Simple Summary
The in vitro antiprotozoal activity of Hibiscus sabdariffa ethanolic extract was evaluated on the ciliate parasite Philasterides dicentrarchi, which causes severe losses in turbot aquaculture. Our results showed that H. sabdariffa extract had successfully inhibited the parasite population growth rate (IC50 = 1.57 mg mL−1), and caused significant changes in the activity of antioxidant enzymes (LOEC = 0.22 mg mL−1), especially glutathione peroxidase, total glutathione, and catalase. The activity of proteases (virulence factors) was also inhibited (IC50 = 0.76 mg mL−1), and the gene expression of catepsin 90 and leishmanolysin proteases was downregulated. Organic acids and phenolic phytochemicals in hibiscus extract are potentially responsible for the antiprotozoal bioactivity herein determined. Therefore, H. sabdariffa extract can be a promising disease-control alternative against the ciliate proliferation, cellular defense mechanisms, and pathogenicity.
Abstract
Philasterides dicentrarchi is an histophagous parasite that infects flatfish, namely turbot (Scophthalmus maximus), and cause significant losses in aquaculture units. The available measures for P. dicentrarchi control have limited efficiency, and some cause harm to fish. Hence, sustainable and natural control strategies are urgently needed. This study evaluated the in vitro bioactivity of the ethanol extract of Hibiscus sabdariffa calyces on P. dicentrarchi population growth rate (PGR), oxidative stress biomarkers (glutathione-S-transferases (GST), glutathione reductase (GR), glutathione peroxidase (GPx), total glutathione (TG) and catalase (CAT), neurotoxicity (acetylcholinesterase, AChE), activity and gene expression of proteases as major virulence factors. H. sabdariffa extract inhibited parasite PGR (IC50 = 1.57 mg mL−1), and caused significant changes in the activity of antioxidant enzymes (LOEC = 0.22 mg mL−1), especially GPx, TG, and CAT. The activity of proteases was also severely inhibited (IC50 = 0.76 mg mL−1), and gene expression of catepsin 90 and leishmanolysin proteases was downregulated. Organic acids and phenolic phytochemicals in hibiscus extract are potentially responsible for the antiprotozoal bioactivity herein determined. Therefore, H. sabdariffa extract can be a promising disease-control alternative against the ciliate proliferation, cellular defense mechanisms and pathogenicity. Still, its applicability in aquaculture settings, and potential effects on farmed fish, should be further elucidated.
1. Introduction
Philasterides dicentrarchi (subclass Scuticociliatia, phylum Ciliophora) are originally free-living protozoa, which can become opportunistic histophagous parasites capable of causing severe infections and mortality outbreaks in several fish species (e.g., Dicentrarchus labrax, Paralichthys olivaceus) [1]. In particular, turbot (Scophthalmus maximus) produced in intensive aquaculture units [2] has been often affected with the disease provoked by this parasite (i.e., scuticociliatosis), that results in great economic and production losses [1].
Despite the critical constraints frequently caused, the mechanisms of P. dicentrarchi infection and pathogenicity have not been so far fully elucidated. Notwithstanding, there are evidences that a potential infection pathway may be associated with the synthesis and release of proteases by the ciliates [3,4]. Such proteases act as virulence factors by promoting the digestion of fish tissues, modulating the function of leukocytes [5], and contributing to overall evasion from the fish immune response [6,7]. Cysteine proteases are one type of proteases likely involved in parasite infectivity, being thereby considered a potential target for the treatment of scuticociliatosis [3,5]. Leishmanolysin proteases are also critical in host invasion and immune system modulation [7], being upregulated in P. dicentrarchi-infected turbots [8]. Besides the proteases, P. dicentrarchi can benefit from a robust antioxidant system that boosts its resilience to reactive oxygen species (ROS) produced along host’s infection and immune response. Folgueira et al. [9] observed that the generation of toxic products, such as ROS, triggered the activity of antioxidant enzymes in P. dicentrarchi (e.g., superoxide dismutase, catalase, glutathione peroxidase) to ensure the parasites’ survival. Therefore, the analysis of ROS production in P. dicentrarchi can be exploited as a biomarker of oxidative stress and also as a target for controlling the proliferation of the parasite.
Since the appearance of scuticociliatosis events in aquaculture units, several mitigation measures of P. dicentrarchi proliferation have been attempted [10]. Nevertheless, none have so far resulted in an effective control or eradication of the parasite, mainly due to its high virulence, genetic variability and endoparasitic behaviour [4]. Some substances previously tested, such as oxyclozanide and niclosamide, demonstrated to be effective against P. dicentrarchi, but they were toxic to zebrafish [10]; while formalin, which is regularly applied, is a known carcinogen [11]. P. dicentrarchi showed to be sensitive to several antiprotozoal drugs in vitro. However, these substances did not successfully treat systemic infections [2], besides contributing for the environmental burden regarding the widespread of antimicrobial resistance. Efforts have been devoted to the development of vaccines against scuticociliatosis through the stimulation of the fish immune system. Although the created vaccines protected turbot against homologous isolates of P. dicentrarchi, this protection becomes low or null against other non-homologous isolates or serotypes, which regularly co-occur in aquaculture units [2].
Currently, the aquaculture is globally seeking for sustainable and environmental-friendly disease management schemes and products, that may help reaching the United Nations Sustainable Development Goals established for aquaculture. In this context, nature-based solutions have been preferably claimed, in which plants can be excellent candidates, since they are a source of (secondary) metabolites with biological activity. These metabolites have a key role in defense mechanisms against phytopathogens (microbes) and predators [12]. Medicinal plants have already been used as feed additives and antimicrobials in aquaculture, and were shown to stimulate fish growth performance, appetite, and digestive activity, as well as to reduce stress and improve the immune system activity [13].
Hibiscus sabdariffa, commonly known as roselle or hibiscus, is an herbaceous shrub of the Malvaceae family used in traditional gastronomy, as well as for medicinal purposes [14]. The medicinal potential of H. sabdariffa has been associated with the abundance of bioactive substances [12,15], such as tannins, phenols, and flavonoids, among which flavonols and anthocyanins are remarkable for its antioxidant and antibacterial properties [16,17]. Indeed, several studies determined the antimicrobial activity of H. sabdariffa extracts against Gram-negative (e.g., Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhi, Salmonella enteritidis) and Gram-positive bacteria (e.g., Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus), fungi (e.g., Candida albicans), and in lesser extent, protozoans (clinical isolate Giardia lamblia) [12,15,18,19,20]. Nevertheless, the antiprotozoal activity of H. sabdariffa has not yet been tested against this parasite or other ciliates.
Hence, we hypothesized that H. sabdariffa may contain extractable chemical compounds with bioactive nature, capable of impairing the proliferation, antioxidant balance, and infectivity of the parasite. Therefore, the goal of the present work was to evaluate the in vitro effect of H. sabdariffa ethanol extract on the population growth rate, oxidative stress responses, proteases activity and differential expression of relevant protease-encoding genes of P. dicentrarchi. In parallel, a qualitative chemical analysis of the crude extract was performed by UHPLC-ESI-MS to find out the potential presence of bioactive compounds. Our major finding was that the H. sabdariffa crude extract exerted an antiprotozoal activity that may be associated with the presence of certain organic acids and polyphenolic compounds. More specifically, it was observed the impairment of biological responses of P. dicentrarchi, which are pivotal to the physiological balance, virulence and survival of the fish parasite, thereby reinforcing that H. sabdariffa has promising properties to be exploited in the future for mitigating the proliferation of this ciliate in turbot aquaculture.
2. Materials and Methods
2.1. Isolation and Culture of Parasites
P. dicentrarchi individuals were isolated from infected turbots in a local Portuguese aquaculture facility, as previously described by Iglesias et al. [21]. The parasites were cultured in the laboratory using T-25 cell culture flasks containing Leibovitz’s L-15 medium supplemented with FBS (10%) and antibiotic-antimycotic solution (10%) [21]. The cultures were kept at 20 °C ± 1 °C during 4 days, being then sub-cultured into fresh medium.
