Production of Biodiesel from Underutilized Algae Oil: Prospects and Current Challenges Encountered in Developing Countries
Abstract
:Simple Summary
Abstract
1. Introduction
2. Concept of Algae Oil as a Source of Biodiesel
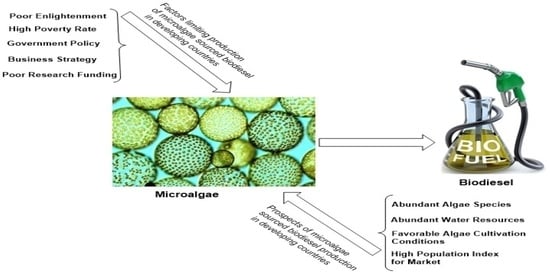
3. Challenges and Prospects
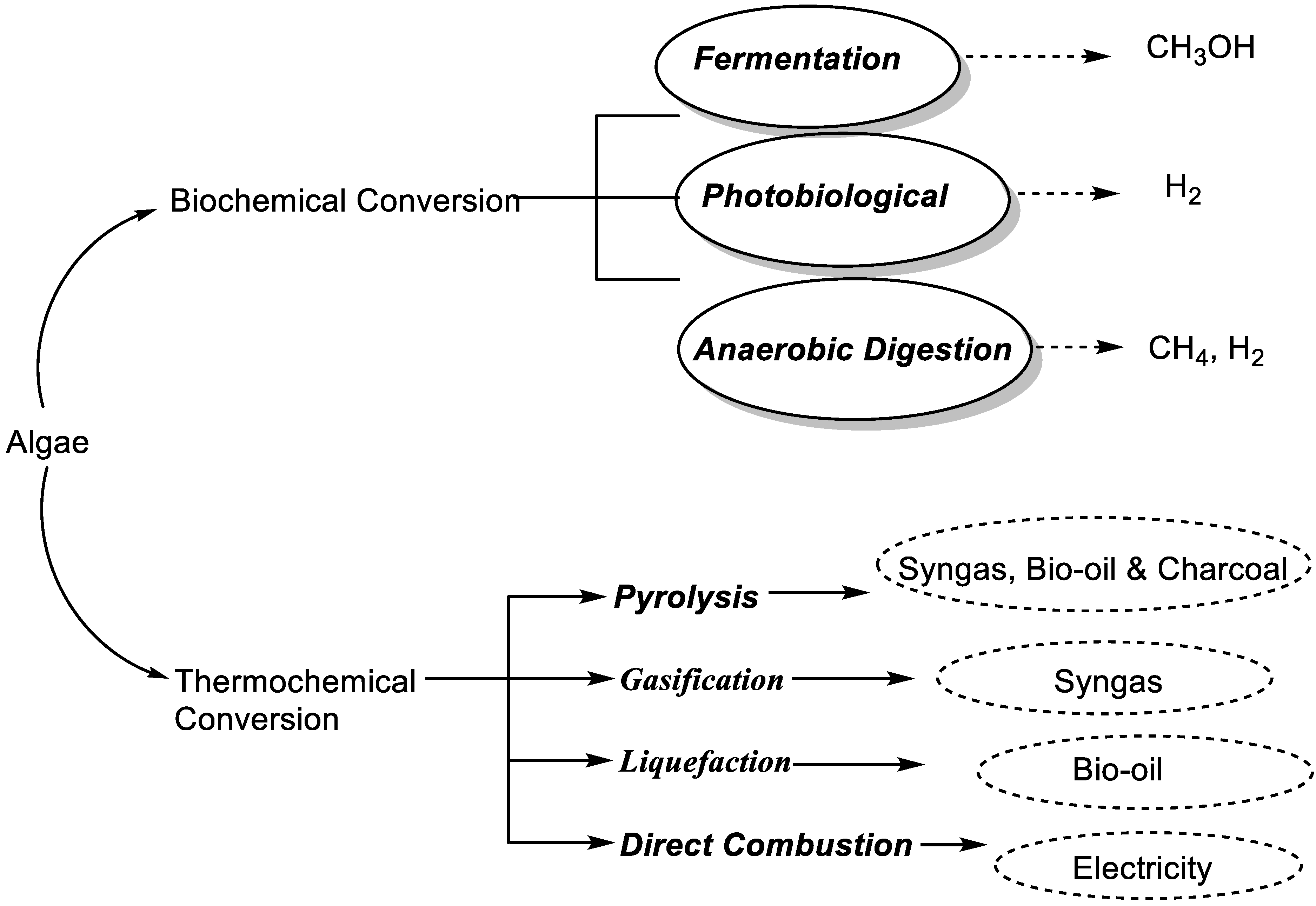
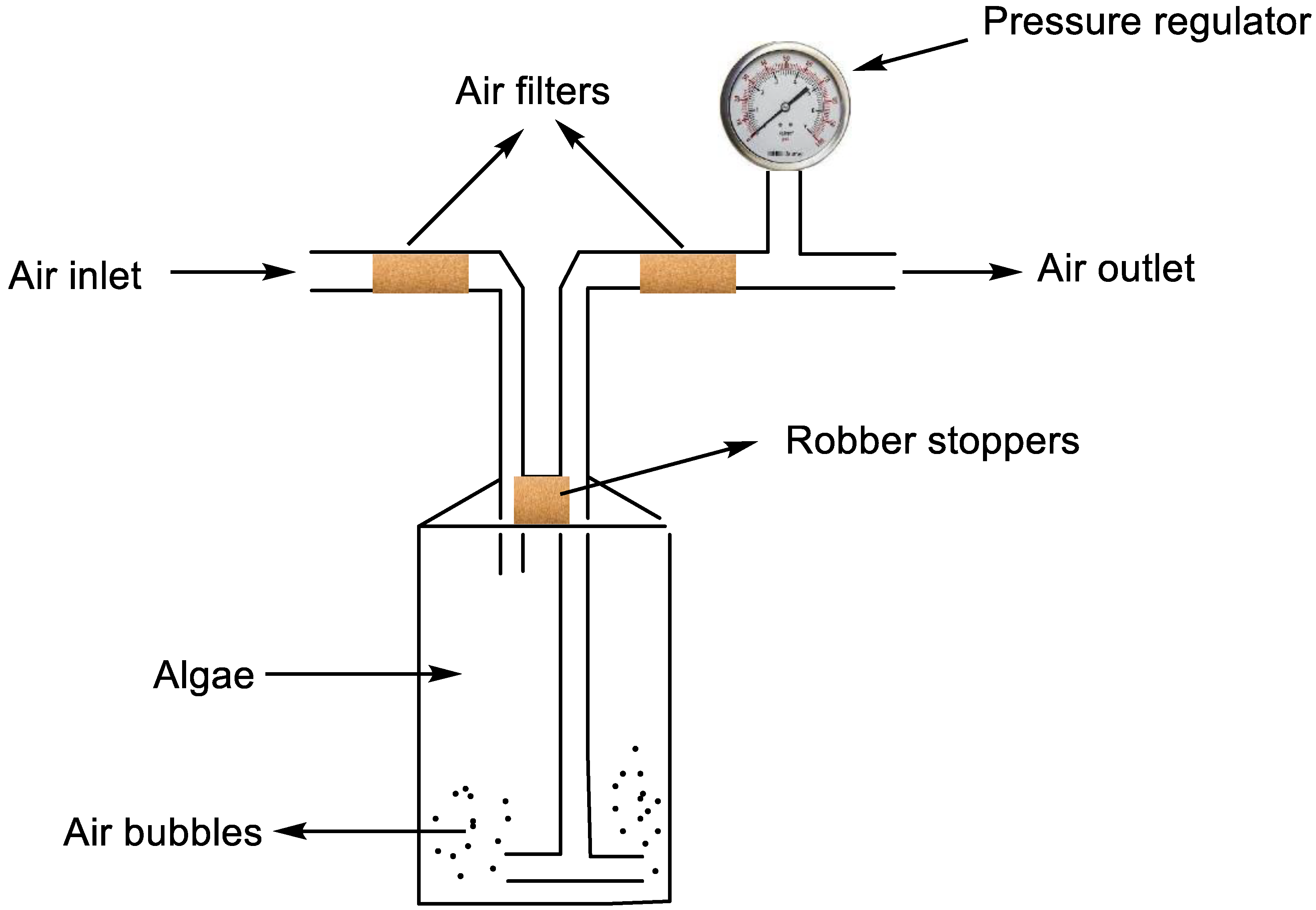
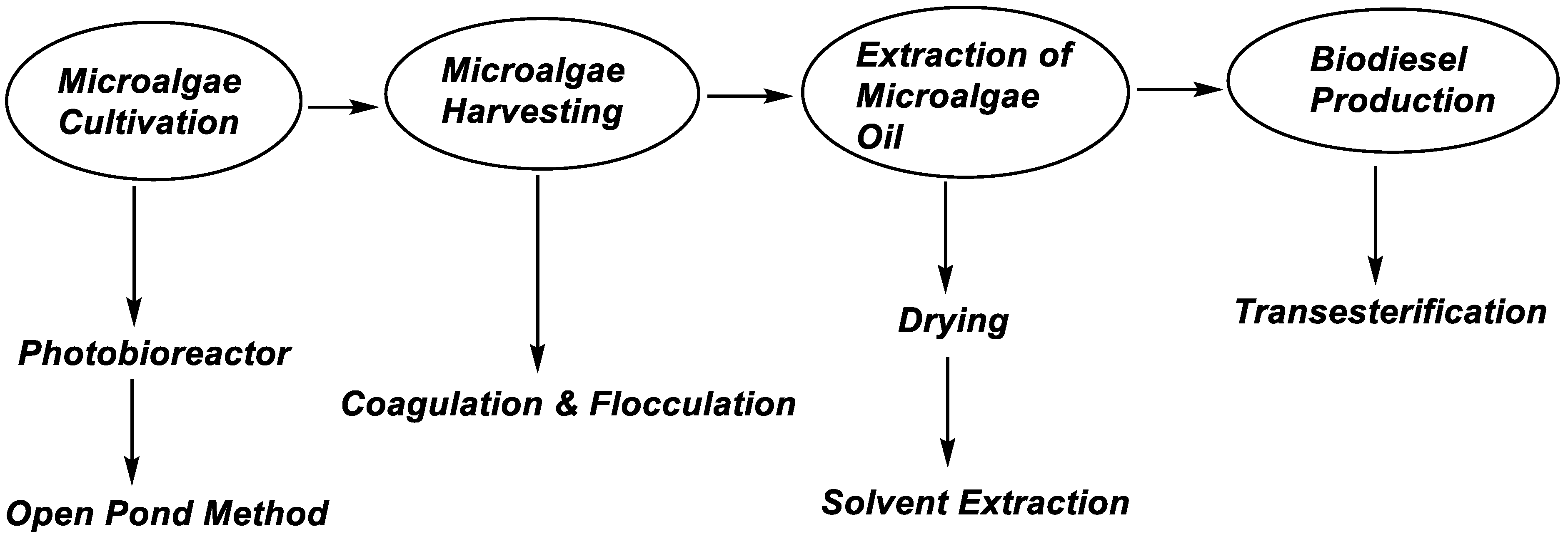
3.1. Microalgae Processing for Biodiesel Production
3.2. Availability of Microalgae Species
3.3. Government Policy and Business Strategy
3.4. Economic Feasibility and Commercialization
4. Current Status and Future Perspective
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajala, O.E.; Aberuagba, F.; Odetoye, T.E.; Ajala, A.M. Biodiesel: Sustainable Energy Replacement to Petroleum-Based Diesel Fuel–A Review. ChemBioEng Rev. 2015, 2, 145–156. [Google Scholar] [CrossRef]
- Ahmad, M.; Elnaggar, A.Y.; Teong, L.K.; Sultana, S.; Zafar, M.; Munir, M.; Hussein, E.E.; Abidin, S.Z.U. Sustainable and eco-friendly synthesis of biodiesel from novel and non-edible seed oil of Monotheca buxifolia using green nano-catalyst of calcium oxide. Energy Convers. Manag. X 2022, 13, 100142. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, R.; Sajjad, W.; Nabi, G.; Hayat, K.M.; Duan, P.; Yao, L. Biodiesel production from algae to overcome the energy crisis. Hayati J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Kukwa, D.T.; Chetty, M. Microalgae: The Multifaceted Biomass of the 21st Century. Biotechnological Applications of Biomass 355. 2021. Available online: https://www.intechopen.com/chapters/73619 (accessed on 3 September 2022).
