Emerging Rhabdoviruses and Human Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Rhabdovirus Structure, Genomic Organisation, Replication, and Taxonomy
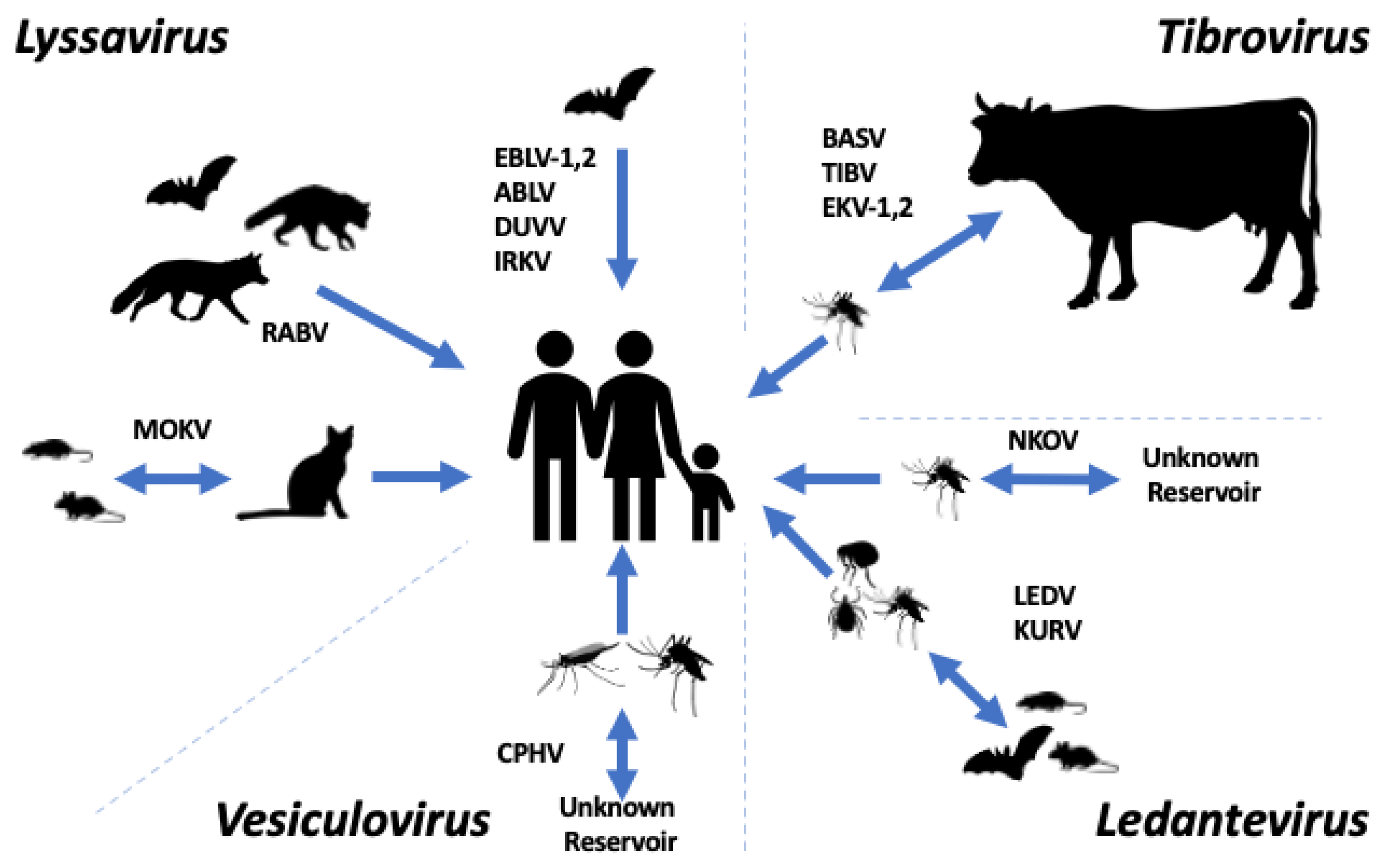
3. Lyssavirus
4. Ledantevirus
5. Tibrovirus
6. Vesiculovirus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Dietzgen, R.G.; Kondo, H.; Goodin, M.M.; Kurath, G.; Vasilakis, N. The family Rhabdoviridae: Mono—And bipartite negative-sense RNA viruses with diverse genome organization and common evolutionary origins. Virus Res. 2017, 227, 158–170. [Google Scholar] [CrossRef]
- Ge, P.; Tsao, J.; Schein, S.; Green, T.J.; Luo, M.; Zhou, Z.H. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 2010, 327, 689–693. [Google Scholar] [CrossRef]
- Roche, S.; Bressanelli, S.; Rey, F.A.; Gaudin, Y. Crystal Structure of the Low-pH Form of the Vesicular Stomatitis Virus Glycoprotein G. Science 2006, 313, 187–191. [Google Scholar] [CrossRef]
- Roche, S.; Rey, F.A.; Gaudin, Y.; Bressanelli, S. Structure of the Prefusion Form of the Vesicular Stomatitis Virus Glycoprotein G. Science 2007, 315, 843–848. [Google Scholar] [CrossRef]
- Zhou, K.; Si, Z.; Ge, P.; Tsao, J.; Luo, M.; Zhou, Z.H. Atomic model of vesicular stomatitis virus and mechanism of assembly. Nat. Commun. 2022, 13, 5980. [Google Scholar] [CrossRef]
- Jenni, S.; Horwitz, J.A.; Bloyet, L.-M.; Whelan, S.P.J.; Harrison, S.C. Visualizing molecular interactions that determine assembly of a bullet-shaped vesicular stomatitis virus particle. Nat. Commun. 2022, 13, 4802. [Google Scholar] [CrossRef]
- Horwitz, J.A.; Jenni, S.; Harrison, S.C.; Whelan, S.P.J. Structure of a rabies virus polymerase complex from electron cryo-microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 2099–2107. [Google Scholar] [CrossRef]
- Jenni, S.; Bloyet, L.-M.; Diaz-Avalos, R.; Liang, B.; Whelan, S.P.J.; Grigorieff, N.; Harrison, S.C. Structure of the Vesicular Stomatitis Virus L Protein in Complex with Its Phosphoprotein Cofactor. Cell Rep. 2020, 30, 53–60.e55. [Google Scholar] [CrossRef]
- Baquero, E.; Albertini, A.A.; Raux, H.; Buonocore, L.; Rose, J.K.; Bressanelli, S.; Gaudin, Y. Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein. PLoS Pathog. 2015, 11, e1004756. [Google Scholar] [CrossRef]
- Belot, L.; Ouldali, M.; Roche, S.; Legrand, P.; Gaudin, Y.; Albertini, A.A. Crystal structure of Mokola virus glycoprotein in its post-fusion conformation. PLoS Pathog. 2020, 16, e1008383. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.C.; Assenberg, R.; Delmas, O.; Verma, A.; Gholami, A.; Talbi, C.; Owens, R.J.; Stuart, D.I.; Grimes, J.M.; Bourhy, H. Rhabdovirus Matrix Protein Structures Reveal a Novel Mode of Self-Association. PLoS Pathog. 2008, 4, e1000251. [Google Scholar] [CrossRef] [PubMed]