2.2. Preparation of H. sabdariffa Extract
Dried calyces of H. sabdariffa originally cultured in Egypt were obtained in a local provider (ADP, Lda., Aveiro, Portugal). The calyces were mechanically grounded using a Bosch MKM6000 grinder (Aveiro, Portugal). The powder was immersed in ethanol 96% (1:10 powder:ethanol) and left for 24 h in an orbital shaker at 125 rpm and 25 °C. The resulting mixture was centrifuged at 4000 rpm for 15 min at 4 °C, preserving the supernatant, which was afterwards filtered through qualitative filter paper grade 1. The ethanol was evaporated in a Heidolph Hei-VAP Core rotary evaporator (Schwabach, Germany), at 86 rpm, employing 90 mbar pressure, with a bath temperature of 41 °C. The resulting crude extract was weighed, dissolved in acetone (according to the stock concentration of crude extraction solution prepared), and stored in the dark at 4 °C until further processing.
2.3. Analysis of H. sabdariffa Extract Bioactivity
2.3.1. Effect on Parasite Population Growth
P. dicentrarchi was exposed to a range of sub-lethal concentrations of H. sabdariffa crude extract (0.04–2.00 mg mL−1), which were prepared by dilution with culture medium from a stock solution of 1 g mL−1. Three replicates were considered per test concentration, plus the negative control (no added extract) and solvent control (added with the maximum concentration of acetone, i.e., 100 μL mL−1). The exposures were performed in 24-well plates (initial cell density of 1.5 × 104 cells mL−1), during 96 h (i.e., 4 days), at a temperature of 20 °C ± 1 °C. At the end, P. dicentrarchi cells in each well were fixed with glutaraldehyde, and the number of viable cells were counted in a Neubauer chamber. The population growth was calculated from the determined cell density (cells mL−1), following Equation (1).
where CD96 represents the ciliate cell density at 96 h, and CD0 represents the cell density at 0 h of exposure, while Δt represents the duration of the experiment (4 days). The growth rate was subsequently expressed as a percentage of the negative control. The parasites in the microplate wells were microscopically observed in an inverted microscope (Axio Vert.A1, Zeiss, Jena, Germany) at the beginning and end of the assay.
Growth rate = (Log(CD96) − Log(CD0))/Δt
2.3.2. Oxidative Stress Biomarkers
P. dicentrarchi was exposed to a range of concentrations of the plant extract (0.14–1.94 mg mL−1) in 24-well plates, for 2 h, at a temperature of 20 °C ± 1 °C, for biomarker analyses. The number of replicates per concentration and controls (negative and solvent controls) was 9. At the end of the exposure, the cells were removed from the wells, washed twice in phosphate buffer saline 1×, and resuspended in potassium phosphate buffer (0.1 M, pH 7.4). Cell suspensions were homogenized using an ultrasonic homogenizer (Branson Ultrasonics Sonifier S-250A, Branson Ultrasonics™, Danbury, CT, USA). The post-mitochondrial supernatant was obtained by centrifugation (10,000× g, 15 min, 4 °C), aliquoted and stored at −80 °C until the measurement of the biomarker activities. The assessed biomarkers were glutathione-S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx), total glutathione (TG), catalase (CAT), and acetylcholinesterase (AChE).
The enzymes activities were expressed per mg of protein content in the samples. Protein concentration was determined according to the Bradford method [22], adapted to the microplate format, measuring the absorbance at 595 nm and using bovine γ-globulin as a standard. The GST activity was determined through the method of [23], adapted for microplate [24], quantifying the conjugation of the substrate 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione (GSH). The absorbance was measured at 340 nm and GST activity calculated as nmol CDNB conjugate formed per minute per mg of protein (ε = 9.6 × 10−3 M−1 cm−1). The GR activity protocol was adapted from [25] into microplate format. The reaction medium contained potassium phosphate buffer 0.05 M with reduced nicotinamide-adenine dinucleotide phosphate (NADPH) 0.23 mM, oxidised glutathione (GSSG) 1 mM, and diethylenetriaminepentaacetic acid (DTPA) 0.45 mM. NADPH disappearance was measured at 340 nm and determined as mmol of oxidized NADPH (NADP+) produced per minute per mg of protein (ε = 6.22 × 103 M−1 cm−1). GPx activity assay was adapted from [26,27]. The reaction medium was composed of potassium phosphate buffer 0.05 M, with sodium azide 1 mM, ethylenediaminetetraacetic acid (EDTA) 1 mM, reduced glutathione 4 mM, NADPH 0.8 mM, and glutathione reductase 1 U mL−1. The reaction, starting with the addition of hydrogen peroxide (H2O2) 0.16%, was measured for NADPH disappearance, at 340 nm, and the results were determined as mmol of oxidized NADP+ produced per minute per mg of protein (ε = 6.22 × 103 M−1 cm−1). TG was assessed as described by [28], measuring GSH turnover with 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), in the presence of GR. TG results were expressed as μmol of recycled GSH per minute per mg of protein. CAT was measured according to [29], following the change in absorbance at 240 nm caused by dismutation of H2O2, and activity was expressed in μmol of H2O2 consumed per minute per mg of protein (ε = 40 M−1 cm−1). AChE activity was determined by the Ellman’s method, where thiocholine is formed by the AChE present in the samples, which reacts with DTNB, resulting in the development of a yellow colour, which is measured at 414 nm and presented as nmol of thiocholine produced per minute per mg protein [30].
2.3.3. Proteolytic Activity
P. dicentrarchi was exposed to a range of concentrations of H. sabdariffa extract (0.22–1.94 mg mL−1) in 24-well plates, for 2 h, at 20 °C ± 1 °C, in order to test for its proteolytic activity. Nine replicates were tested per each concentration, including the negative and solvent controls. After exposure, the cells were washed, suspended in potassium phosphate buffer (0.1 M, pH 7.4), homogenized, and centrifuged (10,000× g, 15 min, 4 °C), as described above, and stored at −80 °C until further analysis.
The proteolytic activity of the crude extract was evaluated through the use of the casein-fluorescein isothiocyanate (FITC-casein) assay [31]. P. dicentrarchi lysates and FITC-casein solution were loaded onto a 96-well plate and incubated at 37 °C for 60 min. The fluorescence levels were measured at 0 and 60 min of incubation, at 538 nm emission and 485 nm excitation. A standard curve was performed with a FITC isomer solution serially diluted (0.5–2.5 μM), in order to quantify FITC-casein disassembly into FITC and casein isomers in the presence of the crude extract. The activity of proteases was expressed as nmol min−1 mg prot−1.
2.3.4. Expression Analysis of Protease-Encoding Genes by qRT-PCR
The targeted proteases represented some of the main types of enzymes found in P. dicentrarchi, which have also been associated with fish infection, namely, cathepsin cysteine proteases and leishmanolysins [3,5,8].
The parasites (5 × 106 cells mL−1) were exposed to a minimum (0.14 mg mL−1) and a maximum (0.80 mg mL−1) concentration of hibiscus extract, plus the negative control (culture medium without plant extract added), in 12-well microplates containing a total assay volume of 1 mL. Four replicates were considered per treatment and the assay was carried out at 20 °C ± 1 °C, during 4 h. At the end of the exposure, the suspensions with parasites from each test condition/replicate were individually centrifuged at 700× g for 5 min and washed in PBS 1×. The pelleted parasites were subjected to total RNA extraction using the NZY Total RNA Isolation kit (Nzytech, Lisboa, Portugal), following the manufacturer’s instructions. The total RNA was quantified with Qubit® RNA BR Assay Kit, and 1 μg was used as input to perform the cDNA synthesis with NZY First-Strand cDNA Synthesis Kit (Nzytech, Lisboa, Portugal) according to manufacturer indications. The cDNA was stored at −20 °C until further use, being quantified with Qubit® ssDNA Assay kit (Alfagene, Lisboa, Portugal).
