MiR-23a Regulates Skin Langerhans Cell Phagocytosis and Inflammation-Induced Langerhans Cell Repopulation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Epidermal Single-Cell Suspension Preparation
2.3. Flow Cytometry and Antibodies
2.4. LC In Vitro Antigen Uptake Assay
2.5. LC Replenishment during Skin Inflammation
2.6. In Vitro LC Maturation
2.7. RNA Extraction and qRT-PCR
2.8. Bulk RNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. MiR-23a Is Not Required for the Embryonic Development of LCs
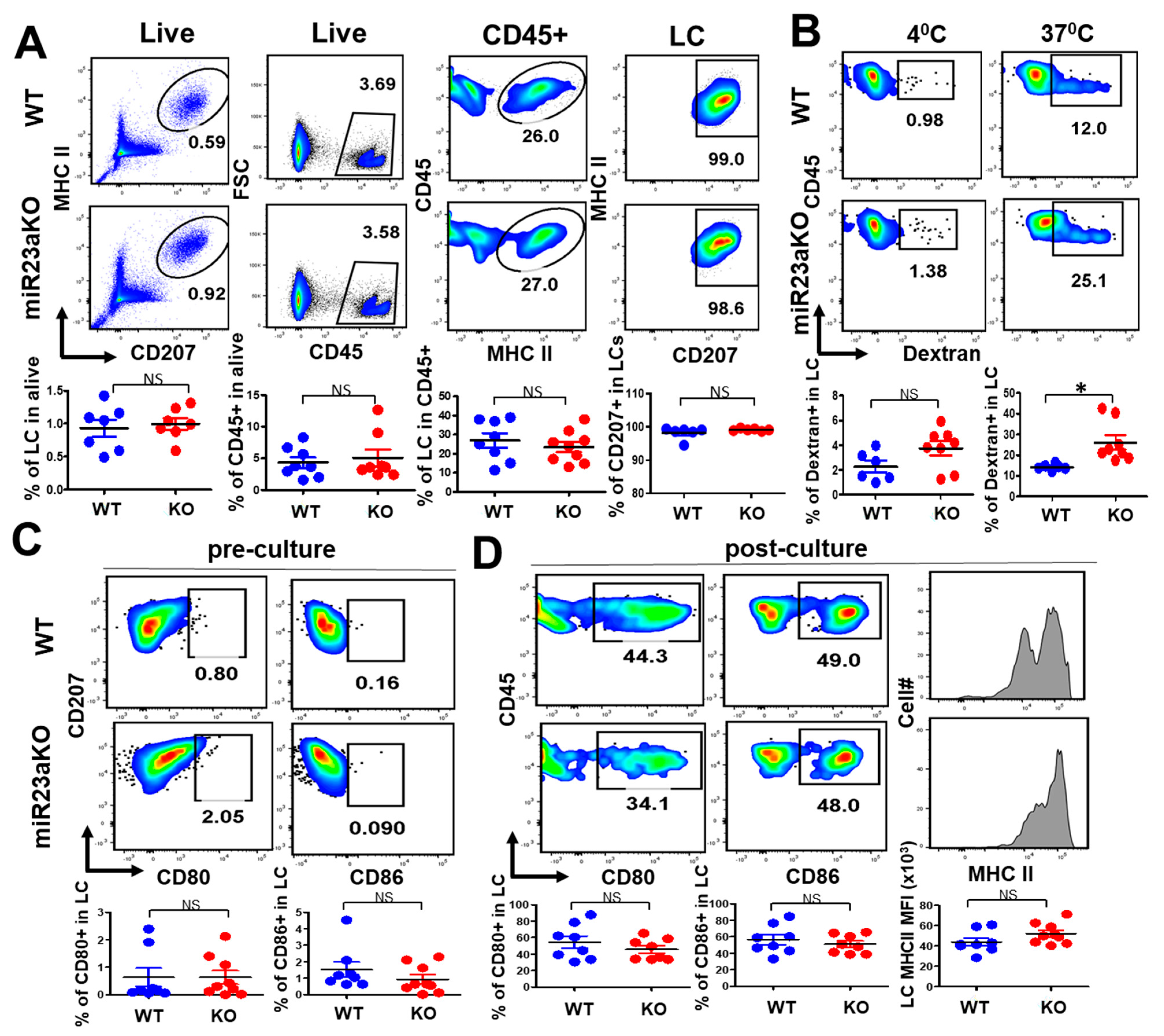
3.2. MiR-23a Is Not Required for LC Postnatal Homeostasis and Differentiation
3.3. MiR-23a Negatively Regulate LC Antigen Uptake but Not LC Maturation
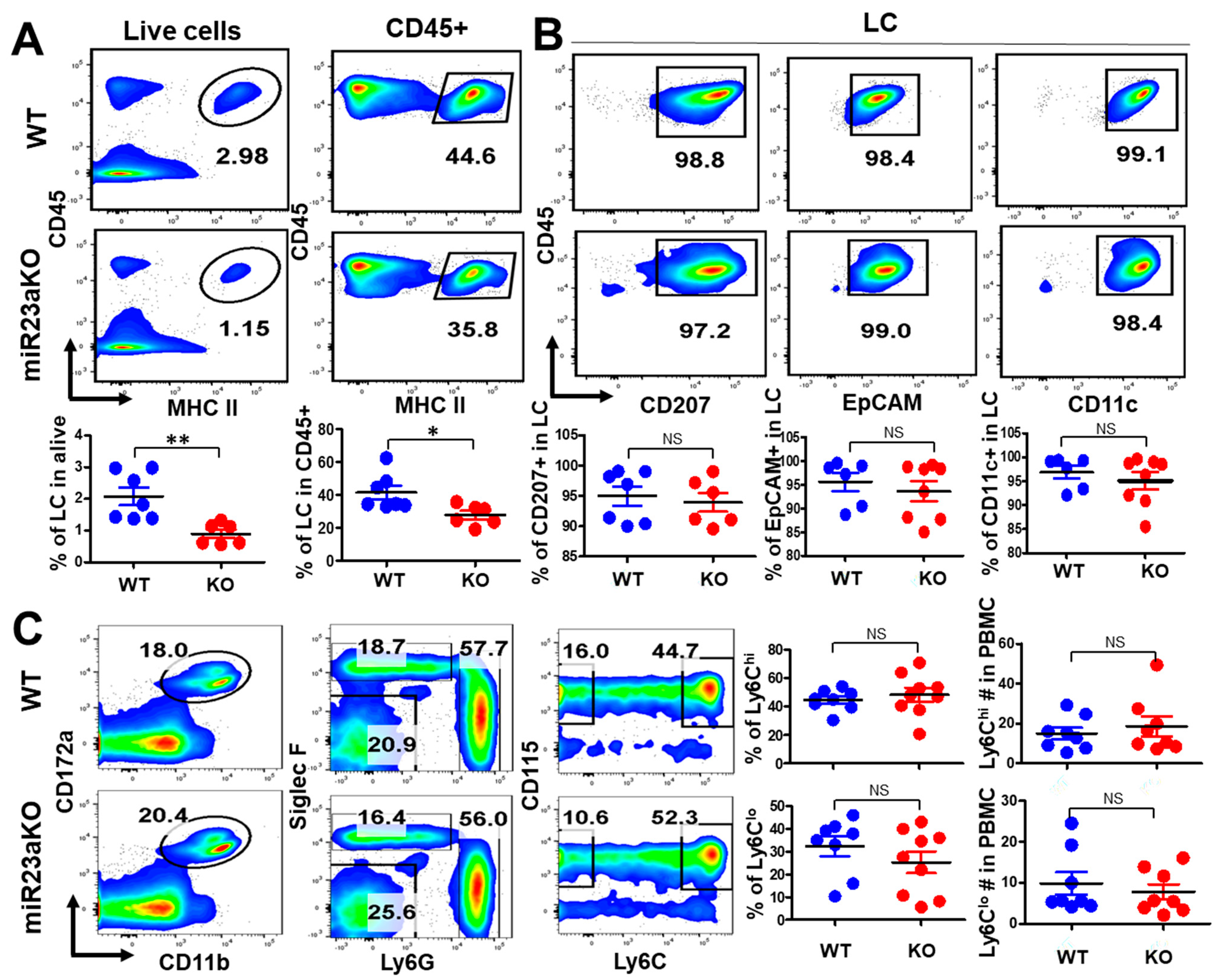
3.4. MiR-23a Deficiency Affects UV-Induced LC Repopulation
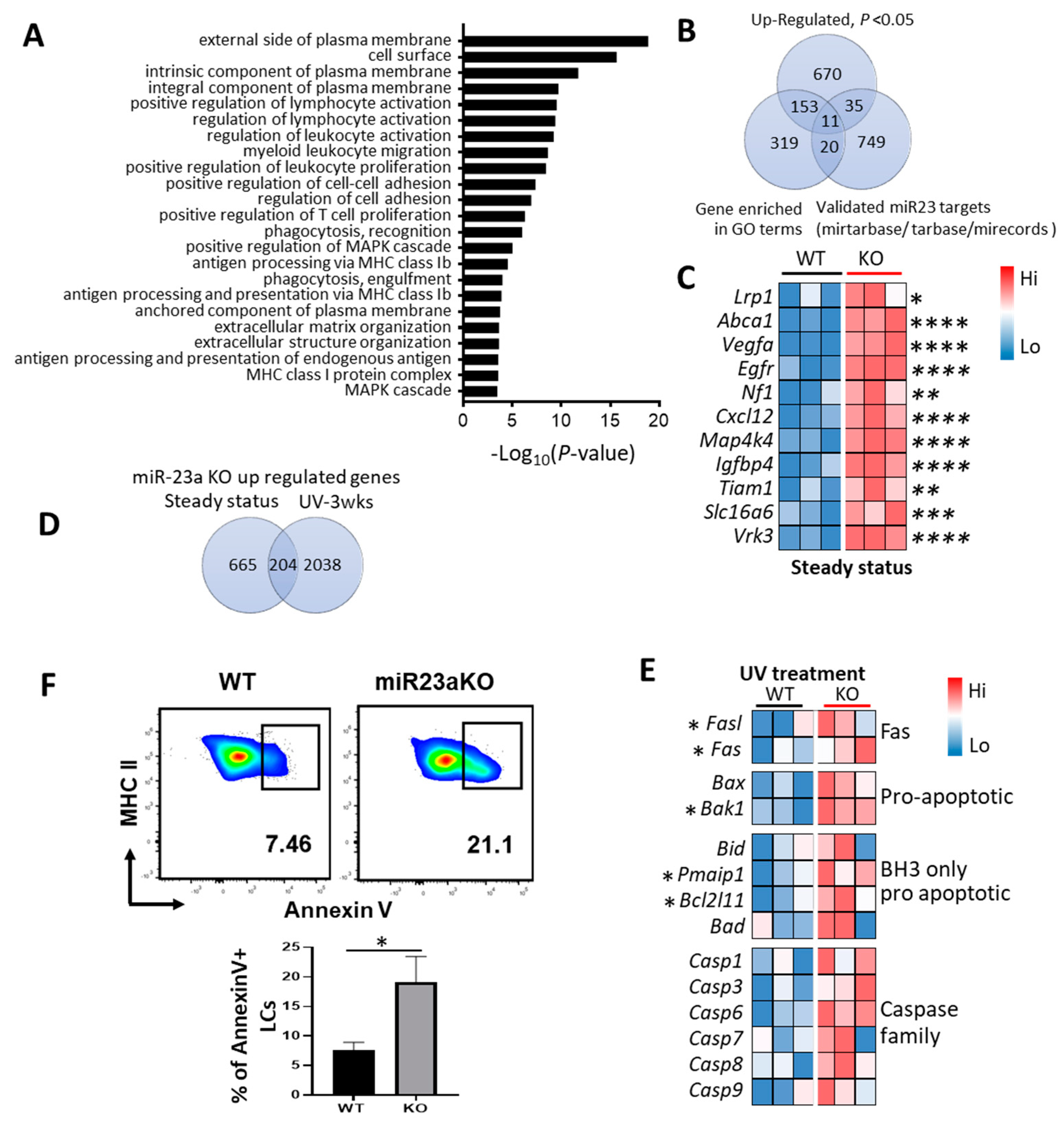
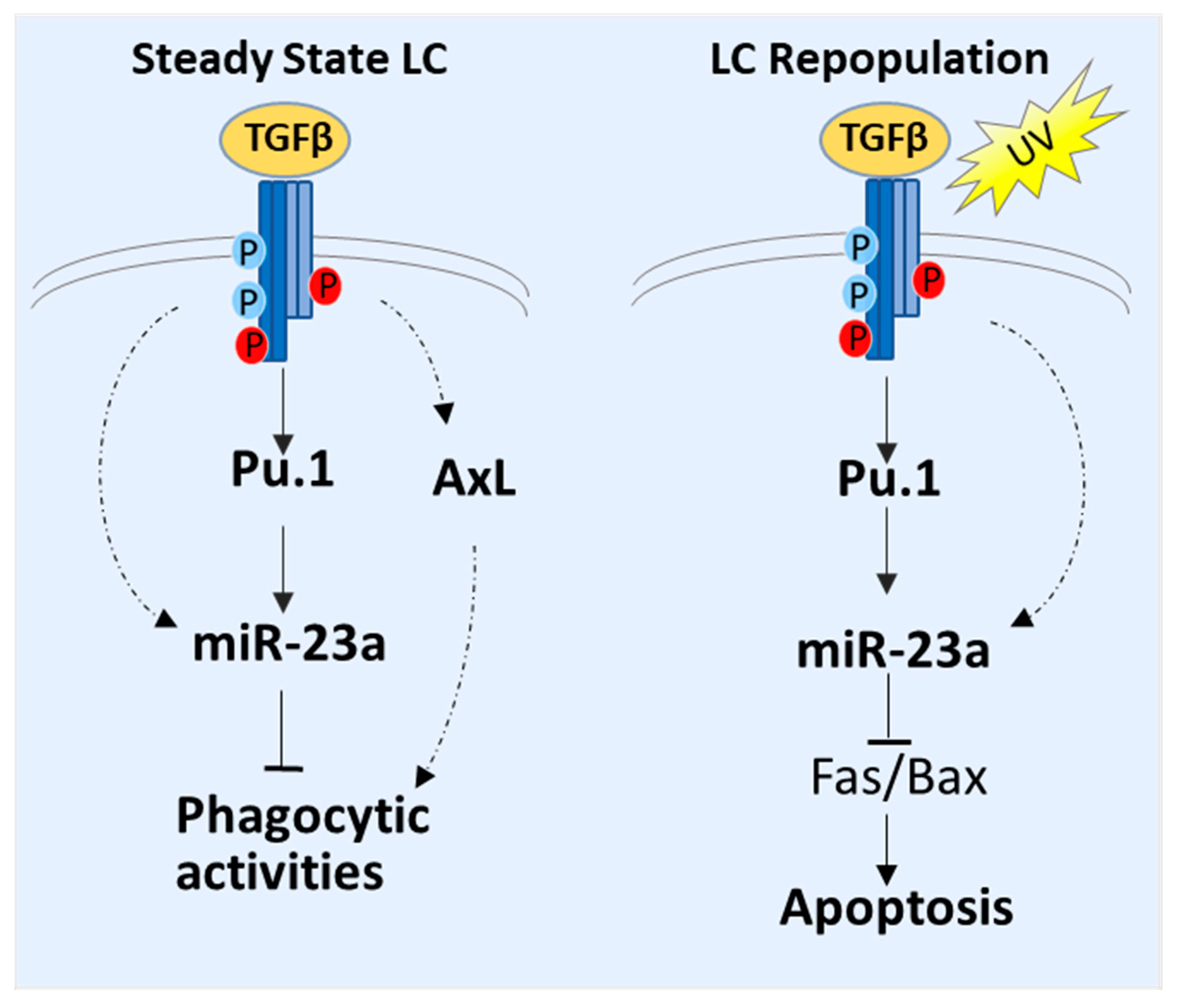
3.5. Molecular Mechanisms of MiR-23a-Mediated LC Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valladeau, J.; Ravel, O.; Dezutter-Dambuyant, C.; Moore, K.; Kleijmeer, M.; Liu, Y.; Duvert-Frances, V.; Vincent, C.; Schmitt, D.; Davoust, J.; et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 2000, 12, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Sere, K.; Baek, J.H.; Ober-Blobaum, J.; Muller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 2012, 37, 905–916. [Google Scholar] [CrossRef]
- Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Mutyambizi, K.; Berger, C.; Edelson, R. The balance between immunity and tolerance: The role of Langerhans cells. Cell. Mol. Life Sci. 2009, 66, 831–840. [Google Scholar] [CrossRef]
- Niedecken, H.; Lutz, G.; Bauer, R.; Kreysel, H. Langerhans cell as primary target and vehicle for transmission of HIV. Lancet 1987, 330, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Hugo, S.; Middel, P.; Blaschke, V.; Heine, A.; Gutgesell, C.; Williams, R.; Neumann, C. Evidence for a role of Langerhans cell-derived IL-16 in atopic dermatitis. J. Allergy Clin. Immunol. 2002, 109, 681–687. [Google Scholar] [CrossRef]