- Roberts, G.W.; Fortier, M.-O.P.; Sturm, B.S.; Stagg-Williams, S.M. Promising pathway for algal biofuels through wastewater cultivation and hydrothermal conversion. Energy Fuels 2013, 27, 857–867. [Google Scholar] [CrossRef]
- Heimann, K. Novel approaches to microalgal and cyanobacterial cultivation for bioenergy and biofuel production. Curr. Opin. Biotechnol. 2016, 38, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win–win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141. [Google Scholar] [CrossRef]
- Kligerman, D.C.; Bouwer, E.J. Prospects for biodiesel production from algae-based wastewater treatment in Brazil: A review. Renew. Sustain. Energy Rev. 2015, 52, 1834–1846. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel production from lignocellulosic biomass using oleaginous microbes: Prospects for integrated biofuel production. Front. Microbiol. 2021, 12, 1–23. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefining Innov. Sustain. Econ. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Adewuyi, A.; Oderinde, R.A.; Rao, B.; Prasad, R.; Anjaneyulu, B. Blighia unijugata and Luffa cylindrica seed oils: Renewable sources of energy for sustainable development in rural Africa. BioEnergy Res. 2012, 5, 713–718. [Google Scholar] [CrossRef]
- Kandasamy, S.; Bhuvanendran, N.; Narayanan, M.; He, Z. Thermochemical conversion of algal biomass. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 281–302. [Google Scholar]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Lee, H.V.; Juan, J.C. Conversion of microalgae biomass to biofuels. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–161. [Google Scholar]
- Hossain, S.Z. Biochemical conversion of microalgae biomass into biofuel. Chem. Eng. Technol. 2019, 42, 2594–2607. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Zulqarnain; Mohd Yusoff, M.H.; Ayoub, M.; Ramzan, N.; Nazir, M.H.; Zahid, I.; Abbas, N.; Elboughdiri, N.; Mirza, C.R.; Butt, T.A. Overview of Feedstocks for Sustainable Biodiesel Production and Implementation of the Biodiesel Program in Pakistan. ACS Omega 2021, 6, 19099–19114. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.E.; Leite, G.B.; Hallenbeck, P.C. Addressing the challenges for sustainable production of algal biofuels: II. Harvesting and conversion to biofuels. Environ. Technol. 2013, 34, 1807–1836. [Google Scholar] [CrossRef]
- Mapping Algae of Sundarban Origin as Lipid Feedstock for Potential Biodiesel Application. Available online: https://www.semanticscholar.org/paper/Mapping-Algae-of-Sundarban-Origin-as-Lipid-for-Barman-Satpati/d3034b0d8fae4cf2f017c697b15f24bb3feaaf27 (accessed on 1 June 2020).
