Simple Summary
In colorectal carcinoma, migration of the cancer cells greatly contributes to the progression of the disease. One of the driving factors for a directional migration could be the presence of direct-current electrical fields, which is known as galvanotaxis. To investigate migration of colorectal cancer cells in direct-current electrical fields, we employed five low-passage cell lines that were derived from surgical specimens. In three out of five cell lines, a preferred cathodal migration was determined. Exposure to electrical fields in vitro had no effect on cellular integrity. Furthermore, we found voltage-gated calcium channels crucial in galvanotaxis. Intracellular signaling pathways based on the kinases MEK and AKT were identified as being involved in the migratory phenotype. We conclude that colorectal cancer cells are capable of galvanotactic migration. The directional migration was dependent on calcium influx and activation of central signaling pathways of colorectal cancer.
Abstract
Several cues for a directional migration of colorectal cancer cells were identified as being crucial in tumor progression. However, galvanotaxis, the directional migration in direct-current electrical fields, has not been investigated so far. Therefore, we asked whether direct-current electrical fields could be used to mobilize colorectal cancer cells along field vectors. For this purpose, five patient-derived low-passage cell lines were exposed to field strengths of 150–250 V/m in vitro, and migration along the field vectors was investigated. To further study the role of voltage-gated calcium channels on galvanotaxis and intracellular signaling pathways that are associated with migration of colorectal cancer cells, the cultures were exposed to selective inhibitors. In three out of five colorectal cancer cell lines, we found a preferred cathodal migration. The cellular integrity of the cells was not impaired by exposure of the cells to the selected field strengths. Galvanotaxis was sensitive to inhibition of voltage-gated calcium channels. Furthermore, signaling pathways such as AKT and MEK, but not STAT3, were also found to contribute to galvanotaxis in our in vitro model system. Overall, we identify electrical fields as an important contributor to the directional migration of colorectal cancer cells.
1. Introduction
Colorectal carcinoma (CRC) represents the third-most-common tumor disease and is the fourth-leading cause of cancer-related deaths in the world [1,2]. In most cases, metastases are the main reason for the high rate of mortality. Common sites of metastasis are the liver and the peritoneum [3]. Metastasization is a complex multi-step process of epithelial–mesenchymal transition, migration, and invasion [4]. Migration of the cancer cells is believed to be multifactorial, driven by several stimuli, such as chemotactic agents and haptotactic and durotactic cues [5]. Another driving force for a directional migration could be galvanotaxis. Galvanotaxis (also referred to as electrotaxis) is the migration of cells along direct-current electrical fields (DCEF), experimentally presented as a permanent stimulus or in a pulsed manner [6]. Under physiological conditions, DCEF arise from transepithelial potentials (TEP) that emerge from asymmetric ion flux over plasma membranes [7,8]. As a result, the membrane of cells is depolarized on the cathodal side and hyperpolarized on the anodal one within the electrical field. Our current understanding also includes a second basic mechanism, a phenomenon termed electromigration [9,10]. It has been shown that membrane-bound electrically charged components such as receptors and ion channels may be electrophoretically distributed to the anode or cathode under DCEF conditions. Hence, via electromigration, membrane-bound receptors may predominantly cluster within the membrane on one or the other side of the cells.
Electrical fields may occur in the colonic epithelium due to its major role in chloride secretion and absorption (reviewed by Negussie et al., Cells 2022 [11]). Since net chloride secretion through basolateral NKCC1 and apical CFTR dominates transepithelial electrolyte transport in the colon mucosa [12], there is a typical lumen-negative TEP of roughly −10 mV in mice [13] and −30 mV in healthy humans [14]. Although there might be some decrease in the lumen-negative TEP along the colonic longitudinal axis, since chloride absorption processes are more relevant in the distal colon [15], it is well conceivable that the lumen-negative TEP over a single cell layer (approx. 100 µm) could create a permanent electrical field of >250 V/m. For this reason, we suppose that it could be possible for cathodic migration of colorectal cancer cells, i.e., towards the lumen, to take place in the human colon.
To the best of our knowledge, no data for the migratory behavior of CRC cells in DCEF have been published so far. However, in various solid malignancies such as brain cancer [16,17,18,19], lung cancer [20,21,22,23], prostate cancer [24], and breast cancer [25,26] migration in the DCEF has been reported. In the migration of cells, the influx of Ca2+ and the intracellular Ca2+ concentration play major roles [5], which also applies to CRC cells [27]. Interestingly, the expression of Ca2+-permeable ion channels has been found to be altered in CRC [28,29,30] and has been associated with tumor progression [27,31]. A high dependency on Ca2+ influx contributes to invasion and migration [27], but may also affect the directional motility in electrical fields [32].
Therefore, we hypothesize that CRC cells exhibit a directional migration in the DCEF. As a biological CRC in vitro model, we employed a panel of patient-derived low-passage cell lines that were established from primary tumors and a metastasis from surgical CRC patients. In the current study, the cells were challenged with different electrical field strengths, and their migration in 2D galvanotaxis chambers was determined. Moreover, the role of Ca2+ influx and the impact of signaling pathways such as PI3K/AKT, Raf/MEK/ERK, and STAT3 on directional migration in the electrical field were investigated.
2. Materials and Methods
2.1. Patient-Derived Low-Passage Cell Lines
Primary CRC resection specimens and a liver metastasis were obtained from surgery at the clinic of general surgery of the Rostock University Medical Center, with informed written patient consent. All procedures were approved by the Ethics Committee of the Rostock University Medical Center (reference numbers II HV 43/2004 and A 45/2007) in accordance with generally accepted guidelines for the use of human material. Establishment of the cell line HROC18 [33] and the other four cell lines has been described in detail before [34]. The in vitro models were either directly established from freshly taken tumor material (HROC18, HROC173, HROC383) or following xenografting (HROC277, HROC277Met2) in immunodeficient mice [35]. All primary tumors were obtained from untreated adenocarcinoma (Table 1). Additionally, HROC277Met2, a cell line derived from a liver metastasis obtained from subsequent surgery of the patient with tumor ID HROC277 was employed in the study. Of the selected CRC models, all but HROC383 were of a microsatellite stable subtype (Table 1). More detailed information on the HROC models can be found in Mullins et al. 2019 [35].

Table 1.
Colorectal cancer and metastasis cell lines [35].
To culture HROC cell lines, Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12; from PAN Biotech, Aidenbach, Germany) with 10% fetal calf serum (FCS, Bio&SELL, Feucht, Germany) was used. Culturing of the cells was done at 37 °C in a 5% CO2 humidified atmosphere. All experiments were performed with ≤50 cell passages. At constant intervals, MycoSPY®—PCR Mycoplasma Test Kit (Biontex, Munich, Germany) was used to test cell culture supernatants for mycoplasma contamination. No contamination with mycoplasmas were found during the project period.