- Lewis, J.; Filler, R.; Smith, D.A.; Golubets, K.; Girardi, M. The contribution of Langerhans cells to cutaneous malignancy. Trends Immunol. 2010, 31, 460–466. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Yu, F.S.; Zhou, L.; Mi, Q.S. TGF-β1-induced transcription factor networks in Langerhans cell development and maintenance. Allergy 2016, 71, 758–764. [Google Scholar] [CrossRef]
- Yao, Y.; Martin, C.; Yin, C.; Guo, C.; Dong, Z.; Zhou, L.; Mi, Q.S. Micro RNAs are required for Langerhans cell, skin- and lung-resident macrophage ontogeny. J. Allergy Clin. Immunol. 2018, 142, 976–978.e972. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Q.; Martin, C.; Yin, C.; Dong, Z.; Mi, Q.S.; Zhou, L. Embryonic Fate Mapping Uncovers the Critical Role of microRNAs in the Development of Epidermal γδ T Cells. J. Investig. Dermatol. 2018, 138, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; Schnorfeil, F.M.; Fehling, H.J.; Bartels, H.; Brocker, T. Dicer-dependent microRNAs control maturation, function, and maintenance of Langerhans cells in vivo. J. Immunol. 2010, 185, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Dubey, R.; Saini, N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24-2 cluster and its implication in human diseases. Mol. Cancer 2010, 9, 232. [Google Scholar] [CrossRef]
- Kurkewich, J.L.; Boucher, A.; Klopfenstein, N.; Baskar, R.; Kapur, R.; Dahl, R. The mirn23a and mirn23b microrna clusters are necessary for proper hematopoietic progenitor cell production and differentiation. Exp. Hematol. 2018, 59, 14–29. [Google Scholar] [CrossRef]