- Beetul, K.; Bibi Sadally, S.; Taleb-Hossenkhan, N.; Bhagooli, R.; Puchooa, D. An investigation of biodiesel production from microalgae found in Mauritian waters. Biofuel Res. J. 2014, 1, 58–64. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Sharma, Y.C. Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst. Bioresour. Technol. 2019, 287, 121357. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pal, A.; Maji, S. Biodiesel production from microalgae oil through conventional and ultrasonic methods. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 806–810. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Dewi, R.N.; Di Livia, K.; Arisa, F.; Cahyono, R.B.; Budiman, A. Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol. Open Chem. 2020, 18, 833–842. [Google Scholar] [CrossRef]
- Kalacheva, G.; Zhila, N.; Volova, T.; Gladyshev, M. The effect of temperature on the lipid composition of the green alga Botryococcus. Microbiology 2002, 71, 286–293. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Agila, E.; Sivakumar, P.; Salam, Z.; Rengasamy, R.; Ani, F.N. Optimization and characterization of biodiesel production from microalgae Botryococcus grown at semi-continuous system. Energy Convers. Manag. 2014, 88, 936–946. [Google Scholar] [CrossRef]
- Faried, M.; Samer, M.; Abdelsalam, E.; Yousef, R.; Attia, Y.; Ali, A. Biodiesel production from microalgae: Processes, technologies and recent advancements. Renew. Sustain. Energy Rev. 2017, 79, 893–913. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Pruvost, J.; Pottier, L.; Legrand, J. Numerical investigation of hydrodynamic and mixing conditions in a torus photobioreactor. Chem. Eng. Sci. 2006, 61, 4476–4489. [Google Scholar] [CrossRef]
- Ranjbar, R.; Inoue, R.; Katsuda, T.; Yamaji, H.; Katoh, S. High efficiency production of astaxanthin in an airlift photobioreactor. J. Biosci. Bioeng. 2008, 106, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.; Benemann, J.R. A realistic technology and engineering assessment of algae biofuel production. Energy Biosci. Inst. 2010, 1, 1–78. [Google Scholar]
- Lam, M.K.; Khoo, C.G.; Lee, K.T. Scale-up and commercialization of algal cultivation and biofuels production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 475–506. [Google Scholar]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Benvenuti, G.; Bosma, R.; Ji, F.; Lamers, P.; Barbosa, M.J.; Wijffels, R.H. Batch and semi-continuous microalgal TAG production in lab-scale and outdoor photobioreactors. J. Appl. Phycol. 2016, 28, 3167–3177. [Google Scholar] [CrossRef] [PubMed]
- Alkarawi, M.A.; Caldwell, G.S.; Lee, J.G. Continuous harvesting of microalgae biomass using foam flotation. Algal Res. 2018, 36, 125–138. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-N.N.; Lai, Y.-Y.; Lu, W.-B.; Chang, J.-S. Exploring the high lipid production potential of a thermotolerant microalga using statistical optimization and semi-continuous cultivation. Bioresour. Technol. 2014, 163, 128–135. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wu, W.T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Lin, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- Moejes, F.W.; Moejes, K.B. Algae for Africa: Microalgae as a source of food, feed and fuel in Kenya. Afr. J. Biotechnol. 2017, 16, 288–301. [Google Scholar] [CrossRef]
- Ajala, E.; Ajala, M.; Akinpelu, G.; Akubude, V. Cultivation and Processing of Microalgae for Its Sustainability as a Feedstock for Biodiesel Production. Niger. J. Technol. Dev. 2021, 18, 322–343. [Google Scholar] [CrossRef]
- Bilanovic, D.; Andargatchew, A.; Kroeger, T.; Shelef, G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations–response surface methodology analysis. Energy Convers. Manag. 2009, 50, 262–267. [Google Scholar] [CrossRef]
- FAO Fisheries & Aquaculture. Aquaculture Department. 2013 Global Aquaculture Production Statistics for the Year. 2011. Available online: https://www.fao.org/fishery/docs/news/GlobalCaptureProductionStatistics2011.pdf (accessed on 3 September 2022).
- FAO. Aquaculture Department Port State Measures. 2016. Available online: https://www.fao.org/documents/card/en/c/I5801E/ (accessed on 3 September 2022).