2.2. Quantification of DNA Synthesis
To gauge the effects of small-molecule inhibitors and calcium channel blockers on proliferation of the cells, a 5-bromo-2′-deoxyuridine (BrdU) incorporation assay kit (Roche Diagnostics GmbH, Mannheim, Germany) was performed as surrogate. In our study, MEK inhibitor trametinib, AKT inhibitors capivasertib and MK-2206, and STAT3 inhibitor niclosamide (all from Selleck Chemicals, Houston, TX, USA) were employed. DMSO was used as a solvent. Additionally, the effects of MgCl2, NiCl2, and verapamil (Tocris bioscience, Bristol, UK; all dissolved in DMEM/F12) were investigated. The cells (5 × 103/well) were seeded in 96-well half-area microplates at equal densities and allowed to adhere overnight in a complete culture medium. On the following day, the culture medium was replaced by a medium supplemented with inhibitors, MgCl2 or NiCl2 at the indicated doses. After an incubation period of 32 h, BrdU was added at a final concentration of 10 µM into the culture media. Another 16 h later, labeling was stopped, and BrdU uptake was measured according to the manufacturer’s instructions.
2.3. Migration in the Direct-Current Electrical Field
To determine the galvanotactic migration of CRC cells, cultures were exposed to direct-current electrical fields (DCEF) as described [19]. For this purpose, the HROC cells (2 × 104) were seeded onto collagen I-coated (Advanced Biomatrix, San Diego, CA, USA) coverslips that were mounted into direct-current (DC) chambers. After 24 h, silver/silver chloride electrodes were placed into the outer reservoirs. Current flow was conducted by agar bridges consisting of 2% agarose (TopVision agarose, ThermoScientific, Waltham, MA, USA) in Ringer’s solution (Braun, Melsungen, Germany). A DC power supply (Standard Power PackP25, Biometra, Göttingen, Germany) was used to apply current for 6 h. During this time, voltage was determined directly at the borders of the cell area, with a 25 mm distance in-between, with a multimeter (Voltcraft VC220, Conrad Electronic, Wollerau, Switzerland) and adjusted during the experiments to maintain a constant electrical field strength.
The positions of the cells prior to and after 6 h of DC stimulation were determined by analyses of microphotographs at eight fixed positions distributed over the whole area, which were taken at the beginning and end of the experiment, employing a Leica DMI 6000 microscope (Leica, Wetzlar, Germany) with Leica Application Suite (v. 2.0.0.13332) software package. An exact overlay of both microphotographs, taken at the start and end, was brought about using the image software GIMP (2.10.30). Subsequently, these pictures were exported for evaluation of cell migration in ImageJ (1.53e).
In ImageJ, five to six cells per field of view were analyzed by encircling cells, including all cell extensions and centering cells. In total, 40 cells per chamber were analyzed. Circle center coordinates were determined at the start (X0/Y0) and six hours later (X1/Y1). Based on these coordinates, the distance of each dimension in the two-dimensional system and overall migration distance () were calculated (this value is referred to as the absolute value of migration; see single-cell data of 0 V/m and 200 V/m in Supplementary Figure S1). The DC electrical field was oriented along the X-axis, with the positive X-axis towards the anodal and the negative X-axis towards the cathodal pole. All n-numbers given in the text and figures correspond to the number of cells that were analyzed in three or more biological independent experiments. To test different strengths of DCEF conditions, three field strengths (150 ± 15 V/m, 200 ± 20 V/m, 250 ± 25 V/m) were applied, all of which ranged within previously used field strengths of in vitro studies [16,18,19,36,37,38].
To examine the impact of Ca2+ influx on galvanotaxis, HROC cell cultures were challenged with MgCl2, NiCl2 or verapamil for the entire duration of the experiment. MgCl2 was used to block Ca2+ influx in an unspecific manner. To inhibit Ca2+ flux via L-Type Ca2+ channels, verapamil was applied. To impair T-type Ca2+ channels, the cell cultures were exposed to NiCl2. Effects of signal transduction pathways associated with migration of CRC cells were addressed employing MK-2206, capivasertib, trametinib or niclosamide. The selected doses of MK-2206 [39,40], capivasertib [41], trametinib [42,43], and niclosamide [44,45] had previously shown antitumoral effects in various in vitro cancer models.
2.4. Fluorescence Microscopy
To estimate cellular integrity after DC stimulation, nuclei and cytoplasm were stained. Therefore, HROC cells were cultured on coverslips as described above. After DC stimulation for 6 h, the coverslips were washed with phosphate-buffered saline (PBS) and fixed afterwards in 3.7% paraformaldehyde overnight at 4 °C. The next day, slides were washed again in PBS and permeabilized with 0.5% TritonX-100 (Carl Roth, Karlsruhe, Germany). After another washing step with PBS, cellular actin was stained using Acti-stain 488 phalloidin (Cytoskeleton, Denver, CO, USA; diluted 1:140 in PBS) for 30 min at room temperature. Subsequently, the cells were washed and embedded with ProLongTM Gold containing DAPI (ThermoFisher Scientific, Waltham, MA, USA) on microscope slides. Fluorescence analysis was performed by laser-scanning microscopy (Leica DMI 6000) with Leica Application Suite (v. 2.0.0.13332) software.
2.5. Statistical Analysis
Statistical analysis was performed with SigmaPlot 13.0. The experimental results are presented in box plots or given as mean ± standard error of the mean (SEM) for the indicated number of cells and experiments. Mean group differences were tested for significance using the nonparametric Kruskal–Wallis test before, for multiple comparisons, subgroups were tested with a post hoc Dunn’s test. To analyze migration velocity and field strength, we used a two-way ANOVA followed by a Bonferroni t-test. A significance level of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Migration of CRC Cells in the DC Eletcrical Field
Five patient-derived colorectal cancer cell lines were employed in our study. In initial studies, the cells were challenged with three different field strengths to test migratory behavior in the direct-current (DC) electrical field (DCEF). Sum vectors showed a motile phenotype independently of DC stimulation (Figure 1A). After DC stimulation based on a field strength of 150 V/m, HROC18 (0 V/m: 0.66 ± 0.04 µm/h vs. 150 V/m: 1.31 ± 0.11 µm/h) and HROC277Met2 (0 V/m: 0.63 ± 0.04 µm/h vs. 150 V/m: 1.14 ± 0.09 µm/h) presented enhanced velocities (p < 0.05 for all, Kruskal–Wallis test with post hoc Dunn’s test; Figure 1B). In both cell lines, a further increase of field strengths had no effect on the migratory velocity of the tumor cells. In HROC173, a field strength of 200 V/m was required to increase velocity in comparison to unstimulated controls (0 V/m: 1.81 ± 0.13 µm/h vs. 200 V/m: 2.12 ± 0.11 µm/h). However, significant differences in the range of 150–250 V/m were found (Figure 1B). Cell line HROC383 presented a stepwise increase of velocity ranging from 0–200 V/m (0 V/m: 1.78 ± 0.1 µm/h vs. 150 V/m: 2.42 ± 0.19 µm/h, 0 V/m vs. 200 V/m: 3.17 ± 0.17 µm/h; 150 V/m vs. 200 V/m). A further increase of the DCEF to 250 V/m had no effect on migration velocity. In contrast, in HROC277, only at a field strength of 200 V/m, a small but significant increase in velocity was found (0 V/m: 0.44 ± 0.01 µm/h vs. 200 V/m: 0.62 ± 0.04 µm/h).