- Kurkewich, J.L.; Bikorimana, E.; Nguyen, T.; Klopfenstein, N.; Zhang, H.; Hallas, W.M.; Stayback, G.; McDowell, M.A.; Dahl, R. The mirn23a microRNA cluster antagonizes B cell development. J. Leukoc. Biol. 2016, 100, 665–677. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Gordon, J.A.; Beloti, M.M.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc. Natl. Acad. Sci. USA 2010, 107, 19879–19884. [Google Scholar] [CrossRef]
- Zeng, H.C.; Bae, Y.; Dawson, B.C.; Chen, Y.; Bertin, T.; Munivez, E.; Campeau, P.M.; Tao, J.; Chen, R.; Lee, B.H. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-β signalling in osteoblasts. Nat. Commun. 2017, 8, 15000. [Google Scholar] [CrossRef]
- Bain, C.C.; Hawley, C.A.; Garner, H.; Scott, C.L.; Schridde, A.; Steers, N.J.; Mack, M.; Joshi, A.; Guilliams, M.; Mowat, A.M.I.; et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun. 2016, 7, ncomms11852. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.-Q.; Li, C.; Lykken, E.; Jiang, S.; Wong, E.; Gong, Z.; Tao, Z.; Zhu, B.; Wan, Y.; et al. MicroRNA-23a Curbs Necrosis during Early T Cell Activation by Enforcing Intracellular Reactive Oxygen Species Equilibrium. Immunity 2016, 44, 568–581. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Feng, Y.-G.; Zhang, C.; Chen, F.; Feng, Y. microRNA-23a in Human Cancer: Its Roles, Mechanisms and Therapeutic Relevance. Cancers 2018, 11, 7. [Google Scholar] [CrossRef]
- Chandran, P.A.; Keller, A.; Weinmann, L.; Seida, A.A.; Braun, M.; Andreev, K.; Fischer, B.; Horn, E.; Schwinn, S.; Junker, M.; et al. The TGF-β-inducible miR-23a cluster attenuates IFN-γ levels and antigen-specific cytotoxicity in human CD8⁺ T cells. J. Leukoc. Biol. 2014, 96, 633–645. [Google Scholar] [CrossRef]