Towards the relative quantification of proteases’ gene expression, a reverse transcription (RT) real-time PCR was run with primers designed from the DNA sequences encoding cathepsin L-like cysteine protease, catepsin 90 protease, and leishmanolysin-family protein (Table 1) of P. dicentrarchi, obtained from Genbank. The primers were synthesized by IDT (Integrated DNA Technologies), being their amplification efficiency between 104.3 and 105.4% (cf. Table 1). The housekeeping gene used for normalizing the qRT-PCR results was β-tubulin. The qRT-PCR reaction contained 1× NZYSpeedy qPCR Green Master Mix (Nzytech, Lisboa, Portugal), 59 ng cDNA, 400 nM of each primer, and RNA-free ultrapure water up to a total reaction volume of 20 μL. Controls with no template were also performed for each primer pair and three biological replicates were performed for each assay. The PCR was performed in a CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Lisboa, Portugal), using the following cycling parameters: 95 °C for 2 min for enzyme activation, 40 cycles of denaturation at 95 °C for 5 s, and annealing at 60 °C for 15 s, and a final extension step at 60 °C for 15 s. In order to confirm the absence of unspecific products of amplification, a melting curve was done under the following conditions: 15 s at 95 °C, 15 s at 55 °C, heating gradually 0.2 °C per 5 s up to 95 °C. The qRT-PCR cycle threshold (Ct) values obtained were expressed as average and standard deviation. For each testing condition was computed the fold change in proteases gene expression following the application of method 2−ΔΔCt [32], being:
where, Ct is the cycle threshold, and PDx represents the target gene x (cf. Table 1).
ΔΔCt = [(Ct(PDx) − Ct(ß-tubulin))extract] − [(Ct(PDx) − Ct(ß-tubulin))negative control]

Table 1.
Proteases, primers, size of expected gene fragments and efficiency of primers in qRT-PCR.
2.4. Chemical Analysis of the Crude Extract
2.4.1. Ultra-High-Performance Liquid Chromatography (UHPLC) Analysis
The separation of the compounds was carried out with a gradient elution program at a flow rate of 0.2 mL min−1, at 30 °C, by using a Hypersil Gold C18 column (100 × 2.1 mm; 1.9 µm) supplied by Thermo Fisher (Thermo Fisher Scientific, San Jose, CA, USA). The injection volume in the UHPLC system was 6 μL and the mobile phase consisted of formic acid 0.1% (A) and acetonitrile (B). The following linear gradient was applied: 0–14.7 min (5–40% B), 14.7–16.6 min (40–100% B), 16.6–18.8 min (100% B) 18.8–24 min (100–5%), followed by re-equilibration of the column for 10 min before the next run. Online detection was carried out in the diode array detector, at 280 nm, and UV spectra in a range of 190–700 nm were recorded.
2.4.2. Electrospray Ionization Mass Spectrometric Detection (ESI–MSn) Analysis
The UHPLC was coupled to an LTQ XL Linear Ion Trap 2D mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA), equipped with an orthogonal electrospray ionization source operating in negative mode. The nitrogen sheath and auxiliary gas were 50 and 10 (arbitrary units), respectively. The spray voltage was 5 kV and the capillary temperature was 275 °C. The capillary and tune lens voltages were set at −28 V and −115 V, respectively. The data acquisition was carried out by using Xcalibur® data system (Thermo Scientific, San Jose, CA, USA). The identification of compounds obtained by chemical analysis of the crude extract was carried out by comparing their chromatographic characteristics (e.g., retention time, UV-Vis maximum absorption (λmax, 190–700 nm), and mass spectrum (m/z)) with data available in the literature.
2.5. Statistical Analysis
Significant differences between the negative control and the solvent control or the extract concentrations were detected through the application of a one-way analysis of variance (one-way ANOVA), followed by the post-hoc multicomparison Dunnett’s or Tukey test (α = 0.05), for the following parameters: population growth rate, oxidative stress biomarkers, protease activity inhibition, differential expression of protease genes. No significant differences were determined between the negative and the solvent controls, thus, the latter was not plotted. The NOEC (no-observed effect concentration) and LOEC (low-observed effect concentration) values were determined for each parameter (except for gene expression outcomes). These analyses were performed using SigmaPlot 14.0. The IC50 (concentration inhibiting 50% of a biological parameter) values were determined by nonlinear regression for the population growth rate and proteases activity, using Graph Pad Prism 8.0 software (GraphPad Software Inc., Boston, MA, USA), being selected the nonlinear model that provided the best fit (higher R2 values) and lower-range confidence intervals.
3. Results
3.1. Effect of Hibiscus Extract on P. dicentrarchi Biological Responses
The growth rate of P. dicentrarchi population had progressively decreased under increasing concentrations of H. sabdrariffa crude extract, being significantly inhibited at a LOEC of 0.29 mg mL−1 with an IC50 of 1.57 mg mL−1 (Figure 1A, Table 2). The steep reduction of the population growth rate under higher doses of the extract was associated with cell disruption and the appearance of aggregates of death cells, as microscopically observed (cf. Figure 1B).
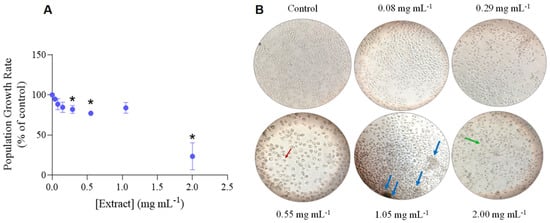
Figure 1.
Effect of Hibiscus sabdariffa ethanol extract on Philasterides dicentrarchi proliferation. (A) Average growth rate of the ciliates under increasing concentrations of hibiscus crude extract ([Extract]). Results are expressed as a percentage of the negative control. The error lines correspond to the standard deviation. * Significantly different from the control (p < 0.05). (B) Photographs of P. dicentrarchi in the negative control and under some concentrations (in mg L−1) of H. sabdariffa extract, obtained in an inverted microscope with 50× or 100× (only for 0.55 mg mL−1 extract) magnification. The red arrow indicates a ciliate with shrunk cell content; the blue arrows highlight agglomerates of dead/disrupted cells; the green arrow points for disrupted cells.

Table 2.
Results of the one-way ANOVA analysis carried out to assess the effects of Hibiscus sabdariffa crude extract in Philasterides dicentrarchi population growth rate, biomarkers, and protease activity. The toxicity point estimates regarding NOEC, LOEC, and IC50 values are also presented (mg mL−1). GST: glutathione-S-transferase; GR: glutathione reductase; GPx: glutathione peroxidase; TG: total glutathione; CAT: catalase; AChE: acetylcholinesterase; PGR: population growth; PA: protease activity; DF: degrees of freedom; SS: sum of squares; MS: mean square; P: probability; NOEC: no-observed effect concentration; LOEC: low-observed effect concentration; IC50: concentration that inhibited a parameter in 50% of the population; CL: confidence limits of the IC50 value; R2: goodness-of-fit of the model adjusted to the experimental data for the derivation of the IC50 value; nd: not determined.
P. dicentrarchi was exposed to several sub-lethal concentrations of H. sabdariffa extract, to evaluate its effect on the ciliates’ redox balance by assaying biotransformation and antioxidant enzyme activities (GST, GR, GPx, TG, CAT), as well as the activity of AChE. A general increase in GST activity was observed in all concentrations, however, only statistically significant at 0.81 mg mL−1 (Figure 2A). There was a significant increase in GR activity at 0.22 and 0.34 mg mL−1, and the lowest concentration caused no effect (Figure 2B). GPx activity remained mostly unchanged at the lowest extract concentrations (0.14 and 0.22 mg mL−1), and significantly increased at 0.34, 0.52, 0.81, 1.25, 1.94 mg mL−1, comparatively to the control (Figure 2C). In terms of TG, there was a significant decrease in TG at concentrations between 0.22 and 1.94 mg mL−1 (Figure 2D). CAT (Figure 2E) exhibited a pattern of increase in activity along increasing concentrations of extract, with the two highest concentrations (1.25 and 1.94 mg mL−1) causing a significant increase comparatively to the control. AChE (Figure 2F) exhibited a pattern of decreasing activity in all concentrations (except the lowest), with a significant decrease in AChE activity on the two highest concentrations (1.25 and 1.94 mg mL−1).
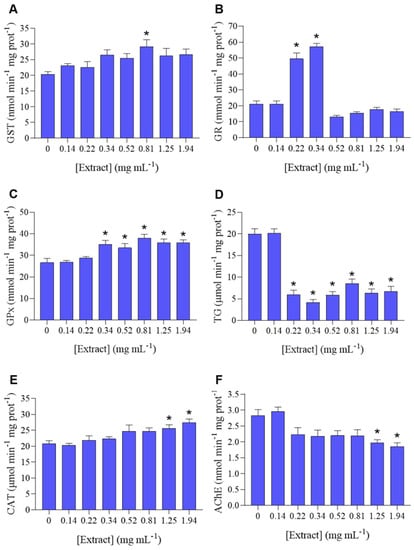
Figure 2.