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Bio/Technol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, L.; Pan, Q.; Yang, X.; Cheng, X. Preventive effect of phycocyanin from Spirulina platensis on alloxan-injured mice. Environ. Toxicol. Pharmacol. 2012, 34, 721–726. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Adhikari, B.; Lv, W.; Chen, H. Nostoc sphaeroides Cyanobacteria: A review of its nutritional characteristics and processing technologies. Crit. Rev. Food Sci. Nutr. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.-S.; Chang, J.-S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, A.L.; Tsai, Z.C.; Shen, Y.M.; Kao, P.H.; Ng, I.S.; Chang, J.S. Expression of synthetic phytoene synthase gene to enhance β-carotene production in Scenedesmus sp. CPC2. Biotechnol. J. 2017, 12, 1700204. [Google Scholar] [CrossRef]
- Tan, K.W.M.; Lee, Y.K. Expression of the heterologous Dunaliella tertiolecta fatty acyl-ACP thioesterase leads to increased lipid production in Chlamydomonas reinhardtii. J. Biotechnol. 2017, 247, 60–67. [Google Scholar] [CrossRef]
- Li, Z.; Meng, T.; Ling, X.; Li, J.; Zheng, C.; Shi, Y.; Chen, Z.; Li, Z.; Li, Q.; Lu, Y. Overexpression of malonyl-CoA: ACP transacylase in Schizochytrium sp. to improve polyunsaturated fatty acid production. J. Agric. Food Chem. 2018, 66, 5382–5391. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, C.; Jin, Y.; Hu, G.-R.; Li, F.-L. Characterization of 3-ketoacyl-coA synthase in a nervonic acid producing oleaginous microalgae Mychonastes afer. Algal Res. 2018, 31, 225–231. [Google Scholar] [CrossRef]
- Kwon, S.; Kang, N.K.; Koh, H.G.; Shin, S.E.; Lee, B.; Jeong, B.r.; Chang, Y.K. Enhancement of biomass and lipid productivity by overexpression of a bZIP transcription factor in Nannochloropsis salina. Biotechnol. Bioeng. 2018, 115, 331–340. [Google Scholar] [CrossRef]
- Osorio, H.; Jara, C.; Fuenzalida, K.; Rey-Jurado, E.; Vásquez, M. High-efficiency nuclear transformation of the microalgae Nannochloropsis oceanica using Tn5 Transposome for the generation of altered lipid accumulation phenotypes. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Salas-Montantes, C.J.; González-Ortega, O.; Ochoa-Alfaro, A.E.; Camarena-Rangel, R.; Paz-Maldonado, L.M.T.; Rosales-Mendoza, S.; Rocha-Uribe, A.; Soria-Guerra, R.E. Lipid accumulation during nitrogen and sulfur starvation in Chlamydomonas reinhardtii overexpressing a transcription factor. J. Appl. Phycol. 2018, 30, 1721–1733. [Google Scholar] [CrossRef]
- Jia, B.; Xie, X.; Wu, M.; Lin, Z.; Yin, J.; Huang, Y.; Hu, Z. Understanding the functions of endogenous DOF transcript factor in Chlamydomonas reinhardtii. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Wang, Y. Microalgae as the Third Generation Biofuel: Production, Usage, Challenges and Prospects. 2013. Available online: https://www.semanticscholar.org/paper/Microalgae-as-the-Third-Generation-Usage%2C-and-Wang/42cd70697003ad5a84cf46112f18dc0660f322f4 (accessed on 1 April 2020).
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. ACS Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef]
- Stecker, T. Algal biofuel sustainability review highlights concerns about water supply. Energy Sustain. 2012. Available online: https://www.scientificamerican.com/article/algal-biofuel-sustainability-review-hightlights-concerns-about-water-safety/ (accessed on 1 October 2012).
- Xu, W.; Yaghi, O.M. Metal–organic frameworks for water harvesting from air, anywhere, anytime. ACS Cent. Sci. 2020, 6, 1348–1354. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Bravo-Sánchez, M.G.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Petriz-Prieto, M.A. Technoeconomic Evaluation of Microalgae Oil Production: Effect of Cell Disruption Method. Fermentation 2022, 8, 301. [Google Scholar] [CrossRef]
- Ahkamiraad, A.; Wang, Y. An agent-based model for zip-code level diffusion of electric vehicles and electricity consumption in New York City. Energies 2018, 11, 640. [Google Scholar] [CrossRef]
- Alternative Fuels Data Center. Alternative Fuels Data Center-Fuel Properties Comparison. US Department of Energy–Energy Efficiency and Renewable Energy–Alternative Fuels Data Center. 2013. Available online: https://afdc.energy.gov/fuels/properties (accessed on 3 September 2022).