- Kong, K.Y.; Owens, K.S.; Rogers, J.H.; Mullenix, J.; Velu, C.S.; Grimes, H.L.; Dahl, R. MIR-23A microRNA cluster inhibits B-cell development. Exp. Hematol. 2010, 38, 629–640.e621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Wang, J.; Li, G.; Weiland, M.; Yu, F.S.; Mi, Q.S.; Gu, J.; Zhou, L. TIM-4 is differentially expressed in the distinct subsets of dendritic cells in skin and skin-draining lymph nodes and controls skin Langerhans cell homeostasis. Oncotarget 2016, 7, 37498–37512. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, G.H.; Yu, Q.; Xu, Y.; Cvetkovski, S.; Wang, X.; Parajuli, N.; Udo-Inyang, I.; Kaplan, D.; Zhou, L.; et al. Smad2/4 Signaling Pathway Is Critical for Epidermal Langerhans Cell Repopulation Under Inflammatory Condition but Not Required for Their Homeostasis at Steady State. Front. Immunol. 2020, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef]
- Greter, M.; Lelios, I.; Pelczar, P.; Hoeffel, G.; Price, J.; Leboeuf, M.; Kundig, T.M.; Frei, K.; Ginhoux, F.; Merad, M.; et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 2012, 37, 1050–1060. [Google Scholar] [CrossRef]
- Iwasaki, H.; Somoza, C.; Shigematsu, H.; Duprez, E.A.; Iwasaki-Arai, J.; Mizuno, S.; Arinobu, Y.; Geary, K.; Zhang, P.; Dayaram, T.; et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 2005, 106, 1590–1600. [Google Scholar] [CrossRef]
- Chorro, L.; Geissmann, F. Development and homeostasis of ‘resident’myeloid cells: The case of the Langerhans cell. Trends Immunol. 2010, 31, 438–445. [Google Scholar] [CrossRef]
- Tripp, C.H.; Chang-Rodriguez, S.; Stoitzner, P.; Holzmann, S.; Stössel, H.; Douillard, P.; Saeland, S.; Koch, F.; Elbe-Bürger, A.; Romani, N. Ontogeny of Langerin/CD207 expression in the epidermis of mice. J. Investig. Dermatol. 2004, 122, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Schuler, G.; Steinman, R.M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 1985, 161, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.R.; West, H.C.; Henderson, S.; Ushakov, D.S.; Santos, E.S.P.; Strid, J.; Chakraverty, R.; Yates, A.J.; Bennett, C.L. A wave of monocytes is recruited to replenish the long-term Langerhans cell network after immune injury. Sci. Immunol. 