Oxidative stress biomarkers in Philasterides dicentrarchi exposed to increasing sub-lethal concentrations of Hibiscus sabdariffa crude extract ([Extract]). (A) Glutathione-S-transferase (GST); (B) Glutathione reductase (GR); (C) Glutathione peroxidase (GPx); (D) Total glutathione (TG); (E). Catalase (CAT); (F) Acetylcholinesterase (AChE). Results are expressed as mean ± standard error. * Significantly different from control (p < 0.05).
In what concerns the impact of hibiscus ethanolic extract on the protease activity of P. dicentrarchi, it was observed a significant inhibition under higher concentrations, allowing the estimation of a LOEC of 0.34 mg mL−1 and an IC50 of 0.76 mg mL−1 (Figure 3A, Table 2). For the evaluation of the differential expression of proteases genes, a preliminary step consisted on the analysis of melting curves for each sample and target gene, which confirmed that no unspecific products were formed during the PCR run. Hence, only one peak was observed at the melting temperature of the β-tubulin (housekeeping/reference gene) and target genes (Figure S1). Cathepsin L-like cysteine protease was significantly up-regulated under the higher concentration of the hibiscus ethanolic extract, whilst catepsin 90 was significantly downregulated (Figure 3B). In contrast, the leishmanolysin-family proteases were significantly down-regulated under both concentrations of the H. sabdariffa crude extract (Figure 3B).
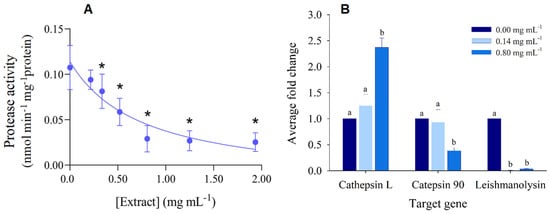
Figure 3.
Effect of hibiscus crude extract on Philasterides dicentrarchi proteases. (A) Protease activity in the presence of increasing concentrations of H. sabdariffa crude extract. The results are expressed as average of protease activity (nmol min−1 mg protein−1). Error lines represent standard deviation. * Significantly different from the control, according to the Dunnett’s test (p < 0.05). (B) Differential expression (normalized relatively to the negative control and the reference gene β-tubulin) of protease-encoding genes under a low and a high concentration of the hibiscus extract. Error lines represent standard deviation. The letters above the bars indicate treatments or concentrations significantly different from each other for the respective target gene, according to the Tukey test (p < 0.05).
3.2. Qualitative Chemical Analysis of Hibiscus Extract
The chemical analysis of H. sabdariffa ethanol crude extract performed through UHPLC-ESI-MSn allowed the identification of two organic acids and fifteen polyphenolic compounds. Among the latter were detected four phenolic acids, six flavonoids, and five anthocyanins. Table 3 lists the tentative identification of the compounds (peak numbered in the chromatogram in Figure 4) and the respective bioactivities reported in the literature.

Table 3.
Hibiscus sabdariffa crude extract compounds tentatively identified by electrospray ionisation mass spectrometry (ESI-MS; [M–H]−), and their respective bioactive function. RT: retention time; λmax: maximum absorbance wavelengths in the UV-VIS spectrum region; nd: not determined. 5-CQA: 5-O-caffeoylquinic acid; FA: formic acid.
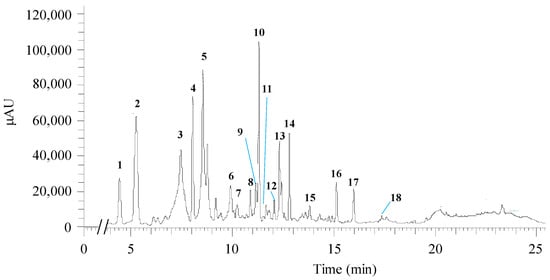
Figure 4.
Chromatographic profile acquired from the qualitative analysis of H. sabdariffa crude ethanolic extract by UHPLC-ESI-MSn. The numbers highlight the base peaks analyzed in Table 3. AU—arbitrary units.
More specifically, at 4.44 min, the ion found with m/z 189 could correspond to hibiscus acid, showing a λmax at 277 nm. A hibiscus acid derivative, hibiscus acid hydroxyethyldimethylesther, was also tentatively identified at 9.91 min. At 5.27 min, the m/z 353 (λmax = 324 nm) was possibly identified as 3-O-caffeoylquinic acid (3-CQA). Another chlorogenic acid was apparently found, 4-O-caffeoylquinic acid (4-CQA), with 8.04 min of retention time, λmax of 325 nm, and m/z 353. Cyanidin 3,5-O-diglucoside was tentatively identified in conjunction with 5-O-caffeoylquinic acid (5-CQA) (retention time: 10.22 min, λmax = 317 nm, m/z: 611, 353). 5-O-caffeoylshikimic acid (m/z: 335) and petunidin-3-O-glucoside (m/z: 479) are the proposed compounds at 10.89 min with λmax = 328 nm.
The 9th peak (Figure 4) was possibly assigned to the flavonoid quercetin-O-sambubioside (RT = 11.16 min, λmax = 354 nm, m/z: 595). At the retention time of 11.31 min, λmax 240 and 325 nm, and m/z 381 the 10th peak (Figure 4) could be associated with the formation of a complex of 5-CQA and formic acid, which was used in the mobile phase for compound elution. Quercetin derivatives could be identified, namely quercetin-3-O-rutinoside (retention time 11.66; λmax 256 and 352 nm; m/z 609) and quercetin 3-O-diglucoside (retention time 12.06 min; λmax 256 and 353 nm; m/z 463). Myricetin was the flavonoid proposed at 13.81 min (peak 15, Figure 4), with λmax 252 and 368 nm, m/z 317. At 15.13 min retention time, the peak could correspond to N-feruloyltyramide (λmax = 240, 291 and 317 nm, and m/z: 312). Quercetin or delphinidin were potentially assigned to the peak at a retention time of 15.97 min, with λmax 255 and 368 nm, and m/z 301; whereas delphinidin-3-O-sambubioside could be recovered at 17.36 min, λmax of 287 nm, and m/z 597.
Overall, among the identified phytochemicals some had previously shown anti-diabetic (hibiscus acid), antioxidant (3-CQA, 4-CQA, 5-CQA, 5-O-caffeoylshikimic acid, petunidin-3-O-glucoside, delphinidin-3-sambubioside, delphinidin-3-O-sambubioside, delphinidine, cyanidin 3,5-O-diglucoside, myricetin, and N-anthocyanins), antimicrobial (e.g., 3-CQA, 4-CQA, 5-CQA, quercetin diglucoside), anti-inflammatory (e.g., cyanidin 3,5-O-diglucoside, quercetin-3-O-diglucoside), and antiprotozoal (quercetin-3-O-rutinoside) activities (Table 3).
4. Discussion
Scuticociliate infections have become an extremely concerning issue in aquaculture units, particularly in turbot aquaculture, given the difficulties in mitigating the outbreaks and rapidly counteract the consequent impacts in the production levels. Thus, it is of paramount importance to optimize or create more sustainable and effective measures to control P. dicentrarchi infections, while promoting a safe and environmentally-protective aquaculture. In this context, plant extracts can potentially eliminate pathogens in fish aquaculture, due to their rich content in secondary and bioactive metabolites. On the other hand, the exploitation of plant-based disease control agents may be more cost-effective and environmentally beneficial, due to their faster biodegradability [54]. Based on these assumptions, the current study focused on bioprospecting H. sabdariffa crude extract and elucidate the qualitative chemical composition, which may sustain its potential antiprotozoal activity against P. dicentrarchi.