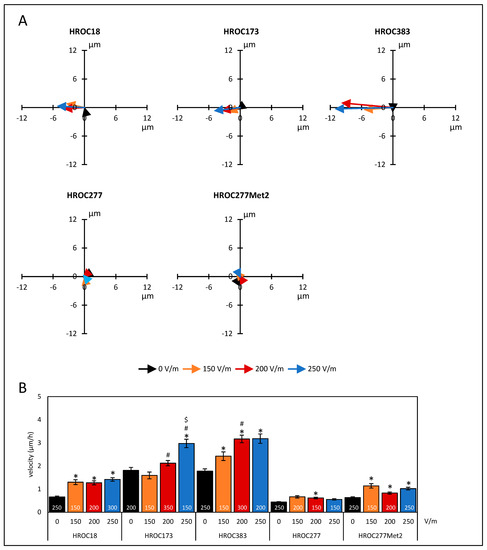
Figure 1.
Migration of colorectal cancer cells under DC electrical field conditions. CRC cells (HROC18, HROC173, HROC383, HROC277, and HROC277Met2) were seeded on collagen-coated coverslips that were mounted in DC chambers. The exact positions of the cells prior to and after DC stimulation were estimated by analyses of microphotographs at eight fixed positions. At each field of view, positions in the two-dimensional system of five to six cells were determined. The distances along the field vectors in the X-dimension and the Y-dimension (perpendicular to the field vectors) and overall migration velocity were calculated. (A) Sum vectors of total distance after six hours of DC stimulation with 150 V/m (orange), 200 V/m (red), 250 V/m (blue), and control cultures w/o DC (black). Positive values in the X-dimension represent anodal migration; negative vales imply a cathodal movement of the cells. (B) Velocities of tumor cells under different DC conditions. Data are represented as mean velocity ± SEM; 150–350 cells for each experimental group were analyzed (exact numbers are given in the columns) based on 3–7 independent experiments; * p < 0.05 versus 0 V/m; # p < 0.05 versus 150 V/m; $ p < 0.05 versus 200 V/m (Kruskal–Wallis test with post hoc Dunn’s test).
Additionally, a two-way ANOVA (factor tumor origin, i.e., HROC277 vs. HROC277Met2, and factor current, ranging from 0–250 V/m) with Bonferroni post hoc test revealed that the metastasis cell line HROC277Met2 exhibited a significantly higher migration velocity, regardless of the selected field strength, than did the cell line HROC277 derived from the primary tumor (p < 0.001; two-way ANOVA followed by Bonferroni t-test).
Regarding the migration along vectors of the DCEF, we examined whether the cells migrated preferentially to the anodal or cathodal pole. For this purpose, the distance of migration in the X-dimension from the beginning to the end of the experiment was calculated (Figure 2A). Cell lines HROC18, HROC173, and HROC383 significantly migrated in the cathodal direction (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). Furthermore, in HROC383, an enhanced migration towards the cathode between 150 V/m-stimulated cultures and those exposed to higher field strengths was found (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). With respect to the absolute values (difference of DC stimulation minus control conditions), the HROC383 cell line presented the overall highest distance (11.09 µm) of migration at 250 V/m (Figure 2B). Neither HROC277 nor the metastasis cell line derived from the same patient presented a galvanotactic phenotype.
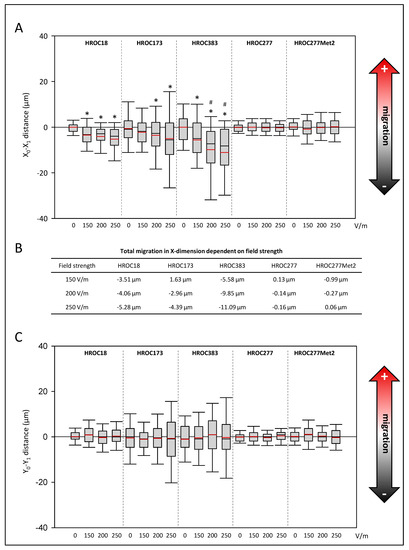
Figure 2.
Effects of DC electrical fields on pole-directed migration of CRC cells. CRC cells (HROC18, HROC173, HROC383, HROC277, and HROC277Met2) were stimulated with DC fields ranging from 0–250 V/m for six hours, and pole-directed migration ((A) X-dimension, (C) Y-dimension) was calculated based on analyses of microphotographs at eight fixed positions on each slide. For each experimental group, 150–350 cells were analyzed based on 3–7 independent experiments. Median is shown as a black-colored line and the mean is red. (B) Total migration in X-dimension was calculated as difference values of DC stimulated cell cultures minus values of control cultures; * p < 0.05 versus control cultures w/o DC; # p < 0.05 versus 150 V/m DC (Kruskal–Wallis test with post hoc Dunn’s test).
With respect to the Y-dimension, no significant differences were found among all the selected cell lines (Figure 2C).
Next, we investigated whether DCEF conditions might affect cellular integrity and viability of our in vitro models, as apoptosis was reported in previous studies (reviewed in [46]). As illustrated by microscopic analysis of cell cultures stimulated with field strength of 200 V/m in comparison to control cultures, no impairments such as apoptotic bodies or nuclear fragmentation were identified (Figure 3; see Supplementary Figures S2–S6 for high-resolution microscopic photographs). Also, there was no abundance of stress fibers in the CRC cells.
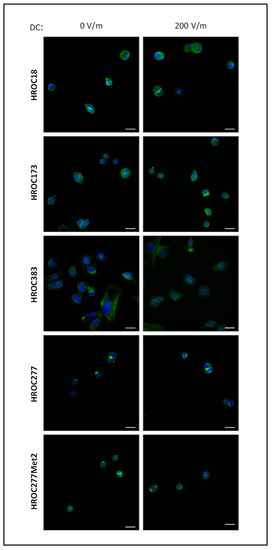
Figure 3.
Fluorescence microscopic images of HROC cell cultures after DC stimulation. HROC cells were exposed to a field strength of 200 V/m for 6 h, or were cultured under control conditions w/o DC stimulation for the same amount of time. Afterwards, Actin was visualized using Acti-stain 488 phalloidin (diluted 1:140 in PBS; shown in green). Nuclei were counterstained with DAPI (blue). Representative images are based on microscopic photographs that were taken at 400× magnification. Bars represent 25 µm.