2019, 4, eaax8704. [Google Scholar] [CrossRef]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef]
- Chopin, M.; Seillet, C.; Chevrier, S.; Wu, L.; Wang, H.; Morse, H.C., 3rd; Belz, G.T.; Nutt, S.L. Langerhans cells are generated by two distinct PU.1-dependent transcriptional networks. J. Exp. Med. 2013, 210, 2967–2980. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic. Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Yancey, P.G.; Blakemore, J.; Ding, L.; Fan, D.; Overton, C.D.; Zhang, Y.; Linton, M.F.; Fazio, S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arter. Thromb. Vasc. Biol. 2010, 30, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Hayes, C.D.; Thome, J.J.; Thorp, E.; Matsushima, G.K.; Herz, J.; Farber, D.L.; Liu, K.; Lakshmana, M.; Tabas, I. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J. Clin. Investig. 2014, 124, 1296–1308. [Google Scholar] [CrossRef]
- Beisiegel, U.; Weber, W.; Ihrke, G.; Herz, J.; Stanley, K.K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature 1989, 341, 162–164. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Wang, J.; Zhang, R.; Zhang, T.; Wu, Y.; Wang, S.; Xing, D. The ABCA1-efferocytosis axis: A new strategy to protect against atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 518, 1–8. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, S.; Guo, Z.; Xing, D.; Chen, W. The crosstalk of ABCA1 and ANXA1: A potential mechanism for protection against atherosclerosis. Mol. Med. 2020, 26, 84. [Google Scholar] [CrossRef]
- Watts, C.; West, M.A.; Zaru, R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin. Immunol. 2010, 22, 124–130. [Google Scholar] [CrossRef]