- Hellert, J.; Buchrieser, J.; Larrous, F.; Minola, A.; de Melo, G.D.; Soriaga, L.; England, P.; Haouz, A.; Telenti, A.; Schwartz, O.; et al. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat. Commun. 2020, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.M.; Fedosyuk, S.; English, S.; Augusto, G.; Berg, A.; Thorley, L.; Haselon, A.-S.; Segireddy, R.R.; Bowden, T.A.; Douglas, A.D. Structure of trimeric pre-fusion rabies virus glycoprotein in complex with two protective antibodies. Cell Host Microbe 2022, 30, 1219–1230.e1217. [Google Scholar] [CrossRef]
- Callaway, H.M.; Zyla, D.; Larrous, F.; de Melo, G.D.; Hastie, K.M.; Avalos, R.D.; Agarwal, A.; Corti, D.; Bourhy, H.; Saphire, E.O. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022, 8, eabp9151. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Shi, M.; Orciari, L.A.; Yager, P.A.; Velasco-Villa, A.; Kuzmina, N.A.; Streicker, D.G.; Bergman, D.L.; Rupprecht, C.E. Molecular Inferences Suggest Multiple Host Shifts of Rabies Viruses from Bats to Mesocarnivores in Arizona during 2001–2009. PLoS Pathog. 2012, 8, e1002786. [Google Scholar] [CrossRef]
- Walker, P.J.; Dietzgen, R.G.; Joubert, D.A.; Blasdell, K.R. Rhabdovirus accessory genes. Virus Res. 2011, 162, 110–125. [Google Scholar] [CrossRef]
- Walker, P.J.; Firth, C.; Widen, S.G.; Blasdell, K.R.; Guzman, H.; Wood, T.G.; Paradkar, P.N.; Holmes, E.C.; Tesh, R.B.; Vasilakis, N. Evolution of Genome Size and Complexity in the Rhabdoviridae. PLoS Pathog. 2015, 11, e1004664. [Google Scholar] [CrossRef]
- Walker, P.J.; Byrne, K.A.; Riding, G.A.; Cowley, J.A.; Wang, Y.; McWilliam, S. The genome of bovine ephemeral fever rhabdovirus contains two related glycoprotein genes. Virology 1992, 191, 49–61. [Google Scholar] [CrossRef]
- Iverson, L.E.; Rose, J.K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 1981, 23, 477–484. [Google Scholar] [CrossRef]
- Abraham, G.; Banerjee, A.K. Sequential transcription of the genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 1976, 73, 1504–1508. [Google Scholar] [CrossRef]
- Simmonds, P.; Adams, M.J.; Benkő, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Prim. 2017, 3, 17091. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Romijn, P.C.; Uieda, W.; Tamayo, H.; da Silva, D.F.; Belotto, A.; da Silva, J.B.; Leanes, L.F. Rabies transmitted by vampire bats to humans: An emerging zoonotic disease in Latin America? Rev. Panam. Salud Pública 2009, 25, 260–269. [Google Scholar] [CrossRef]
- Hemachudha, T.; Laothamatas, J.; Rupprecht, C.E. Human rabies: A disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Kotait, I.; Oliveira, R.d.N.; Carrieri, M.L.; Castilho, J.G.; Macedo, C.I.; Pereira, P.M.C.; Boere, V.; Montebello, L.; Rupprecht, C.E. Non-human primates as a reservoir for rabies virus in Brazil. Zoonoses Public Health 2019, 66, 47–59. [Google Scholar] [CrossRef]