3.2. Ca2+ Influx Is Mandatory for Directional Migration of HROC383 Cell Cultures in the DCEF
Since we aimed to contribute to a better understanding of molecular mechanisms of galvanotaxis in CRC, we tested whether Ca2+ influx is mandatory. To that end, the HROC383 cell line with the highest response to the DCEF was selected as a viable in vitro model. In our experiments, we focused on an intermediate field strength of 200 V/m, as no further increase was achieved with a higher stimulation (Figure 2A). In prior experiments based on DCEF stimulation, BrdU assays were performed to exclude potential harmful doses (Figure 4). Three different conditions to impair transmembraneous Ca2+ influx into the HROC383 cells were used in our study. MgCl2 was employed as an unspecific blocker of transmembraneous Ca2+ influx. To impair ion flux via T-type Ca2+ channels, NiCl2 was used. A third condition included the L-Type Ca2+-channel blocker verapamil. At the selected doses in experiments regarding galvanotaxis (11.5 mM MgCl2, 100 µM verapamil, and 50 µM NiCl2), no effects on cell proliferation were determined (Figure 4A–C).
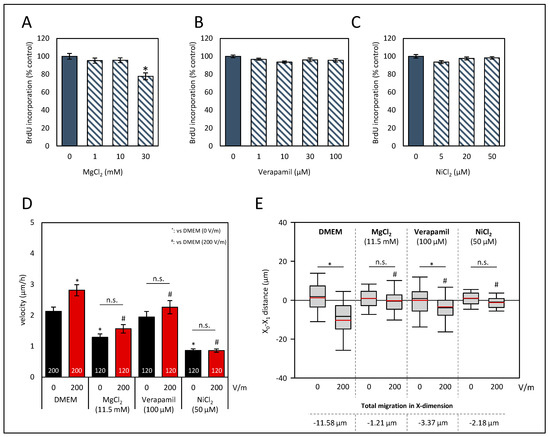
Figure 4.
Effects of Ca2+ influx perturbation on proliferation and galvanotaxis of HROC383 cell cultures. (A–C) HROC383 cells growing in 96-well half-area microplates were exposed to MgCl2, verapamil or NiCl2 at the indicated doses for 48 h before DNA synthesis was assessed with the BrdU incorporation assay. One hundred percent BrdU incorporation corresponds to cells cultured with solvent (cell culture medium) only. Data are presented as mean ± SEM (n ≥ 12 separate cultures); * p < 0.05 versus control cultures (Kruskal–Wallis test with post hoc Dunn’s test). (D) The velocity of tumor cells under DCEF conditions (200 V/m, 6 h) and control conditions. Cells were treated with MgCl2, verapamil, NiCl2, and DMEM, respectively, at the indicated doses. Data are represented as mean velocity ± SEM; 120–200 cells for each experimental group from three to five independent experiments were analyzed; n.s., no significant difference; * p < 0.05 vs. DMEM w/o DC; # p < 0.05 vs. DMEM with 200 V/m DC (Kruskal–Wallis test with post hoc Dunn’s test). (E) The pole-directed migration was estimated, and total migration in X-dimension was calculated as the difference in the values of DC-stimulated cell cultures and the values of control cultures. n.s., no significant difference; * p < 0.05 vs. control cultures w/o DC; # p < 0.05 vs. DMEM with 200 V/m DC (Kruskal–Wallis test with post hoc Dunn’s test).
Our data show that both MgCl2 and NiCl2 not only prevented a DC-stimulated increase of the velocity of HROG383 cells (Figure 4D), but also reduced the velocity in comparison to solvent-treated cultures (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). The latter finding was not surprising, given that calcium influx is mandatory for cellular migration [47]. Interestingly, the addition of verapamil had no effect on the velocity of cultures w/o DC stimulation (Figure 4D). However, no increase in the velocity after DC stimulation was determined and, in comparison to DC-stimulated control cultures, velocity was also found to be reduced (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test).
As illustrated in Figure 4E, X-dimensional migration was analyzed. Both treatments, that with MgCl2 and that with NiCl2, abolished galvanotaxis of HROC383 cells (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). Exposure of the cells to verapamil did not entirely prevent cathodal movement, but in comparison to control cultures w/o verapamil, X-dimensional migration was significantly reduced (p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). With respect to the Y-dimension, no significant differences were found (Supplementary Figure S7). In summary, our data indicate that Ca2+ influx greatly contributes to a directional migration in galvanotaxis.
3.3. Effects of Intracellular Signaling Cascades on Galvanotaxis of HROC383 Cell Cultures
In addition to Ca2+ flux over the plasma membrane, various intracellular signaling pathways are known to contribute to galvanotaxis. Based on studies on other tumor entities [17,19,48,49] and keratinocytes [50], we proposed that the phosphatidylinositol 3-kinase (PI3K)/AKT pathway could be a key player in the directional migration of HROC383 cells in DCEFs. Therefore, two small-molecule kinase inhibitors that act as specific AKT blockers were chosen for investigation (allosteric AKT inhibitor MK-2206 and capivasertib, an ATP-competitive inhibitor). Furthermore, the closely linked Raf/MEK/ERK pathways may also contribute to galvanotaxis [48]. To specifically target this pathway, trametinib, a MEK inhibitor, was selected. A third pathway in CRC that has been associated with migration is based on signal transducer and activator of transcription 3 (STAT3) [51], which could be targeted with niclosamide [52].
Again, assays on proliferation were performed to determine well-tolerated doses that presented only minor biological effects (Figure 5A–D). Based on these studies, 1 µM MK-2206, 10 µM capivasertib, 1 nM trametinib, and 0.1 µM niclosamide were selected for subsequent analysis.
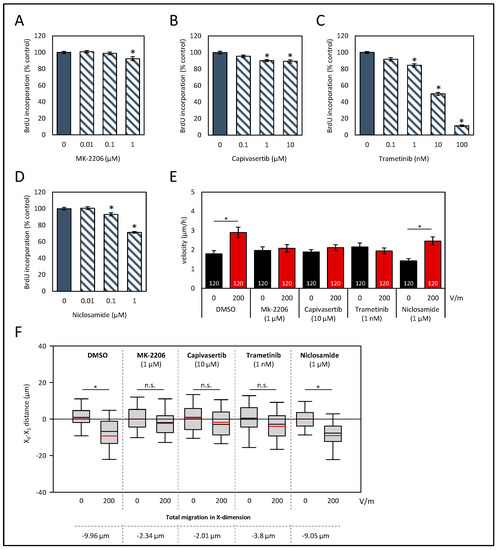
Figure 5.
Effects of signaling pathway inhibitors on proliferation and galvanotaxis of HROC383 cells. (A–D) HROC383 cells growing in 96-well half-area microplates were treated with AKT inhibitors MK-2206 or capivasertib, MEK inhibitor trametinib, or STAT3 inhibitor niclosamide at the indicated doses for 48 h, before DNA synthesis was assessed with the BrdU incorporation assay. One hundred percent BrdU incorporation corresponds to cells cultured with solvent only. Data are presented as mean ± SEM (n ≥ 12 separate cultures); * p < 0.05 versus control cultures (Kruskal–Wallis test with post hoc Dunn’s test). (E) The velocities of tumor cells under DCEF conditions (200 V/m, 6 h) and control conditions. Cells were treated with inhibitors or solvent (DMSO), respectively, at the indicated doses. Data are represented as mean velocity ± SEM. As illustrated in the columns, 120 cells for each experimental group from three independent experiments were analyzed; * p < 0.05 versus control w/o DC (Kruskal–Wallis test with post hoc Dunn’s test). (F) The pole-directed migration was estimated, and total X-dimension migration was calculated. * p < 0.05 vs. control cultures w/o DC; n.s., no significant difference; (Kruskal–Wallis test with post hoc Dunn’s test).