- Tripolitsioti, D.; Kumar, K.S.; Neve, A.; Migliavacca, J.; Capdeville, C.; Rushing, E.J.; Ma, M.; Kijima, N.; Sharma, A.; Pruschy, M.; et al. MAP4K4 controlled integrin β1 activation and c-Met endocytosis are associated with invasive behavior of medulloblastoma cells. Oncotarget 2018, 9, 23220–23236. [Google Scholar] [CrossRef] [PubMed]
- Kearns, M.T.; Dalal, S.; Horstmann, S.A.; Richens, T.R.; Tanaka, T.; Doe, J.M.; Boe, D.M.; Voelkel, N.F.; Taraseviciene-Stewart, L.; Janssen, W.J.; et al. Vascular endothelial growth factor enhances macrophage clearance of apoptotic cells. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2012, 302, L711–L718. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.; Traynor, D.; Sander, S.P.; Veltman, D.M.; Pachebat, J.A.; Kay, R.R. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife 2015, 4, e04940. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Ohyagi, H.; Onai, N.; Sato, T.; Yotsumoto, S.; Liu, J.; Akiba, H.; Yagita, H.; Atarashi, K.; Honda, K.; Roers, A.; et al. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity 2013, 39, 584–598. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. J. Immunol. 2017, 199, 2937–2947. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95′s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, M.; Gao, F.; Liu, W.; Yang, Y.; Liu, H.; Cheng, Y.; Liu, C.; Cai, J. Up-regulated expression of miR-23a/b targeted the pro-apoptotic Fas in radiation-induced thymic lymphoma. Cell Physiol. Biochem. 2013, 32, 1729–1740. [Google Scholar] [CrossRef]
- Sabirzhanov, B.; Zhao, Z.; Stoica, B.A.; Loane, D.J.; Wu, J.; Borroto, C.; Dorsey, S.G.; Faden, A.I. Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10055–10071. [Google Scholar] [CrossRef] [PubMed]