- Regnault, B.; Evrard, B.; Plu, I.; Dacheux, L.; Troadec, E.; Cozette, P.; Chrétien, D.; Duchesne, M.; Vallat, J.-M.; Jamet, A.; et al. First Case of Lethal Encephalitis in Western Europe Due to European Bat Lyssavirus Type 1. Clin. Infect. Dis. 2022, 74, 461–466. [Google Scholar] [CrossRef]
- Amengual, B.; Whitby, J.E.; King, A.; Cobo, J.S.; Bourhy, H. Evolution of European bat lyssaviruses. J. Gen. Virol. 1997, 78, 2319–2328. [Google Scholar] [CrossRef]
- Fooks, A.R.; McElhinney, L.M.; Pounder, D.J.; Finnegan, C.J.; Mansfield, K.; Johnson, N.; Brookes, S.M.; Parsons, G.; White, K.; McIntyre, P.G.; et al. Case report: Isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J. Med. Virol. 2003, 71, 281–289. [Google Scholar] [CrossRef]
- McElhinney, L.M.; Marston, D.A.; Wise, E.L.; Freuling, C.M.; Bourhy, H.; Zanoni, R.; Moldal, T.; Kooi, E.A.; Neubauer-Juric, A.; Nokireki, T.; et al. Molecular Epidemiology and Evolution of European Bat Lyssavirus 2. Int. J. Mol. Sci. 2018, 19, 156. [Google Scholar] [CrossRef]
- Fooks, A. The challenge of new and emerging lyssaviruses. Expert Rev. Vaccines 2004, 3, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Familusi, J.B.; Moore, D.L. Isolation of a rabies related virus from the cerebrospinal fluid of a child with ‘aseptic meningitis’. Afr. J. Med. Sci. 1972, 3, 93–96. [Google Scholar]
- Familusi, J.B.; Osunkoya, B.O.; Moore, D.L.; Kemp, G.E.; Fabiyi, A. A fatal human infection with Mokola virus. Am. J. Trop. Med. Hyg. 1972, 21, 959–963. [Google Scholar] [CrossRef]
- Kgaladi, J.; Wright, N.; Coertse, J.; Markotter, W.; Marston, D.; Fooks, A.R.; Freuling, C.M.; Müller, T.F.; Sabeta, C.T.; Nel, L.H. Diversity and Epidemiology of Mokola Virus. PLoS Negl. Trop. Dis. 2013, 7, e2511. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Markotter, W.; Mohale, D.K.; Shumba, W.; Wandeler, A.I.; Nel, L.H. Mokola virus in domestic mammals, South Africa. Emerg. Infect. Dis. 2007, 13, 1371–1373. [Google Scholar] [CrossRef]
- Tignor, G.H.; Murphy, F.A.; Clark, H.F.; Shope, R.E.; Madore, P.; Bauer, S.P.; Buckley, S.M.; Meredith, C.D. Duvenhage Virus: Morphological, Biochemical, Histopathological and Antigenic Relationships to the Rabies Serogroup. J. Gen. Virol. 1977, 37, 595–611. [Google Scholar] [CrossRef]
- Paweska, J.T.; Blumberg, L.H.; Liebenberg, C.; Hewlett, R.H.; Grobbelaar, A.A.; Leman, P.A.; Croft, J.E.; Nel, L.H.; Nutt, L.; Swanepoel, R. Fatal Human Infection with Rabies-related Duvenhage Virus, South Africa. Emerg. Infect. Dis. J. 2006, 12, 1965. [Google Scholar] [CrossRef]
- van Thiel, P.P.; van den Hoek, J.A.; Eftimov, F.; Tepaske, R.; Zaaijer, H.J.; Spanjaard, L.; de Boer, H.E.; van Doornum, G.J.; Schutten, M.; Osterhaus, A.D.; et al. Fatal case of human rabies (Duvenhage virus) from a bat in Kenya: The Netherlands, December 2007. Eurosurveillance 2008, 13, 1–2. [Google Scholar] [CrossRef]
- Field, H.E. Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa. Zoonoses Public Health 2018, 65, 742–748. [Google Scholar] [CrossRef]
