 | 11.36 |
| 31 | 90 | 15 | 0.5 | 1 | 1 | −1 | 15.59 | 10.38 |
| 32 | 90 | 10 | 2 | 1 | 0 | 0 | 12.26 | 12.26 |
| β Coefficients | Model | |
|---|---|---|
| Yield | Sugar Concentration | |
| β0 | 10.662 | 10.678 |
| β1 | 1.343 | 1.196 |
| β2 | 0.185 * | 5.195 |
| β3 | 0.353 | 0.433 |
| β12 | 0.378 | 0.268 |
| β13 | 0.412 | 0.412 |
| β23 | - | 0.405 |
| β22 | −0.642 | −0.542 |
| [H2SO4] (% (v/v)) | Time (min) | Biomass/Acid Ratio (% (w/v)) | Yield (g/100 g) | Sugar Concentration (g/L) | |
|---|---|---|---|---|---|
| Prediction Experimental | 3.5 | 90 | 10 | 12.84 11.41 ± 0.04 | 12.72 11.45 ± 0.03 |
| Prediction error (%) | ≈11 | ≈13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.; Pardilhó, S.; Dias, J.M.; Pires, J.C.M. Microalgae to Bioenergy: Optimization of Aurantiochytrium sp. Saccharification. Biology 2023, 12, 935. https://doi.org/10.3390/biology12070935
Oliveira J, Pardilhó S, Dias JM, Pires JCM. Microalgae to Bioenergy: Optimization of Aurantiochytrium sp. Saccharification. Biology. 2023; 12(7):935. https://doi.org/10.3390/biology12070935
Chicago/Turabian StyleOliveira, Joana, Sara Pardilhó, Joana M. Dias, and José C. M. Pires. 2023. "Microalgae to Bioenergy: Optimization of Aurantiochytrium sp. Saccharification" Biology 12, no. 7: 935. https://doi.org/10.3390/biology12070935
APA StyleOliveira, J., Pardilhó, S., Dias, J. M., & Pires, J. C. M. (2023). Microalgae to Bioenergy: Optimization of Aurantiochytrium sp. Saccharification. Biology, 12(7), 935. https://doi.org/10.3390/biology12070935