The results achieved showed that the hibiscus extract had significantly depleted P. dicentrarchi population growth rate (Figure 1A, Table 2) up to 76.7% inhibition at 2.00 mg mL−1, causing high mortality of the individuals under higher extract concentrations (Figure 1B). As a matter of fact, a decrease in the population growth rate of the ciliates is normally resulting from an impairment on their reproduction and survival rates [55]. The literature is rife of studies (cf. Table 4 for a summary of the most significant works published) proving the antimicrobial activity of H. sabdariffa extracts against Gram-negative and Gram-positive bacteria, and fungi, but the effect on protozoa has been barely evaluated (e.g., [12,15,18,19,20,36]).

Table 4.
Summary of studies assessing effects of plant-based products on the growth and mortality of pathogenic microorganisms. LOEC: low observed effect concentration; MIC: minimum inhibitory concentration (in growth assays with bacteria); * MIC80 (minimum inhibitory concentration causing 80% growth inhibition).
Jabeur et al. [35,36] confirmed the antibacterial and antifungal activities of the hydroethanolic extract and infusion (Minimum Inhibitory Concentrations, MIC: 0.075–0.60 mg mL−1) of H. sabdariffa against a panel of bacteria and fungi species that represent a public health concern. Similarly, Youns et al. [20] determined that the methanol extract of H. sabdariffa exhibited an inhibitory activity against the growth of E. coli, P. aeruginosa, K. pneumoniae, S. typhi, B. subtilis, and S. aureus (MIC: 6.25–12.5 mg mL−1), having an effect equivalent or greater than 0.04 mg mL−1 gentamicin. In turn, the same authors observed that 0.5 mg mL−1 H. sabdariffa methanolic extract caused 72% mortality of the flagellated protozoa G. lamblia, after 72 h of exposure [20], thereby evidencing the antiprotozoal effect of hibiscus. Broadly, the LOEC value herein determined for the P. dicentrarchi population growth rate (0.29 mg mL−1) is within or one-to-two orders of magnitude below the MIC values estimated for bacteria and fungi exposed to extracts of hibiscus prepared with different solvents (Table 4). In fact, the polarity of the solvents used during the extraction procedure constrains the type and amount of the chemical compounds recovered [36]. Consequently, a certain variability on the bioactivity level of the hibiscus extracts is expected, depending on the selected solvent. In the current study was used ethanol since it is considered a green solvent, i.e., it is safe, non-toxic and presents a low environmental risk [56]. Moreover, ethanol has been proving enhanced effectiveness to extract compounds with antimicrobial activity, as compared to other solvents, such as methanol and acetone [57]. Besides solvent polarity, the species- and strains-specific sensitivity can also assume a wide range and trigger different profiles of biological responses, especially when the test organisms (prokaryotes vs. eukaryotes) present distinct physiological and metabolic complexities. In fact, according to Budiño et al. [2] different strains can co-exist in the same aquaculture unit, and these strains may present varying levels of infectivity and sensitivity to anti-protozoal agents. Notwithstanding, the outcome achieved regarding the inhibition of P. dicentrarchi population growth rate suggests that the H. sabdariffa extract may be a valuable alternative in the future to control its proliferation in fish farming.
Species across distinct taxa have developed antioxidant, detoxification, and repair systems to avoid the accumulation of oxidative damage, in which ROS are biologically relevant substances [58,59]. Although the redox system is highly conserved between species, there are significant differences among taxa. In bacteria and invertebrates, the oxidative stress mechanism is speculated to have a major role in the adaptive response to abiotic stressors. However, in protozoa, this mechanism is still mostly unknown [58]. It has been postulated that GST is responsible for the detoxification of xenobiotics, by directly catalyzing biotransformation reactions, besides having an important role in the cellular antioxidant defense [60]. In the present study, the H. sabdariffa ethanolic crude extract caused an overall increase in GST activity (Figure 2A), suggesting that this is a biotransformation mechanism activated by the testing compound within the organism.
GR is a highly specific enzyme responsible for reducing oxidized glutathione (GSSG) into two molecules of reduced glutathione (GSH), thereby maintaining the cellular redox balance by keeping the ratio of GSH to GSSG high [25]. In turn, GPx is responsible for catalyzing the reduction of H2O2, or organic hydroperoxides, into water or corresponding alcohols, using GSH as an electron donor, and oxidizing this molecule into GSSG [61]. GPx and GR maintain the glutathione cycle, as the continual conversion of GSH into GSSG (and vice-versa) protect cells from oxidative injury by removing free radicals produced in the metabolism of xenobiotics [62]. TG was used in the present study as an additional indicator to understand the way P. dicentrarchi manages the exposure to H. sabdariffa extract. Broadly, the tested extract caused a dose-dependent increase in GPx activity and a transient increase in the GR activity (Figure 2B–D), suggesting that H. sabdariffa extract caused oxidative stress in P. dicentrarchi, which triggered the antioxidant system. While the glutathione cycle is ubiquitous, there are organism-specific variations [63]. However, there is still little knowledge on how protozoal species regulate the GSH/GSSG ratio, despite the variability among protozoal species. For instance, apicomplexan protozoa have a well-developed glutathione system, while the amitochondrial protozoans Entamoeba histolytica, Giardia, and Trichomonas do not possess a glutathione metabolism [64]. Plasmodium species have a parasite-specific GR, but contain no true GPx, with the most GPx-like protein being thioredoxin peroxidase [65]. GR and GPx gene expression was identified in the ciliate Euplotes crassus [66], whereas GST and GPx were identified in the ciliate Tetrahymena thermophila [67]. Despite this knowledge for the mentioned parasites, there are currently no such studies unravelling the P. dicentrarchi glutathione metabolism.
CAT represents one of the first lines of antioxidant defense and an immediate protective response to ROS resulting from environmental stress. CAT catalyzes the conversion of H2O2 into water and molecular oxygen in cells exposed to environmental stress [68]. In this work, CAT exhibited a dose-dependent increase in activity, which was significantly promoted under the two highest concentrations (1.25 and 1.94 mg mL−1) (Figure 2E), confirming the activation of the antioxidant system as a result of the exposure to the hibiscus extract. A previous study showed that P. dicentrarchi antioxidant defense was triggered when subjected to natural polyphenolic compounds, however, this field remains vastly unexplored. The exposure of P. dicentrarchi to the natural polyphenol resveratrol caused dose-dependent CAT inhibition and GST induction, as well as a significant reduction in oxygen consumption and increased ROS production, which are indicative of oxidative stress and inability to eliminate ROS [69].
The cholinesterase activity of P. dicentrarchi was significantly decreased under 1.25 and 1.94 mg mL−1 H. sabdariffa ethanolic extract (Figure 2F). The activity of cholinesterases is usually considered a marker of neuronal function in ecotoxicity. However, the presence and function of cholinesterases in protozoa is barely understood [70], and no studies were found regarding P. dicentrarchi cholinesterases. It has been speculated that the fibrillar structure connecting the bases of cilia is a primitive conducting organ analogous to the nervous system. Thus, the presence of cholinesterase in ciliate protozoa could be correlated with motility, as well as reproduction [71], what could partly underpin the observed significant depletion of the population growth rate. The presence of the cholinergic system in Colpoda sp. seems to be connected to cell-to-cell interaction other than cell motility [72]. Moreover, AChE-like proteins have been detected in E. crassus and Paramecium primaurelia [72,73]. AChE expression has been found in Trypanosoma evansi, possibly with the purpose of regulating acetylcholine, similarly to mammals [74].
P. dicentrarchi secrete proteases, most predominantly cysteine proteases of the cathepsin L, B and D protease subfamilies, which mediate host entry, the spread of the infection, and the disruption or modification of the function of immune cells and molecules [3,5]. In particular, cysteine proteases can degrade haemoglobin, as well as type-I collagen, a protein component of the vertebrate tissues [3], thereby favoring the progressive digestion of fish tissues. Previous studies have reported a dose-dependent increase in the activity of the protease caspase-3-like in P. dicentrarchi incubated with turbot leucocytes [5], followed by the apoptosis of these immune cells. This outcome evidenced that proteases may be a tool for ciliates to evade the fish immune response and sustain their histophagous behavior. In the present study, the H. sabdariffa ethanolic extract caused a significant dose-dependent decrease in the activity of P. dicentrarchi proteases (Figure 3A, Table 2), reaching an inhibition of 73% at 0.81 mg mL−1 of extract.