In solvent-treated cell cultures, DC stimulation of 200 V/m led to an increase in the velocity of the cells (Figure 5E; p < 0.05, Kruskal–Wallis test with post hoc Dunn’s test). All cell cultures, except for those exposed to niclosamide, lacked an increase in velocity after DC stimulation. Regarding the pole-directed migration, both AKT inhibitors and trametinib abolished the cathodal migration (Figure 5F). Exposure of the cells to the STAT3 inhibitor niclosamide had no effect on galvanotaxis. Here, as in in DC-stimulated control cultures (−9.96 µm), a high cathodal migration of roughly −9 µm was observed. No significant differences regarding a Y-dimensional migration were determined (Supplementary Figure S7B).
4. Discussion
To an increasing extent, the impact of electrical fields as a major driving force in migration of cells has been recognized. With respect to CRC, previous studies on migration have focused on environmental cues based on chemical or physical signals, while, to the best of our knowledge, investigation on the motility of CRC cells in DC electrical fields has been neglected so far.
The results of this study showed that CRC cells exhibit a galvanotactic phenotype. Based on patient-derived in vitro models that were established from primary tumors, three out of four in vitro models presented a cathodal migration under DCEF conditions. In principle, in the DCEF, cells may migrate in a directional manner (cathodal or anodal pole) or show no directness at all. In the DCEF, the minus pole attracts cations outside of the cells, and the transmembrane potential decreases. As a result, the membrane becomes further depolarized on these parts of the cells, whereas on the anodal side, the membrane becomes hyperpolarized due to an asymmetric ion influx via voltage-gated ion channels [36]. So far, it has not yet been conclusively clarified which biophysical mechanisms account for the predominant direction [53,54]. Of the five selected patient-derived cell lines, only HROC383 was classified as MSI-high, whereas all other cell lines were of a MSS subtype. Since the cells of both subtypes showed a cathodal migration, no implication of a link between the molecular status and galvanotaxis was suggested.
Our data indicate an important role for voltage-gated Ca2+-permeable ion channels in CRC cells, since these greatly contributed to the directional migration in HROC383 cell cultures. The unspecific Ca2+ influx was abolished by high levels of MgCl2 in the culture media, whereas Ca2+ influx via T-Type Ca2+ channels was inhibited by NiCl2 substitution. Furthermore, in experiments, including ones involving the L-Type Ca2+ channel blocker verapamil, cathodal migration of HROC383 was found only to be attenuated and not entirely blocked. Nonetheless, galvanotaxis was found to be reduced in comparison to the untreated control. As Ca2+-permeable channels are often found to be upregulated in CRC (summarized in Ref. [55]), which results in high intracellular Ca2+ levels [56], influx via these ion channels could play an important role in galvanotaxis of this tumor entity. The mechanism of Ca2+ influx as a response to DC stimulation is complex and far from full understanding [53,54]. In human osteoblasts, inhibition of T-type and L-type Ca2+ channels had no effect on galvanotaxis, but store-operated channels (SOCs) were identified as crucial [57]. In contrast, Babona-Pilipos and colleagues reported that inhibition of T-type and L-type Ca2+ channels reduced the velocity of neural precursor cells but did not affect the directness of the migration [58]. Another study, on murine fibroblasts, described an attenuation of a cathodal migration by inhibition of SOCs and T-type Ca2+ channels, but not for L-type channels [59]. Furthermore, Ca2+ influx via Ca2+ carriers (e.g., Na+/Ca2+ exchangers, Ca2+ ATPases), and the potential role of Ca2+-permeable TRP channels have been neglected so far. In addition, hyperpolarization of the plasma membrane on the anodal side may attract intracellular Ca2+ by passive electrochemical diffusion. As a result, the cytoplasmic Ca2+ concentration on the anodal side of the cell may increase, and thus may substantially contribute to the ion distribution in the cell [6]. Furthermore, in a small set of experiments, an electrophoretic distribution of membrane-bound receptors and ion channels within the DCEF was reported, which may additionally alter the migratory behavior [20,60]. Therefore, further studies that systematically investigate the role of Ca2+-mediated galvanotaxis need to be conducted to elucidate the molecular mechanisms, an effort which may also help to identify future targets for the treatment of CRC.
In our study, we were able to include two cell models that were established from the same patient: HROC277, based on tissue of the primary tumor, and HROC277Met2, which was derived from a liver metastasis one year later. Interestingly, both cell lines presented no galvanotaxis at all. However, we found an increase in the motility of the HROC277Met2 cell cultures. As both cell lines were exposed to the same field strengths, one may speculate that the redistribution of ion channels and altered opening properties may orchestrate an overall higher velocity of the metastasis-derived cells. The overall low response to DCEFs is in line with the principal author’s previously published data on the migration of a brain metastases cell line derived from CRC [19].
Other findings included that a DCEF of 200 V/m seems to have had no immediate effect on the cellular integrity of the HROC cultures, and that no hint on direct induction of nuclear fragmentation as part of the apoptosis cascade was found. The field strengths of 150–250 V/m (transferred to the cellular level ~200 µV/µm) are in a physiological range that can be assumed to be present in the colon in vivo. Apoptosis induction seems highly dependent on the cell type and experimental conditions (e.g., field strength and environment) [54]. Field strengths up to 400 V/m in smooth muscle cells of rabbits [61], human keratinocytes [62], and olfactory bulb neural progenitor cells [63] did not lead to apoptosis, or even prevented cell death. In contrast, in the B16 melanoma cell line, induction of apoptosis by application of high-voltage (7.5 kV/m) pulses was reported [64]. Moreover, in other studies based on high-voltage electrical fields, apoptosis was frequently detected [65,66,67].
Furthermore, we asked whether signaling pathways that were associated with the motility of CRC cells and galvanotaxis in various tumor entities may also affect the directional migration in our patient-derived in vitro model. The PI3K/AKT pathway was identified as a key player in galvanotaxis in various cell entities [17,19,48,49,68]. Based on experiments using two AKT inhibitors with different mechanisms of action, we could also validate the comprehensive role of this pathway in galvanotaxis for CRC. In agreement with previously published studies on brain cancer cells [48,49], keratinocytes [69], and corneal epithelial cells [9], perturbation of the Raf/MEK/ERK pathway results in an impairment of DC-stimulated migration. Interestingly, Huang et al. 2016 reported only an effect on the motility of the cancer cells, but, after inhibition of MEK1/2, not in the directionality [49]. Of the five selected patient-derived cell lines, only HROC383 cells harbored a mutation in B-Raf [35]. Oncogenic B-Raf was reported to contribute to an increased migration of CRC cells [70,71]; therefore, the mutation in HROC383 may also be a reason for the large, directed migration of this CRC model. In line with reported studies [52,72,73,74], niclosamide exhibited antitumoral effects on CRC cells in a dose-dependent manner. However, the migratory behavior in the DCEF was not impaired. In marked contrast, in lung cancer cells, STAT3 was identified as crucial for galvanotaxis [21]. After stimulating human T cells with a DCEF of 150 V/m, a decrease in the phosphorylation of STAT3 was determined [75]. The authors linked this finding to the polarization of the immune cells.