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae biodiesel production in China: A preliminary economic analysis. Renew. Sustain. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.; Martins, A.; Freitas, M.; Caetano, N. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to produce cost-effective third-generation biofuel from microalgae. Front. Energy Res. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Liao, K.; Lu, Q.; Zhou, W. Microalgae biotechnology as a promising pathway to ecofriendly aquaculture: A state-of-the-art review. J. Chem. Technol. Biotechnol. 2021, 96, 837–852. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, S.R. Performance and emission characteristics of biodiesel from different origins: A review. Renew. Sustain. Energy Rev. 2013, 21, 633–658. [Google Scholar] [CrossRef]
- Koizumi, T. Biofuels and Food Security: Biofuel Impact on Food Security in Brazil, Asia and Major Producing Countries; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Kang, K.E.; Chung, D.-P.; Kim, Y.; Chung, B.-W.; Choi, G.-W. High-titer ethanol production from simultaneous saccharification and fermentation using a continuous feeding system. Fuel 2015, 145, 18–24. [Google Scholar] [CrossRef]
- Mandegari, M.; Petersen, A.M.; Benjamin, Y.; Görgens, J.F. Sugarcane biofuel production in South Africa, Guatemala, the Philippines, Argentina, Vietnam, Cuba, and Sri Lanka. In Sugarcane Biofuels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 319–346. [Google Scholar]
- Subramaniam, Y.; Masron, T.A. The impact of economic globalization on biofuel in developing countries. Energy Convers. Manag. X 2021, 10, 100064. [Google Scholar] [CrossRef]
- Costelli, C. Genetic and Phylogenetic Characterization of Microalgae Strains in View of Their Exploitation for CO2 Capture and Biofuel Production. Available online: https://www.semanticscholar.org/paper/Genetic-and-phylogenetic-characterization-of-in-of-Costelli/596615600fbd2b5ca8c29f3c8c475990491b243c (accessed on 1 December 2015).
- Andrade, D.S.; Telles, T.S.; Castro, G.H.L. The Brazilian microalgae production chain and alternatives for its consolidation. J. Clean. Prod. 2020, 250, 119526. [Google Scholar] [CrossRef]
- dos Santos, M.G.B.; Duarte, R.L.; Maciel, A.M.; Abreu, M.; Reis, A.; de Mendonça, H.V. Microalgae biomass production for biofuels in brazilian scenario: A critical review. BioEnergy Res. 2021, 14, 23–42. [Google Scholar] [CrossRef]
- Matos, Â.P. Advances in microalgal research in Brazil. Braz. Arch. Biol. Technol. 2021, 64, 1–15. [Google Scholar] [CrossRef]
- Sampaio, R.M.; Bonacelli, M.B.M. Biodiesel in Brazil: Agricultural R&D at petrobras biocombustível. J. Technol. Manag. Innov. 2018, 13, 66–74. [Google Scholar]
- Gerber, N. Bioenergy and Rural Development in Developing Countries: A Review of Existing Studies. ZEF Discuss. Papar Developmet Policy. 2008. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1162142 (accessed on 3 September 2022).
- Zentou, H.; Rosli, N.S.; Wen, C.H.; Abdul Azeez, K.; Gomes, C. The viability of biofuels in developing countries: Successes, failures, and challenges. Iran. J. Chem. Chem. Eng. (IJCCE) 2019, 38, 173–182. [Google Scholar]
- Widenhorn, S. Braving the Storm: How Are Global Biofuel Policies Sustained Despite Being Contested? An Analysis of the Biofuel Discourses of the EU, Brazil and Mozambique. 2013. Available online: https://www.recercat.cat/handle/2072/240812 (accessed on 3 September 2022).