- Gould, A.R.; Kattenbelt, J.A.; Gumley, S.G.; Lunt, R.A. Characterisation of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Res. 2002, 89, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Morgan, J.; Allworth, A. A human case of encephalitis due to a lyssavirus recently identified in fruit bats. Commun. Dis. Intell. 1996, 20, 504. [Google Scholar]
- Hanna, J.N.; Carney, I.K.; Deverill, J.E.; Botha, J.A.; Smith, G.A.; Serafin, I.L.; Harrower, B.J.; Tannenberg, A.E.G.; Fitzpatrick, P.F.; Searle, J.W. Australian bat lyssavirus infection: A second human case, with a long incubation period. Med. J. Aust. 2000, 172, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.R.; Nourse, C.; Vaska, V.L.; Calvert, S.; Northill, J.A.; McCall, B.; Mattke, A.C. Australian Bat Lyssavirus in a Child: The First Reported Case. Pediatrics 2014, 133, e1063–e1067. [Google Scholar] [CrossRef]
- Si, D.; Marquess, J.; Donnan, E.; Harrower, B.; McCall, B.; Bennett, S.; Lambert, S. Potential Exposures to Australian Bat Lyssavirus Notified in Queensland, Australia, 2009−2014. PLoS Negl. Trop. Dis. 2016, 10, e0005227. [Google Scholar] [CrossRef]
- Leonova, G.; Belikov, S.; Kondratov, I.; Krylova, N.; Pavlenko, E.; Romanova, E.; Chentsova, I.; Petukhova, S. A fatal case of bat lyssavirus infection in Primorye Territory of the Russian Far East. Rabies Bull. Eur. 2009, 33, 5–8. [Google Scholar]
- Chen, T.; Miao, F.M.; Liu, Y.; Zhang, S.F.; Zhang, F.; Li, N.; Hu, R.L. Possible Transmission of Irkut Virus from Dogs to Humans. Biomed. Environ. Sci. 2018, 31, 146–148. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhao, J.; Zhang, F.; Hu, R. Isolation of Irkut Virus from a Murina leucogaster Bat in China. PLoS Negl. Trop. Dis. 2013, 7, e2097. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Guzman, H.; Widen, S.G.; Firth, C.; Wood, T.G.; Holmes, E.C.; Tesh, R.B.; Vasilakis, N.; Walker, P.J. Ledantevirus: A Proposed New Genus in the Rhabdoviridae has a Strong Ecological Association with Bats. Am. Soc. Trop. Med. Hyg. 2015, 92, 405–410. [Google Scholar] [CrossRef]
- Cropp, C.B.; Prange, W.C.; Monath, T.P. LeDantec virus: Identification as a rhabdovirus associated with human infection and formation of a new serogroup. J. Gen. Virol. 1985, 66 Pt 12, 2749–2754. [Google Scholar] [CrossRef]
- Lelli, D.; Prosperi, A.; Moreno, A.; Chiapponi, C.; Gibellini, A.M.; De Benedictis, P.; Leopardi, S.; Sozzi, E.; Lavazza, A. Isolation of a novel Rhabdovirus from an insectivorous bat (Pipistrellus kuhlii) in Italy. Virol. J. 2018, 15, 37. [Google Scholar] [CrossRef]
- Woodruff, A.W.; Ansdell, V.E.; Bowen, E.T. Le Dantec virus infection in a patient who had not been to West Africa. Br. Med. J. 1977, 2, 1632–1633. [Google Scholar] [CrossRef]