Since transepithelial potentials (TEP) could be the primary source of DC electrical fields in the colon, targeting key players that give rise to the asymmetric ion distribution could be of value in preventing a directed migration of CRC cells. The lumen-negative TEP is amiloride-sensitive [14]; it could be an intriguing question whether amiloride may promote intraepithelial infiltrative tumor growth. It is attempting to speculate whether an increased anion secretion and/or cation absorption could constrain intramural tumor cell infiltration.
Our study shed light on electrical fields as novel cues for the directional migration of CRC cells. The process of directed cell migration is driven by several stimuli. Hence, various signals must be integrated into the total outcome of the direction and velocity of the cells. As our study is based on in vitro 2D cultures, follow-up investigations should subsequently expand the experimental approaches to include 3D in vitro studies, or even in vivo models of CRC, in which several cues of migration may affect a migratory phenotype.
5. Conclusions
In summary, in patient-derived CRC cell lines, we demonstrated for the first time that these cells could be stimulated to a cathodal migration in DCEFs under in vitro conditions. Our data indicate that Ca2+ influx in general, and specifically via voltage-gated Ca2+ channels, highly contributed to galvanotaxis. In addition, inhibition of the signaling pathways PI3K/AKT and Raf/MEK/ERK abolished cathodal migration, whereas signaling via STAT3 did not seem to be involved in the galvanotactic migration of CRC cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12071032/s1, Figure S1: Galvanotaxis of CRC cells in the DE electrical field. Figure S2: Fluorescence microscopic images of HROC18 cells (original magnification ×630). Figure S3: Fluorescence microscopic images of HROC173 cells (original magnification ×630). Figure S4: Fluorescence microscopic images of HROC383 cells (original magnification ×630). Figure S5: Fluorescence microscopic images of HROC277 cells (original magnification ×630). Figure S6: Fluorescence microscopic images of HROC277Met2 cells (original magnification ×630). Figure S7: Y-dimensional migration of HROC383 colorectal cancer cells in the direct-current electrical field (DCEF).
Author Contributions
Conceptualization, F.L., M.L., K.P. and T.K.; formal analysis, F.L.; investigation, K.P., A.E., T.S. and F.L.; resources, M.L.; data curation, K.P., A.E., T.S. and F.L.; writing—original draft preparation, F.L.; writing—review and editing, T.K., M.L., R.K. and R.J.; visualization, T.S. and F.L.; project administration, F.L.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by an intramural grant from the University Medicine Rostock FORUN Program to F.L. (Project no.: 889027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Andreas Prestel.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Djamgoz, M.B.A. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J. Cell Sci. 2004, 117 Pt 9, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.G.; Pockett, S. The relationship between local field potentials (LFPs) and the electromagnetic fields that give rise to them. Front. Syst. Neurosci. 2014, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.L.; Levin, M.; Oudin, M.J. Bioelectric Control of Metastasis in Solid Tumors. Bioelectricity 2019, 1, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Pu, J.; Forrester, J.V.; McCaig, C.D. Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB J. 2002, 16, 857–859. [Google Scholar] [CrossRef]
- Sarkar, A.; Kobylkevich, B.M.; Graham, D.M.; Messerli, M.A. Electromigration of cell surface macromolecules in DC electric fields during cell polarization and galvanotaxis. J. Theor. Biol. 2019, 478, 58–73. [Google Scholar] [CrossRef]
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712. [Google Scholar] [CrossRef]
- Bachmann, O.; Juric, M.; Seidler, U.; Manns, M.P.; Yu, H. Basolateral ion transporters involved in colonic epithelial electrolyte absorption, anion secretion and cellular homeostasis. Acta Physiol. 2011, 201, 33–46. [Google Scholar] [CrossRef]
- Flores, C.A.; Melvin, J.E.; Figueroa, C.D.; Sepúlveda, F.V. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J. Physiol. 2007, 583 Pt 2, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.C.; Powell, D.W.; Croom, R.D.; Berschneider, H.M.; Boucher, R.C.; Knowles, M.R. Colonic and esophageal transepithelial potential difference in cystic fibrosis. Gastroenterology 1989, 96, 1041–1048. [Google Scholar] [CrossRef]
- Zhu, J.-X.; Xue, H.; Ji, T.; Xing, Y. Cellular localization of NKCC2 and its possible role in the Cl- absorption in the rat and human distal colonic epithelia. Transl. Res. 2011, 158, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.I.; Zhu, K.; Sun, Y.H.; Hegyi, B.; Zeng, Q.; Murphy, C.J.; Small, J.V.; Chen-Izu, Y.; Izumiya, Y.; Penninger, J.M.; et al. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 2015, 6, 8532. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.G.; Carroll, S.L.; Mokarram, N.; Bellamkonda, R.V. Electrotaxis of Glioblastoma and Medulloblastoma Spheroidal Aggregates. Sci. Rep. 2019, 9, 5309. [Google Scholar] [CrossRef]
- Clancy, H.; Pruski, M.; Lang, B.; Ching, J.; McCaig, C.D. Glioblastoma cell migration is directed by electrical signals. Exp. Cell Res. 2021, 406, 112736. [Google Scholar] [CrossRef]
- Lange, F.; Venus, J.; Shams Esfand Abady, D.; Porath, K.; Einsle, A.; Sellmann, T.; Neubert, V.; Reichart, G.; Linnebacher, M.; Köhling, R.; et al. Galvanotactic Migration of Glioblastoma and Brain Metastases Cells. Life 2022, 12, 580. [Google Scholar] [CrossRef]
- Yan, X.; Han, J.; Zhang, Z.; Wang, J.; Cheng, Q.; Gao, K.; Ni, Y.; Wang, Y. Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics 2009, 30, 29–35. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Lu, C.; Sun, Q.; Zhao, S.; Jiao, L.; Han, R.; Lin, C.; Jiang, J.; Zhao, M.; et al. Caveolin-1-mediated STAT3 activation determines electrotaxis of human lung cancer cells. Oncotarget 2017, 8, 95741–95754. [Google Scholar] [CrossRef]