In order to get a closer understanding of the influence of hibiscus extract on the protease activity inhibition, the expression of specific P. dicentrarchi protease genes was analyzed. Although the expression of cathepsin L-like cysteine protease was notably stimulated by the hibiscus extract, the catepsin 90 protease was significantly downregulated at 0.80 mg mL−1. Hence, a successful outcome was accomplished for catepsin 90, but not entirely to cathepsin L-like cysteine. Notwithstanding, it should be further analyzed the efficiency range of this extract towards the inhibition of this and other proteases, in tandem with the expression profiles along fish infection. In fact, despite the previously reported role of cathepsins in host infection by P. dicentrarchi [3,4,5], they were already found to be downregulated in ciliates infecting turbot [8] and the American lobster [75]. In opposition, leishmanolysins were found to be upregulated in ciliates isolated from infected turbot [8]. Leishmanolysins are important virulence-mediator metalloproteases originally found on the membrane of Leishmania bacterial cells [76]. Leishmanolysins (e.g., gp63) are able to destroy cells and molecules, such as molecules involved in the humoral immune response of the host [77], thereby helping the parasite to evade host immune system. In our study, the expression of these proteases was downregulated under both concentrations of the hibiscus ethanolic extract (Figure 3B). Therefore, the hibiscus extract can block this process, by enhance the susceptibility of P. dicentrarchi to the turbot immune system, hence reducing its infectivity.
Plants are rich in protease inhibitors that mediate defense mechanisms against predators and phytopathogens. Such protease inhibitors can also assume an active role as signaling molecules in several metabolic pathways [78]. Protease inhibitors have been previously identified in H. sabdariffa [79], namely roseltides, which are cysteine-rich knottin-type protease inhibitors [80,81]. Besides, protease inhibitors against angiotensin I converting enzyme have been also detected, such as flavones, elastase, trypsin, and alpha-chymotrypsin [82]. Considering that the use of protease inhibitors has been highlighted as a valuable alternative for the treatment of protozoan infections (e.g., [83]), the inhibitory effect of the hibiscus extract is significantly attractive to build-up sustainable measures for controlling the virulent action of P. dicentrarchi.
The overall antiprotozoal effect of the ethanolic extract of hibiscus calyces can be attributed to the presence of organic acids, phenolic acids, flavonoids, and anthocyanins with bioactive nature (Table 3). Considering that the produced extract is a complex mixture of such bioactive phytochemicals, a synergistic effect from their combined action is likely to further induce the observed damages in several biological responses of the ciliates.
Previous studies had reported the chemical composition of H. sabdariffa extracts and, despite some variations on the identified compounds and their concentrations, they are consensual in highlighting the abundance of organic acids and, particularly, of phenolic compounds (e.g., [14,15]). The ethanolic extract of H. sabdariffa analyzed by Borrás-Linares et al. [15] presented organic acids like hibiscus acid, hydroxycitric acid, citric acid, and protocatechuic acid, among which the hibiscus acid was also identified in our study. Hibiscus acids are derived from (2S,3R)-2-hydroxycitric acid, whose biosynthetic pathway has not yet been unraveled, since hibiscus acid(-derived) substances are not synthesized by all plants [84]. Notwithstanding, the hibiscus acid has been pointed out as a fulcral organic acid for the bioactivity of H. sabdariffa extract [14,15,36].
Within the phenolic compounds (i.e., phenolic acids, flavonoids, and anthocyanins), phenolic acids are usually the most represented in hibiscus extracts, with particular abundance of 3-CQA, 4-CQA, 5-CQA [14,15,36,85], which were tentatively assigned to several compound peaks recovered from our ethanolic crude extract (cf. Figure 4, Table 3). 5-CQA has been shown to have antimicrobial action against E. coli, S. aureus, Enterococcus faecium, Proteus vulgaris, P. aeruginosa, K. pneumoniae and C. albicans [44]. 4-CQA has strong antioxidant activity, as well as antimicrobial and anticancer activities [86].
Flavonoids are other class of phenolic compounds detected in the extract, being quercetin, quercetin-3-O-rutinoside, quercetin-O-sambubioside, quercetin-3-O-diglucoside, myricetin, and N-feruloyltyramide, some of the flavonoids likewise determined in related works (e.g., [15,33,35,36,39]). Quercetin-3-O-diglucoside is a naturally occurring form of the flavonol quercetin with anti-inflammatory activity [49,87], while quercetin-3-O-rutinoside is composed of quercetin and rutinose, having anti-allergenic, anti-inflammatory, antitumor, antibacterial, and antiprotozoal activities [50].
Anthocyanins have been frequently identified in extracts of H. sabdariffa and/or within the Hibiscus genus, being the major responsible compounds for the hibiscus pigment. The often detected anthocyanins are delphinidin-3-glucoside, cyanidin-3-glucoside, delphinidin-3-sambubioside and cyanidin-3-sambubioside [36,84,88,89,90,91]. Similarly, our ethanolic extract presented derivative compounds, such as cyaniding-3,5-O-diglucoside, petunidin-3-O-glucoside, delphinidin, and delphinidin-3-O-sambubioside (Table 3). The cyaniding-3,5-O-diglucoside has anti-inflammatory properties [92], whilst petunidin-3-O-glucoside and delphinidin have high antioxidant and/or antimicrobial activity [93,94].
Thus, the phytochemicals detected in H. sabdariffa ethanolic crude extract may explain its effects on P. dicentrarchi population growth rate, oxidative stress, neurotoxicity and proteases inhibition, due to their known antioxidant, antimicrobial, and, most importantly, antiprotozoal activity.
5. Conclusions
H. sabdariffa ethanolic crude extract was able to damage P. dicentrarchi at the molecular (biochemical), cellular (gene expression), individual (reproduction and mortality), and population (proliferation) level. The highest extract concentration caused a 76.7% decline in the population growth rate, which was linked to a severe cell disruption and mortality of the individuals. Moreover, it was demonstrated that sub-lethal concentrations of H. sabdariffa extract caused oxidative stress and cholinesterase inhibition in P. dicentrarchi, with expressive and significant shifts in the activity of the enzymes. These results are further validated by over 73% inhibition of the protozoal protease activity, and the expression inhibition of genes encoding proteases that mediate the parasites’ infectivity.
The mixture of organic acids and phenolic phytochemicals tentatively identified in H. sabdariffa ethanolic extract may trigger its antiprotozoal activity, making this extract efficacious towards the mitigation of P. dicentrarchi proliferation, impairment of its redox balance and general inhibition of crucial virulence factors. Hence, this extract shows favourable properties and should be further investigated to unravel its effect on farmed fish, as well as to evaluate its applicability in sustainable disease-control alternatives for intensive aquaculture settings engaged with high standards of quality, security and welfare in turbot production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12070912/s1, Figure S1: Melt curves obtained in qRT-PCR runs for β-tubulin (reference gene), and cathepsin L-like cysteine, catepsin 90 and leishmanolysin protease genes.