- Ashraf, S.; Jerome, H.; Bugembe, D.L.; Ssemwanga, D.; Byaruhanga, T.; Kayiwa, J.T.; Downing, R.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Shepherd, J.G.; et al. Emerging viruses are an underestimated cause of undiagnosed febrile illness in Uganda. medRxiv 2023, 130, S17. [Google Scholar] [CrossRef]
- Vázquez-Morón, S.; Juste, J.; Ibáñez, C.; Aznar, C.; Ruiz-Villamor, E.; Echevarría, J.E. Asymptomatic rhabdovirus infection in meridional serotine bats (Eptesicus isabellinus) from Spain. Dev. Biol. 2008, 131, 311–316. [Google Scholar]
- Kading, R.C.; Gilbert, A.T.; Mossel, E.C.; Crabtree, M.B.; Kuzmin, I.V.; Niezgoda, M.; Agwanda, B.; Markotter, W.; Weil, M.R.; Montgomery, J.M.; et al. Isolation and molecular characterization of Fikirini rhabdovirus, a novel virus from a Kenyan bat. J. Gen. Virol. 2013, 94, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, D.; Williams, M.C.; Simpson, D.I.; West, R.; Mutere, F.A. Mount Elgon bat virus: A hitherto undescribed virus from Rhinolophus hildebrandtii eloquens K. Anderson. Arch. Gesamte Virusforsch. 1969, 26, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ghedin, E.; Rogers, M.B.; Widen, S.G.; Guzman, H.; Travassos da Rosa, A.P.A.; Wood, T.G.; Fitch, A.; Popov, V.; Holmes, E.C.; Walker, P.J.; et al. Kolente virus, a rhabdovirus species isolated from ticks and bats in the Republic of Guinea. J. Gen. Virol. 2013, 94, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.L.; Bennett, A.J.; Kityo, R.; Kuhn, J.H.; Chapman, C.A. Kanyawara Virus: A Novel Rhabdovirus Infecting Newly Discovered Nycteribiid Bat Flies Infesting Previously Unknown Pteropodid Bats in Uganda. Sci. Rep. 2017, 7, 5287. [Google Scholar] [CrossRef] [PubMed]
- Binger, T.; Annan, A.; Drexler, J.F.; Müller, M.A.; Kallies, R.; Adankwah, E.; Wollny, R.; Kopp, A.; Heidemann, H.; Dei, D.; et al. A Novel Rhabdovirus Isolated from the Straw-Colored Fruit Bat Eidolon helvum, with Signs of Antibodies in Swine and Humans. J. Virol. 2015, 89, 4588–4597. [Google Scholar] [CrossRef]
- Salaun, J.J.; Rickenbach, A.; Brès, P.; Brottes, H.; Germain, M.; Eouzan, J.P.; Ferrara, L. The Nkolbisson virus (YM 31-65), a new prototype of arbovirus isolated in Cameroun. Ann. Inst. Pasteur 1969, 116, 254–260. [Google Scholar]
- Grard, G.; Fair, J.N.; Lee, D.; Slikas, E.; Steffen, I.; Muyembe, J.-J.; Sittler, T.; Veeraraghavan, N.; Ruby, J.G.; Wang, C.; et al. A Novel Rhabdovirus Associated with Acute Hemorrhagic Fever in Central Africa. PLoS Pathog. 2012, 8, e1002924. [Google Scholar] [CrossRef] [PubMed]
- Gubala, A.; Davis, S.; Weir, R.; Melville, L.; Cowled, C.; Boyle, D. Tibrogargan and Coastal Plains rhabdoviruses: Genomic characterization, evolution of novel genes and seroprevalence in Australian livestock. J. Gen. Virol. 2011, 92, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Caì, Y.; Yú, S.; Jangra, R.K.; Postnikova, E.N.; Wada, J.; Tesh, R.B.; Whelan, S.P.J.; Lauck, M.; Wiley, M.R.; Finch, C.L.; et al. Human, Nonhuman Primate, and Bat Cells Are Broadly Susceptible to Tibrovirus Particle Cell Entry. Front. Microbiol. 2019, 10, 856. [Google Scholar] [CrossRef]