- Li, Y.; Yu, W.-K.; Chen, L.; Chan, Y.-S.; Liu, D.; Fong, C.-C.; Xu, T.; Zhu, G.; Sun, D.; Yang, M. Electrotaxis of tumor-initiating cells of H1975 lung adenocarcinoma cells is associated with both activation of stretch-activated cation channels (SACCs) and internal calcium release. Bioelectrochemistry 2018, 124, 80–92. [Google Scholar] [CrossRef]
- Chang, H.F.; Cheng, H.T.; Chen, H.Y.; Yeung, W.K.; Cheng, J.Y. Doxycycline inhibits electric field-induced migration of non-small cell lung cancer (NSCLC) cells. Sci. Rep. 2019, 9, 8094. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Mycielska, M.; Madeja, Z.; Fraser, S.P.; Korohoda, W. Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltagegated Na+ channel activity. J. Cell Sci. 2001, 114 Pt 14, 2697–2705. [Google Scholar] [CrossRef]
- Garg, A.A.; Jones, T.H.; Moss, S.M.; Mishra, S.; Kaul, K.; Ahirwar, D.K.; Ferree, J.; Kumar, P.; Subramaniam, D.; Ganju, R.K.; et al. Electromagnetic fields alter the motility of metastatic breast cancer cells. Commun. Biol. 2019, 2, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hum, N.R.; Reid, B.; Sun, Q.; Loots, G.G.; Zhao, M. Electric Fields at Breast Cancer and Cancer Cell CollectiveGalvanotaxis. Sci. Rep. 2020, 10, 8712. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, S.; Huang, S.; Deng, R.; Ding, Y.; Wu, Y.; Li, X.; Wang, A.; Wang, S.; Chen, W.; et al. A Complex Role for Calcium Signaling in Colorectal Cancer Development and Progression. Mol. Cancer Res. 2019, 17, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Nagaba, Y.; Cross, H.S.; Wrba, F.; Zhang, L.; Guggino, S.E. The mRNA of L-type calcium channel elevated in colon cancer: Protein distribution in normal and cancerous colon. Am. J. Pathol. 2000, 157, 1549–1562. [Google Scholar] [CrossRef]
- Pérez-Riesgo, E.; Gutiérrez, L.G.; Ubierna, D.; Acedo, A.; Moyer, M.P.; Núñez, L.; Villalobos, C. Transcriptomic Analysis of Calcium Remodeling in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 922. [Google Scholar] [CrossRef]
- Ibrahim, S.; Dakik, H.; Vandier, C.; Chautard, R.; Paintaud, G.; Mazurier, F.; Lecomte, T.; Guéguinou, M.; Raoul, W. Expression Profiling of Calcium Channels and Calcium-Activated Potassium Channels in Colorectal Cancer. Cancers 2019, 11, 561. [Google Scholar] [CrossRef]
- Villalobos, C.; Sobradillo, D.; Hernández-Morales, M.; Núñez, L. Calcium remodeling in colorectal cancer. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Trollinger, D.R.; Isseroff, R.R.; Nuccitelli, R. Calcium channel blockers inhibit galvanotaxis in human keratinocytes. J. Cell Physiol. 2002, 193, 10144. [Google Scholar] [CrossRef] [PubMed]
- Maletzki, C.; Gock, M.; Randow, M.; Klar, E.; Huehns, M.; Prall, F.; Linnebacher, M. Establishment and characterization of cell lines from chromosomal instable colorectal cancer. World J. Gastroenterol. 2015, 21, 164–176. [Google Scholar] [CrossRef]
- Matschos, S.; Bürtin, F.; Kdimati, S.; Radefeldt, M.; Krake, S.; Prall, F.; Engel, N.; Krohn, M.; Micheel, B.; Kreutzer, M.; et al. The HROC-Xenobank-A High Quality Assured PDX Biobank of >100 Individual Colorectal Cancer Models. Cancers 2021, 13, 5882. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Micheel, B.; Matschos, S.; Leuchter, M.; Bürtin, F.; Krohn, M.; Hühns, M.; Klar, E.; Prall, F.; Linnebacher, M. Integrated Biobanking and Tumor Model Establishment of Human Colorectal Carcinoma Provides Excellent Tools for Preclinical Research. Cancers 2019, 11, 1520. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; IJspeert, C.; Shen, A.Q. Voltage-gated ion channels mediate the electrotaxis of glioblastoma cells in a hybrid PMMA/PDMS microdevice. APL Bioeng. 2020, 4, 036102. [Google Scholar] [CrossRef]
- Yao, L.; Shanley, L.; McCaig, C.; Zhao, M. Small applied electric fields guide migration of hippocampal neurons. J. Cell Physiol. 2008, 216, 527–535. [Google Scholar] [CrossRef]
- Moarefian, M.; Davalos, R.V.; Burton, M.D.; Jones, C.N. Electrotaxis-on-Chip to Quantify Neutrophil Migration Towards Electrochemical Gradients. Front. Immunol. 2021, 12, 674727. [Google Scholar] [CrossRef]
- Agarwal, E.; Chaudhuri, A.; Leiphrakpam, P.D.; Haferbier, K.L.; Brattain, M.G.; Chowdhury, S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer 2014, 14, 145. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, G.; Qiu, Z. Akt inhibitor MK-2206 reduces pancreatic cancer cell viability and increases the efficacy of gemcitabine. Oncol. Lett. 2020, 19, 1999–2004. [Google Scholar] [CrossRef]
- Davies, B.R.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.-H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J.; et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.; Franz, B.; Maletzki, C.; Linnebacher, M.; Hühns, M.; Jaster, R. Biological and molecular effects of small molecule kinase inhibitors on low-passage human colorectal cancer cell lines. Biomed Res. Int. 2014, 2014, 568693. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Ghosh, S.; Powell, R.; Roszik, J.; Park, Y.; Sobieski, M.; Sorokin, A.; Stephan, C.; Kopetz, S.; Ellis, L.M.; et al. Combining MEK and SRC inhibitors for treatment of colorectal cancer demonstrate increased efficacy in vitro but not in vivo. PLoS ONE 2023, 18, e0281063. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Lee, Y.-H.; Min, B.S.; Choi, J. Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis. Sci. Rep. 2019, 9, 11336. [Google Scholar] [CrossRef]
- Luo, F.; Luo, M.; Rong, Q.-X.; Zhang, H.; Chen, Z.; Wang, F.; Zhao, H.-Y.; Fu, L.-W. Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 245. [Google Scholar] [CrossRef]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell Physiol. 2018, 233, 1860–1876. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-C.; Kuo, G.-H.; Chang, S.-W.; Tsai, P.-J. Ca2+ signaling in cytoskeletal reorganization, cell migration, and cancer metastasis. BioMed Res. Int. 2015, 2015, 409245. [Google Scholar] [CrossRef]