Author Contributions
Conceptualization, C.R.M.; methodology, A.C., I.D., C.C., A.M.S.S., A.M.V.M.S. and C.R.M.; investigation, A.C., I.D., A.M.V.M.S. and C.R.M.; resources, A.M.S.S., A.M.V.M.S. and C.R.M.; writing—original draft preparation, A.C.; writing—review and editing, A.C., I.D., A.M.S.S., A.M.V.M.S. and C.R.M.; supervision, C.R.M.; funding acquisition, A.M.V.M.S. and C.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mar 2020 through PT 2020 and the European Maritime and Fisheries Fund (EMFF) to the MAXIAQUA project (MAR-02.02.01-FEAMP-0019). Ana Carvalho worked under a fellowship granted by the MAXIAQUA Project. Catarina R. Marques is funded by national funds (OE), through FCT (Decree-Law 57/2016, of August 29, changed by Law 57/2017 of July 19). We are grateful to the financial support to CESAM by FCT/MCTES (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020), through national funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We are grateful to the support of all professionals of Stolt Sea Farm—Portugal for the continuous availability, dedication, engagement and collaborative work. Some BioRender.com images were used to create the Graphical Abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.M.; Cho, J.B.; Kim, S.K.; Nam, Y.K.; Kim, K.H. Occurrence of scuticociliatosis in olive flounder Paralichthys olivaceus by Phiasterides dicentrarchi (Ciliophora: Scuticociliatida). Dis. Aquat. Org. 2004, 62, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Budiño, B.; Leiro, J.; Cabaleiro, S.; Lamas, J. Characterization of Philasterides dicentrarchi isolates that are pathogenic to turbot: Serology and cross-protective immunity. Aquaculture 2012, 364–365, 130–136. [Google Scholar] [CrossRef]
- Paramá, A.; Iglesias, R.; Álvarez, M.F.; Leiro, J.; Ubeira, F.M.; Sanmartín, M.L. Cysteine proteinase activities in the fish pathogen Philasterides dicentrarchi (Ciliophora: Scuticociliatida). Parasitology 2004, 128, 541–548. [Google Scholar] [CrossRef]
- Shin, S.P.; Han, S.Y.; Han, J.E.; Jun, J.W.; Kim, J.H.; Park, S.C. Expression and characterization of cathepsin L-like cysteine protease from Philasterides dicentrarchi. Parasitol. Int. 2014, 63, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Paramá, A.; Castro, R.; Lamas, J.; Sanmartín, M.L.; Santamarina, M.T.; Leiro, J. Scuticociliate proteinases may modulate turbot immune response by inducing apoptosis in pronephric leucocytes. Int. J. Parasitol. 2007, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dzik, J.M. Molecules released by helminth parasites involved in host colonization. Acta Biochim. Pol. 2006, 53, 33–64. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Caffrey, C.; Kelly, B.; Loke, P.; Sajid, M. Proteases in parasitic diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 497–536. [Google Scholar] [CrossRef]
- Valle, A.; Leiro, J.M.; Pereiro, P.; Figueras, A.; Novoa, B.; Dirks, R.P.H.; Lamas, J. Interactions between the parasite Philasterides dicentrarchi and the immune system of the turbot Scophthalmus maximus. A transcriptomic analysis. Biology 2020, 9, 337. [Google Scholar] [CrossRef]
- Folgueira, I.; Lamas, J.; de Felipe, A.P.; Sueiro, R.A.; Leiro, J.M. Identification and Molecular Characterization of Superoxide Dismutases Isolated From A Scuticociliate Parasite: Physiological Role in Oxidative Stress. Sci. Rep. 2019, 9, 13329:1–13329:14. [Google Scholar] [CrossRef]
- Dai, J.R.; Wang, W.; Liang, Y.S.; Li, H.J.; Guan, X.H.; Zhu, Y.C. A novel molluscicidal formulation of niclosamide. Parasitol. Res. 2008, 103, 405–412. [Google Scholar] [CrossRef]
- Cogliano, V.J.; Grosse, Y.; Baan, R.A.; Straif, K.; Secretan, M.B.; Ghissassi, F.E. Meeting Report: Summary of IARC Monographs on Formaldehyde, 2-Butoxyethanol, and 1-tert-Butoxy-2-Propanol. Environ. Health Perspect. 2005, 113, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Esherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.; Chen, Q. Effects of garlic powder on feeding attraction activity, growth and digestive enzyme activities of Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 2020, 26, 390–399. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, L.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crop. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Mak, Y.W.; Chuah, L.O.; Ahmad, R.; Bhat, R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. J. King Saud Univ. Sci. 2013, 25, 275–282. [Google Scholar] [CrossRef]
- Higginbotham, K.L.; Burris, K.P.; Zivanovic, S.; Davidson, P.M.; Stewart, C.N. Antimicrobial activity of Hibiscus sabdariffa aqueous extracts against Escherichia coli O157:H7 and Staphylococcus aureus in a microbiological medium and milk of various fat concentrations. J. Food Prot. 2014, 77, 262–268. [Google Scholar] [CrossRef]
- Mensah, J.K.; Golomeke, D. Antioxidant and antimicrobial activities of the extracts of the calyx of Hibiscus sabdariffa Linn. Curr. Sci. Perspect. 2015, 1, 69–76. [Google Scholar]
- Youns, M.A.D.; Hashim, A.I.; Suliman, I.S.; Alla, A.B.A. In vitro evaluation of the antimicrobial and antioxidant activity of Hibiscus sabdariffa leaves. J. Dent. Med. Sci. 2018, 17, 69–74. [Google Scholar]
- Iglesias, R.; Paramá, A.; Álvarez, M.F.; Leiro, J.; Aja, C.; Sanmartín, M.L. In vitro growth requirements for the fish pathogen Philasterides dicentrarchi (Ciliophora, Scuticociliatida). Vet. Parasitol. 2003, 111, 19–30. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Guilhermino, L. Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol. Biochem. 2002, 26, 149–156. [Google Scholar] [CrossRef]
- Schaedle, M.; Bassham, J.A. Chloroplast Glutathione Reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Cichoski, A.J.; Rotta, R.B.; Scheuermann, G.; Cunha Junior, A.; Smanioto Barin, J. Investigation of glutathione peroxidase activity in chicken meat under different experimental conditions. Food Sci. Technol. 2012, 32, 661–667. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Claiborne, A. Handbook of Methods for Oxygen Radical Research, 1st ed.; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; Catalase Activity; pp. 283–284. ISBN 9781351072922. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cupp-Enyard, C.; Aldrich, S. Use of the Protease Fluorescent Detection Kit to Determine Protease Activity. J. Vis. Exp. 2009, 30, 1514:1–1514:3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Molina, V.M.; Alonso-Villaverde, C.; Joven, J.; Menéndez, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J. Sep. Sci. 2009, 32, 3441–3448. [Google Scholar] [CrossRef]
- Ramirez-Rodrigues, M.M.; Plaza, M.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Caleja, C.; Calhelha, R.C.; Soković, M.; Catarino, L.; Barros, L.; Ferreira, I.C.F.R. Exploring the chemical and bioactive properties of: Hibiscus sabdariffa L. calyces from Guinea-Bissau (West Africa). Food Funct. 2019, 10, 2234–2243. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure-activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 2011, 59, 502–507. [Google Scholar] [CrossRef]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-Caffeoylquinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT Food Sci. Technol. 2006, 65, 471–479. [Google Scholar] [CrossRef]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef]
- Long, Q.; Chen, H.; Yang, W.; Zhang, L. Delphinidin-3-sambubioside from Hibiscus sabdariffa. L attenuates hyperlipidemia in high fat diet-induced obese rats and oleic acid-induced steatosis in HepG2 cells. Bioengineered 2021, 12, 3837–3849. [Google Scholar] [CrossRef]
- Lazalde-Cruz, R.; Miranda-Romero, L.A.; Tirado-González, D.N.; Carrillo-Díaz, M.I.; Medina-Cuéllar, S.E.; Mendoza-Martínez, G.D.; Lara-Bueno, A.; Tirado-Estrada, G.; Salem, A.Z.M. Potential Effects of Delphinidin-3-O-sambubioside and Cyanidin-3-O-sambubioside of Hibiscus sabdariffa L. in Ruminant Meat and Milk Production and Quality. Animals 2021, 11, 2827. [Google Scholar] [CrossRef]
- Segura-Carretero, A.; Puertas-Mejía, M.A.; Cortacero-Ramírez, S.; Beltrán, R.; Alonso-Vilaverde, C.; Joven, J.; Dinelli, G.; Fernández-Gutiérrez, A. Selective extraction, separation, and identification of anthocyanins from Hibiscus sabdariffa L. using solid phase extraction-capillary electrophoresis-mass spectrometry (time-of-flight/ion trap). Electrophoresis 2008, 29, 2852–2861. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Alonso-Villaverde, C.; Aragonès, G.; Rodríguez-Medina, I.; Rull, A.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Camps, J.; Joven, J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mitra, A. The antioxidant and antimicrobial activity properties of the methanolic extract from Cocos nucifera mesocarp. Food Chem. 2008, 107, 994–999. [Google Scholar] [CrossRef]