- Stremlau, M.H.; Andersen, K.G.; Folarin, O.A.; Grove, J.N.; Odia, I.; Ehiane, P.E.; Omoniwa, O.; Omoregie, O.; Jiang, P.-P.; Yozwiak, N.L.; et al. Discovery of Novel Rhabdoviruses in the Blood of Healthy Individuals from West Africa. PLoS Negl. Trop. Dis. 2015, 9, e0003631. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Pān, H.; Chiu, C.Y.; Stremlau, M. Human Tibroviruses: Commensals or Lethal Pathogens? Viruses 2020, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.R.; Singh, K.R.; Dhanda, V.; Bhatt, P.N. Experimental transmission of Chandipura virus by mosquitoes. Indian J. Med. Res. 1967, 55, 1306–1310. [Google Scholar] [PubMed]
- Marriott, A.C. Complete genome sequences of Chandipura and Isfahan vesiculoviruses. Arch. Virol. 2005, 150, 671–680. [Google Scholar] [CrossRef]
- Tesh, R.B.; Modi, G.B. Development of a continuous cell line from the sand fly Lutzomyia longipalpis (Diptera: Psychodidae), and its susceptibility to infection with arboviruses. J. Med. Entomol. 1983, 20, 199–202. [Google Scholar] [CrossRef]
- Mavale, M.S.; Fulmali, P.V.; Geevarghese, G.; Arankalle, V.A.; Ghodke, Y.S.; Kanojia, P.C.; Mishra, A.C. Venereal transmission of Chandipura virus by Phlebotomus papatasi (Scopoli). Am. J. Trop. Med. Hyg. 2006, 75, 1151–1152. [Google Scholar] [CrossRef]
- Mavale, M.S.; Geevarghese, G.; Ghodke, Y.S.; Fulmali, P.V.; Singh, A.; Mishra, A.C. Vertical and Venereal transmission of Chandipura Virus (Rhabdoviridae) by Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 909–911. [Google Scholar] [CrossRef]
- Dhanda, V.; Rodrigues, F.M.; Ghosh, S.N. Isolation of Chandipura virus from sandflies in Aurangabad. Indian J. Med. Res. 1970, 58, 179–180. [Google Scholar]
- Fontenille, D.; Traore-Lamizana, M.; Trouillet, J.; Leclerc, A.; Mondo, M.; Ba, Y.; Digoutte, J.P.; Zeller, H.G. First isolations of arboviruses from phlebotomine sand flies in West Africa. Am. J. Trop. Med. Hyg. 1994, 50, 570–574. [Google Scholar] [CrossRef]
- Rao, B.L.; Basu, A.; Wairagkar, N.S.; Gore, M.M.; Arankalle, V.A.; Thakare, J.P.; Jadi, R.S.; Rao, K.A.; Mishra, A.C. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet 2004, 364, 869–874. [Google Scholar] [CrossRef]
- Chadha, M.S.; Arankalle, V.A.; Jadi, R.S.; Joshi, M.V.; Thakare, J.P.; Mahadev, P.V.; Mishra, A.C. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am. J. Trop. Med. Hyg. 2005, 73, 566–570. [Google Scholar] [CrossRef]
- John, T.J. Chandipura virus, encephalitis, and epidemic brain attack in India. Lancet 2004, 364, 2175. [Google Scholar] [CrossRef]
- Rao, P.N.; Kumar, P.A.; Rao, T.A.; Prasad, Y.A.; Rao, C.J.; Rajyam, L.; Sarma, M.; Ashok, G. Role of Chandipura virus in an” epidemic brain attack” in Andhra Pradesh, India. J. Pediatr. Neurol. 2004, 2, 131–143. [Google Scholar]