- Sabirzhanov, B.; Makarevich, O.; Barrett, J.; Jackson, I.L.; Faden, A.I.; Stoica, B.A. Down-Regulation of miR-23a-3p Mediates Irradiation-Induced Neuronal Apoptosis. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells As Immune Regulators within the Skin. Front. Immunol. 2017, 8, 1941. [Google Scholar] [CrossRef]
- Bauer, T.; Zagórska, A.; Jurkin, J.; Yasmin, N.; Köffel, R.; Richter, S.; Gesslbauer, B.; Lemke, G.; Strobl, H. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. J. Exp. Med. 2012, 209, 2033–2047. [Google Scholar] [CrossRef]
- Schug, J.; McKenna, L.B.; Walton, G.; Hand, N.; Mukherjee, S.; Essuman, K.; Shi, Z.; Gao, Y.; Markley, K.; Nakagawa, M.; et al. Dynamic recruitment of microRNAs to their mRNA targets in the regenerating liver. BMC Genom. 2013, 14, 264. [Google Scholar] [CrossRef]
- Lillis, A.P.; Muratoglu, S.C.; Au, D.T.; Migliorini, M.; Lee, M.J.; Fried, S.K.; Mikhailenko, I.; Strickland, D.K. LDL Receptor-Related Protein-1 (LRP1) Regulates Cholesterol Accumulation in Macrophages. PLoS ONE 2015, 10, e0128903. [Google Scholar] [CrossRef]
- Mueller, P.A.; Kojima, Y.; Huynh, K.T.; Maldonado, R.A.; Ye, J.; Tavori, H.; Pamir, N.; Leeper, N.J.; Fazio, S. Macrophage LRP1 (Low-Density Lipoprotein Receptor-Related Protein 1) Is Required for the Effect of CD47 Blockade on Efferocytosis and Atherogenesis-Brief Report. Arter. Thromb. Vasc. Biol. 2022, 42, e1–e9. [Google Scholar] [CrossRef]
- Kemmerer, M.; Wittig, I.; Richter, F.; Brune, B.; Namgaladze, D. AMPK activates LXRalpha and ABCA1 expression in human macrophages. Int. J. Biochem. Cell Biol. 2016, 78, 1–9. [Google Scholar] [CrossRef]
- Keshet, Y.; Seger, R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Biol. 2010, 661, 3–38. [Google Scholar] [CrossRef]