- Li, F.; Chen, T.; Hu, S.; Lin, J.; Hu, R.; Feng, H. Superoxide mediates direct current electric field-induced directional migration of glioma cells through the activation of AKT and ERK. PLoS ONE 2013, 8, e61195. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hoffmann, G.; Wheeler, B.; Schiapparelli, P.; Quinones-Hinojosa, A.; Searson, P. Cellular microenvironment modulates the galvanotaxis of brain tumor initiating cells. Sci. Rep. 2016, 6, 21583. [Google Scholar] [CrossRef]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Z.-G.; Tian, X.-Q.; Sun, D.-F.; Liang, Q.-C.; Zhang, Y.-J.; Lu, R.; Chen, Y.-X.; Fang, J.-Y. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef]
- Wu, M.M.; Zhang, Z.; Tong, C.W.S.; Yan, V.W.; Cho, W.C.S.; To, K.K.W. Repurposing of niclosamide as a STAT3 inhibitor to enhance the anticancer effect of chemotherapeutic drugs in treating colorectal cancer. Life Sci. 2020, 262, 118522. [Google Scholar] [CrossRef]
- Cortese, B.; Palamà, I.E.; D’Amone, S.; Gigli, G. Influence of electrotaxis on cell behaviour. Integr. Biol. 2014, 6, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Med. Sci. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Tajada, S.; Villalobos, C. Calcium Permeable Channels in Cancer Hallmarks. Front. Pharmacol. 2020, 11, 968. [Google Scholar] [CrossRef]
- Wu, L.; Lin, W.; Liao, Q.; Wang, H.; Lin, C.; Tang, L.; Lian, W.; Chen, Z.; Li, K.; Xu, L.; et al. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020, 33, 108327. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Ziebart, J.; Kirschstein, T.; Sellmann, T.; Porath, K.; Kühl, F.; Delenda, B.; Bahls, C.; van Rienen, U.; Bader, R.; et al. Human Osteoblast Migration in DC Electrical Fields Depends on Store Operated Ca2+-Release and Is Correlated to Upregulation of Stretch-Activated TRPM7 Channels. Front. Bioeng. Biotechnol. 2019, 7, 422. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Liu, N.; Pritchard-Oh, A.; Mok, A.; Badawi, D.; Popovic, M.R.; Morshead, C.M. Calcium influx differentially regulates migration velocity and directedness in response to electric field application. Exp. Cell Res. 2018, 368, 202–214. [Google Scholar] [CrossRef]
- Guo, L.; Xu, C.; Li, D.; Zheng, X.; Tang, J.; Bu, J.; Sun, H.; Yang, Z.; Sun, W.; Yu, X. Calcium Ion Flow Permeates Cells through SOCs to Promote Cathode-Directed Galvanotaxis. PLoS ONE 2015, 8, e0139865. [Google Scholar] [CrossRef]
- Allen, G.M.; Mogilner, A.; Theriot, J.A. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 2013, 23, 560–568. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.; He, G.; Liu, J.; Feng, J. Electrical stimulation inhibits neointimal hyperplasia after abdominal aorta balloon injury through the PTEN/p27Kip1 pathway. Acta Biochim. Biophys. Sin. 2010, 42, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Iqbal, S.A.; Colthurst, J.; Volk, S.W.; Bayat, A. Electrical stimulation enhances epidermal proliferation in human cutaneous wounds by modulating p53-SIVA1 interaction. J. Investig. Dermatol. 2015, 135, 1166–1174. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Liu, M.; Song, W.; Wu, Q.; Fan, Y. Potential protective effect of biphasic electrical stimulation against growth factor-deprived apoptosis on olfactory bulb neural progenitor cells through the brain-derived neurotrophic factor-phosphatidylinositol 3’-kinase/Akt pathway. Exp. Biol. Med. 2013, 238, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, N.; Takeda, M.; Ishikawa, T.; Kinjo, A.; Hayasaka, T.; Imai, Y.; Yamaguchi, T. Activation of caspases and apoptosis in response to low-voltage electric pulses. Oncol. Rep. 2010, 23, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, J.; Wu, S.; Feng, H.; Sun, S.; Pan, J.; Zhang, J.; Beebe, S.J. Synergistic effects of nanosecond pulsed electric fields combined with low concentration of gemcitabine on human oral squamous cell carcinoma in vitro. PLoS ONE 2012, 7, e43213. [Google Scholar] [CrossRef]
- Qi, F.; Wang, Y.; Ma, T.; Zhu, S.; Zeng, W.; Hu, X.; Liu, Z.; Huang, J.; Luo, Z. Electrical regulation of olfactory ensheathing cells using conductive polypyrrole/chitosan polymers. Biomaterials 2013, 34, 1799–1809. [Google Scholar] [CrossRef]
- Chen, X.; Yin, S.; Hu, C.; Chen, X.; Jiang, K.; Ye, S.; Feng, X.; Fan, S.; Xie, H.; Zhou, L.; et al. Comparative study of nanosecond electric fields in vitro and in vivo on hepatocellular carcinoma indicate macrophage infiltration contribute to tumor ablation in vivo. PLoS ONE 2014, 9, e86421. [Google Scholar] [CrossRef]
- Meng, X.; Arocena, M.; Penninger, J.; Gage, F.H.; Zhao, M.; Song, B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp. Neurol. 2011, 227, 210–217. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Liu, J.; Guo, X.; Huang, J.; Jiang, X.; Zhang, Y.; Huang, Y.; Fan, D.; Zhang, J. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Makrodouli, E.; Oikonomou, E.; Koc, M.; Andera, L.; Sasazuki, T.; Shirasawa, S.; Pintzas, A. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: A comparative study. Mol. Cancer 2011, 10, 118. [Google Scholar] [CrossRef]
- Ma, Z.; Qi, Z.; Gu, C.; Yang, Z.; Ding, Y.; Zhou, Y.; Wang, W.; Zou, Q. BRAFV600E mutation promoted the growth and chemoresistance of colorectal cancer. Am. J. Cancer Res. 2023, 13, 1486–1497. [Google Scholar] [PubMed]
- Suliman, M.A.; Zhang, Z.; Na, H.; Ribeiro, A.L.L.; Zhang, Y.; Niang, B.; Hamid, A.S.; Zhang, H.; Xu, L.; Zuo, Y. Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family. Int. J. Mol. Med. 2016, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zheng, H.; Hu, W.; Zhou, B.; Dai, X.; Zhang, Y.; Liu, Z.; Wu, X.; Zhao, C.; Liang, G. Niclosamide inhibition of STAT3 synergizes with erlotinib in human colon cancer. Onco Targets Ther. 2017, 10, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Cerles, O.; Benoit, E.; Chéreau, C.; Chouzenoux, S.; Morin, F.; Guillaumot, M.-A.; Coriat, R.; Kavian, N.; Loussier, T.; Santulli, P.; et al. Niclosamide Inhibits Oxaliplatin Neurotoxicity while Improving Colorectal Cancer Therapeutic Response. Mol. Cancer Ther. 2017, 16, 300–311. [Google Scholar] [CrossRef]
- Arnold, C.A.; Rajnicek, A.M.; Hoare, J.I.; Pokharel, S.M.; Mccaig, C.D.; Barker, R.N.; Wilson, H.M. Physiological strength electric fields modulate human T cell activation and polarization. Sci. Rep. 2019, 9, 17604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).