- Rodrigues, J.J.; Singh, P.B.; Dave, D.S.; Prasan, R.; Ayachit, V.; Shaikh, B.H.; Pavri, K.M. Isolation of Chandipura virus from the blood in acute encephalopathy syndrome. Indian J. Med. Res. 1983, 77, 303–307. [Google Scholar]
- Fajs, L.; Humolli, I.; Saksida, A.; Knap, N.; Jelovšek, M.; Korva, M.; Dedushaj, I.; Avšič-Županc, T. Prevalence of Crimean-Congo hemorrhagic fever virus in healthy population, livestock and ticks in Kosovo. PLoS ONE 2014, 9, e110982. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Ross, T.M.; Evans, J.D. West Nile virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Marston, L.D. The perpetual challenge of antimicrobial resistance. JAMA 2014, 311, 1853–1854. [Google Scholar] [CrossRef] [PubMed]


| Virus | Reservoir | Distribution |
|---|---|---|
| Australian bat lyssavirus | Bats | Australia |
| Duvenhage virus | Bats | South Africa, Zimbabwe, Kenya |
| European bat lyssavirus 1 | Bats | Europe, Russia |
| European bat lyssavirus 2 | Bats | Europe |
| Irkut virus | Bats | Russia, China |
| Mokola virus | Rodents | Nigeria, South Africa |
| Rabies virus | Various mammals | Global |
| Virus | Clinical Syndrome | Reservoir/Vector | Distribution | References |
|---|---|---|---|---|
| ABLV | Encephalitis | Bats | Australia | [41,42,43,44,45,46] |
| DUVV | Encephalitis | Bats | South Africa, Kenya | [38,39,40] |
| EBLV-1 | Encephalitis | Bats | Europe, Russia | [29,30] |
| EBLV-2 | Encephalitis | Bats | Europe | [31,32] |
| IRKV | Encephalitis | Bats | Russia, China | [47,48,49] |
| MOKV | Encephalitis | Rodents | Nigeria, South Africa | [34,35,36] |
| RABV | Encephalitis | Various mammals | Global | [23,24,26] |
| LEDV | Acute febrile illness | Unknown | Africa | [51,53,54] |
| NKOV | Acute febrile illness | Mosquitoes | Cameroon | [61] |
| KURV | Unknown | Bats | Ghana | [60] |
| BASV | Hemorrhagic fever | Unknown | Central Africa | [62] |
| EKV-1 | Unknown/subclinical | Unknown | Nigeria | [65] |
| EKV-2 | Febrile illness/subclinical | Unknown | Nigeria, Angola | [65,66] |
| CHPV | Fever, encephalopathy | Sandflies | India, Senegal | [67,70,71,72,73,74,75,76,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, J.G.; Davis, C.; Streicker, D.G.; Thomson, E.C. Emerging Rhabdoviruses and Human Infection. Biology 2023, 12, 878. https://doi.org/10.3390/biology12060878
Shepherd JG, Davis C, Streicker DG, Thomson EC. Emerging Rhabdoviruses and Human Infection. Biology. 2023; 12(6):878. https://doi.org/10.3390/biology12060878
Chicago/Turabian StyleShepherd, James G., Chris Davis, Daniel G. Streicker, and Emma C. Thomson. 2023. "Emerging Rhabdoviruses and Human Infection" Biology 12, no. 6: 878. https://doi.org/10.3390/biology12060878
APA StyleShepherd, J. G., Davis, C., Streicker, D. G., & Thomson, E. C. (2023). Emerging Rhabdoviruses and Human Infection. Biology, 12(6), 878. https://doi.org/10.3390/biology12060878