- Loeb, G.B.; Khan, A.A.; Canner, D.; Hiatt, J.B.; Shendure, J.; Darnell, R.B.; Leslie, C.S.; Rudensky, A.Y. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 2012, 48, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Niisato, M.; Kawasaki, Y.; Karaman, S.; Robciuc, M.R.; Shibata, Y.; Ishida, Y.; Nishio, R.; Masuda, T.; Sugai, T.; et al. VEGF-C/VEGFR-3 signalling in macrophages ameliorates acute lung injury. Eur. Respir. J. 2022, 59, 2100880. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Jost, P.J.; Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Dixit, V.M. Caspases: Intracellular signaling by proteolysis. Cell 1997, 91, 443–446. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Ma, S.; Liu, M.; Xu, Z.; Li, Y.; Guo, H.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2016, 7, 13502–13519. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Parajuli, N.; Wang, Q.; Khalasawi, N.; Peng, H.; Zhang, J.; Yin, C.; Mi, Q.-S.; Zhou, L. MiR-23a Regulates Skin Langerhans Cell Phagocytosis and Inflammation-Induced Langerhans Cell Repopulation. Biology 2023, 12, 925. https://doi.org/10.3390/biology12070925
Wang J, Parajuli N, Wang Q, Khalasawi N, Peng H, Zhang J, Yin C, Mi Q-S, Zhou L. MiR-23a Regulates Skin Langerhans Cell Phagocytosis and Inflammation-Induced Langerhans Cell Repopulation. Biology. 2023; 12(7):925. https://doi.org/10.3390/biology12070925
Chicago/Turabian StyleWang, Jie, Nirmal Parajuli, Qiyan Wang, Namir Khalasawi, Hongmei Peng, Jun Zhang, Congcong Yin, Qing-Sheng Mi, and Li Zhou. 2023. "MiR-23a Regulates Skin Langerhans Cell Phagocytosis and Inflammation-Induced Langerhans Cell Repopulation" Biology 12, no. 7: 925. https://doi.org/10.3390/biology12070925
APA StyleWang, J., Parajuli, N., Wang, Q., Khalasawi, N., Peng, H., Zhang, J., Yin, C., Mi, Q.-S., & Zhou, L. (2023). MiR-23a Regulates Skin Langerhans Cell Phagocytosis and Inflammation-Induced Langerhans Cell Repopulation. Biology, 12(7), 925. https://doi.org/10.3390/biology12070925