- García-Marino, M.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Colour and pigment composition of red wines obtained from co-maceration of Tempranillo and Graciano varieties. Anal. Chim. Acta 2010, 660, 134–142. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.M.; DeWitt, C.A.M. Analysis of phenolic compounds in commercial dried grape pomace by high-performance liquid chromatography electrospray ionization mass spectrometry’. Food Sci. Nutr. 2014, 2, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Academic Press: London, UK; Elsevier: London, UK, 2019; pp. 457–479. ISBN 978-0-12-813820-5. [Google Scholar]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, L.; Wang, M.H. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: Involvement of AP-1 and MAP kinase signalling pathways. Chem. Biol. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M.; el-Din, S.H.; Saleh, S.; El-Lakkany, N.M.; Hammam, O.A.; Salem, M.B.; Botros, S.S. Metabolic profile and hepatoprotective activity of the anthocyanin-rich extract of Hibiscus sabdariffa calyces. Pharm. Biol. 2016, 54, 3172–3181. [Google Scholar] [CrossRef]
- Osman, A.G.; Chittiboyina, A.G.; Khan, I.A. Cytoprotective role of dietary phytochemicals against cancer development via induction of phase II and antioxidant enzymes. In Advances in Molecular Toxicology, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2016; Volume 10, pp. 99–137. ISBN 978-0-12-804700-2. [Google Scholar]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2015, 433, 50–61. [Google Scholar] [CrossRef]
- Jack, J.D.; Gilbert, J.J. Effects of Metazoan Predators on Ciliates in Freshwater Plankton Communities. J. Eukaryot. Microbiol. 1997, 44, 194–199. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Costantini, D. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage between Mechanistic and Evolutionary Approaches, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-54662-4. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978–0–19–871747–8. [Google Scholar]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Chan, H.W.; Yu, L.C. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology 2006, 226, 126–130. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Banyal, H.S.; Sharma, S.K. Glutathione metabolism in parasitic protozoa. J. Parasit. Dis. 2007, 31, 92–102. [Google Scholar]
- Müller, S. Role and regulation of glutathione metabolism in Plasmodium falciparum. Molecules 2015, 20, 10511–10534. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.J.; Lee, J.S.; Lee, Y.M. Acute effects of heavy metals on the expression of glutathione-related antioxidant genes in the marine ciliate Euplotes crassus. Mar. Pollut. Bull. 2014, 85, 455–462. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, Q.; Gong, J. Comparative transcriptomics reveals distinct gene expressions of a model ciliated protozoan feeding on bacteria-free medium, digestible, and digestion-resistant bacteria. Microorganisms 2020, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Poll. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.; Morais, P.; Arranz, J.A.; Sanmartín, M.L.; Orallo, F.; Leiro, J. Resveratrol promotes an inhibitory effect on the turbot scuticociliate parasite Philasterides dicentrarchi by mechanisms related to cellular detoxification. Vet. Parasitol. 2009, 161, 307–315. [Google Scholar] [CrossRef]
- Amaroli, A. Neurotransmitters or Biomediators? The Cholinergic System in Protozoa. EC Microbiol. 2017, 7, 40–41. [Google Scholar]
- Bullock, T.H.; Nachmansohn, D. Choline esterase in primitive nervous systems. J. Cell. Comp. Physiol. 1942, 20, 239–242. [Google Scholar] [CrossRef]
- Trielli, F.; Amaroli, A.; Sifredi, F.; Marchi, B.; Falugi, C.; Corrado, M.U.D. Effects of xenobiotic compounds on the cell activities of Euplotes crassus, a single-cell eukaryotic test organism for the study of the pollution of marine sediments. Aquat. Toxicol. 2007, 83, 272–283. [Google Scholar] [CrossRef]
- Corrado, M.U.D.; Politi, H.; Trielli, F.; Angelini, C.; Falugi, C. Evidence for the presence of a mammalian-like cholinesterase in Paramecium primaurelia (Protista, ciliophora) developmental cycle. J. Exp. Zool. 1999, 283, 102–105. [Google Scholar] [CrossRef]
- Wolkmer, P.; da Silva, C.B.; Paim, F.C.; Da Silva, A.S.; Tavares, K.C.S.; Lazzarotto, C.R.; Palma, H.E.; Thomé, G.R.; Miletti, L.C.; Schetinger, M.R.C.; et al. Biochemistry detection of acetylcholinesterase activity in Trypanosoma evansi and possible functional correlations. Exp. Parasitol. 2012, 132, 546–549. [Google Scholar] [CrossRef]
- Acorn, A.R.; Fraser Clark, K.; Jones, S.; Després, B.M.; Munro, S.; Cawthorn, R.J.; Greenwood, S.J. Analysis of expressed sequence tags (ESTs) and gene expression changes under different growth conditions for the ciliate Anophryoides haemophila, the causative agent of bumper car disease in the American lobster (Homarus americanus). J. Inverteb. Pathol. 2011, 107, 146–154. [Google Scholar] [CrossRef]
- Murase, L.S.; Souza, J.V.P.; Neto, Q.A.L.; Mello, T.F.P.M.; Cardoso, B.M.; Lera-Nonose, D.S.S.L.; Teixeira, J.J.V.T.; Lonardoni, M.V.C.; Demarchi, I.G. The role of metalloproteases in Leishmania species infection in the New World: A systematic review. Parasitology 2018, 145, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Thiakaki, M.; Kolli, B.; Chang, K.P.; Soteriadou, K. Down-regulation of gp63 level in Leishmania amazonensis promastigotes reduces their infectivity in BALB/c mice. Microbes Infect. 2006, 8, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Abu-Tarboush, H.M.; Ahmed, S.A.B. Studies on karkade (Hibiscus sabdariffa): Protease inhibitors, phytate, in vitro protein digestibility and gossypol content. Food Chem. 1996, 56, 15–19. [Google Scholar] [CrossRef]
- Loo, S.; Kam, A.; Xiao, T.; Nguyen, G.K.T.; Liu, F.; Tam, J.P. Identification and characterization of roseltide, a knottin-type neutrophil elastase inhibitor derived from Hibiscus sabdariffa. Sci. Rep. 2016, 6, 39401. [Google Scholar] [CrossRef]
- Kam, A.; Loo, S.; Fan, J.S.; Sze, S.K.; Yang, D.; Tam, J.P. Roseltide rT7 is a disulfide-rich, anionic, and cell-penetrating peptide that inhibits proteasomal degradation. J. Biol. Chem. 2019, 294, 19604–19615. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Wabel, N.; Blunden, G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phytother. Res. 2005, 19, 369–375. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Rosenthal, P.J.; Swenerton, R.; Doyle, P. Development of protease inhibitors for protozoan infections. Curr. Opin. Infect. Dis. 2008, 21, 668–672. [Google Scholar] [CrossRef]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Ferro, V.A.; Drummond, R.M. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef]
- Alonso, J.; Zamilpa, A.; Aguilar, F.A.; Herrera-Ruiz, M.; Tortoriello, J.; Jimenez-Ferrer, E. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J. Ethnopharmacol. 2012, 139, 751–756. [Google Scholar] [CrossRef]
- Ganzon, J.G.; Chen, L.G.; Wang, C.C. 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 2018, 26, 985–993. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Tong, X.; Terahara, N.; Luo, D.; Fujii, M. Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces apoptosis in human leukemia cells through reactive oxygen species-mediated mitochondrial pathway. Arch. Biochem. Biophys. 2005, 440, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Abeda, H.Z.; Kouassi, M.K.; Yapo, K.D.; Koffi, E.; Sie, R.S.; Kone, M.; Kouakou, H.T. Production and enhancement of anthocyanin in callus line of roselle (Hibiscus sabdariffa L.). Int. J. Recent Biotechnol. 2014, 2, 45–56. [Google Scholar]
- Opletal, L.; Chocholousova-Havlikova, L.; Siatka, T.; Cahliková, L.; Locarek, M.; Ali, B.H.; Manoj, P.; Ramkumar, A.; Al Suleimani, Y.M.; Al Za’abi, M.; et al. Preparation and validated analysis of anthocyanin concentrate from the calyces of Hibiscus sabdariffa. Nat. Prod. Commun. 2017, 12, 43–45. [Google Scholar] [CrossRef]
- Gandhi, S.P.; Lokhande, K.B.; Swamy, V.K.; Nanda, R.K.; Chitlange, S.S. Computational data of phytoconstituents from Hibiscus rosa-sinensis on various anti-obesity targets. Data Brief 2019, 24, 103994. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).