| Algae Species | Oil Content (% Dry Weight) |
|---|---|
| Ankistrodesmus TR-87 | 28–40 |
| Botryococcus braunii | 29–75 |
| Dunaliella tertiolecta | 36–42 |
| Tetraselmis suecica | 15–32 |
| Neochloris oleoabundans | 35–54 |
| Chlorella emersonii | 28–32 |
| Botyococcus braunii | 25–80 |
| Chlorella vulgaris | 14–22 |
| Euglena gracilis | 14–20 |
| Neochloris oleoabundans | 35–54 |
| Phaeodactylum tricornutum | 20–30 |
| Pleurochrysis carterae | 30–50 |
| Prymnesium parvum | 22–38 |
| Schizochytriumsp | 50–77 |
| Scenedesmus dimorphus | 16–40 |
| Fatty Acid | Synechocystis pevalekii | Spirulina plantensis | Chlorococcum infusionum | Cladophora crystallina | Navicula minima |
|---|---|---|---|---|---|
| C14:0 | 1.00 | 13.27 | 8.50 | 8.80 | 2.70 |
| C16:0 | 34.20 | 21.10 | 25.62 | 39.10 | 26.40 |
| C16:1 | 3.80 | 9.32 | 5.46 | 2.80 | 18.70 |
| C18:1 | 29.80 | 11.27 | 15.66 | 31.80 | 25.30 |
| C18:2 | 14.90 | 0.24 | 7.50 | 11.60 | 2.20 |
| Microalgae | Cultivation Process | Amount of Biomass (g L−1) | Oil Yield (% wt wt−1) | Oil Production (mg L−1 day−1) | Reference |
|---|---|---|---|---|---|
| Desmodesmus sp. F2 | Batch | 3.32 | 64.10 | 263 | [38] |
| Desmodesmus sp. F2 | Semi-continuous | 3.99 | 45.60 | 302 | [38] |
| Chlorella sp. | Batch | 2.15 | 44.80 | 124 | [39] |
| Chlorella sp. | Semi-continuous | 1.10 | 45.10 | 139 | [39] |
| Nannochloropsis oculate | Semi-continuous | 1.00 | 30.70 | 151 | [40] |
| Nannochloropsis sp. | Batch | 3.83 | 19.30 | 74 | [41] |
| Microalgal Strains | Genetic Modification | Performance | Reference |
|---|---|---|---|
| Nannochloropsis oceanica | Malonyl CoA-acylcarrier protein transacylase. | Neutral lipid content increased by 31%. | [54] |
| Chlamydomonas reinhardtii | C. reinhardtii transformed with acyl-ACP thioesterases. | Lipid content increased by ~56%. | [55] |
| Schizochytrium sp. | Overexpression of malonyl-CoA: ACP transacylase (MAT) in Schizochytrium. | Increase in polyunsaturated fatty acids and lipids by 10.1%. | [56] |
| Mychonastes afer | Cloning and expression of 3-ketoacyl-coA synthase gene from M. afer (MaKCS)in Saccharomyces cerevisiae BY4741. | Increased lipid content, especially nervonic acid under stress conditions of high light and low nitrogen. | [57] |
| Nannochloropsis salina | Overexpression of basic leucine zipper in N. salina. | Improvement in both growth and accumulation of lipid. | [58] |
| Nannochloropsis oceanic | Transposome complex Tn5 containing anti-biotic resistance cassette was inserted in N. oceania generating random mutant strain. | High accumulation of intracellular lipids. | [59] |
| Chlamydomonas reinhardtii | Phospholipase A2 (PLA2) gene knockout. | Increased lipid production by 64.25%. | [60] |
| Chlamydomonas reinhardtii | Overexpression of a DNA-binding-with-one-finger (Dof) transcription factor. | Increase in fatty acid production in sulfur deficient medium by 15.58% and in nitrogen by 17.02%. | [61] |
| Chlamydomonas reinhardtii | Cloning of crDOF from Chlamydomonas reinhardtii and construction of transgenic lines. Overexpression of crDOF. | Increased intracellular lipid content. | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewuyi, A. Production of Biodiesel from Underutilized Algae Oil: Prospects and Current Challenges Encountered in Developing Countries. Biology 2022, 11, 1418. https://doi.org/10.3390/biology11101418
Adewuyi A. Production of Biodiesel from Underutilized Algae Oil: Prospects and Current Challenges Encountered in Developing Countries. Biology. 2022; 11(10):1418. https://doi.org/10.3390/biology11101418
Chicago/Turabian StyleAdewuyi, Adewale. 2022. "Production of Biodiesel from Underutilized Algae Oil: Prospects and Current Challenges Encountered in Developing Countries" Biology 11, no. 10: 1418. https://doi.org/10.3390/biology11101418
APA StyleAdewuyi, A. (2022). Production of Biodiesel from Underutilized Algae Oil: Prospects and Current Challenges Encountered in Developing Countries. Biology, 11(10), 1418. https://doi.org/10.3390/biology11101418