Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Study Design
2.2. Study Procedures
2.3. Study Measures
2.4. Data Analysis
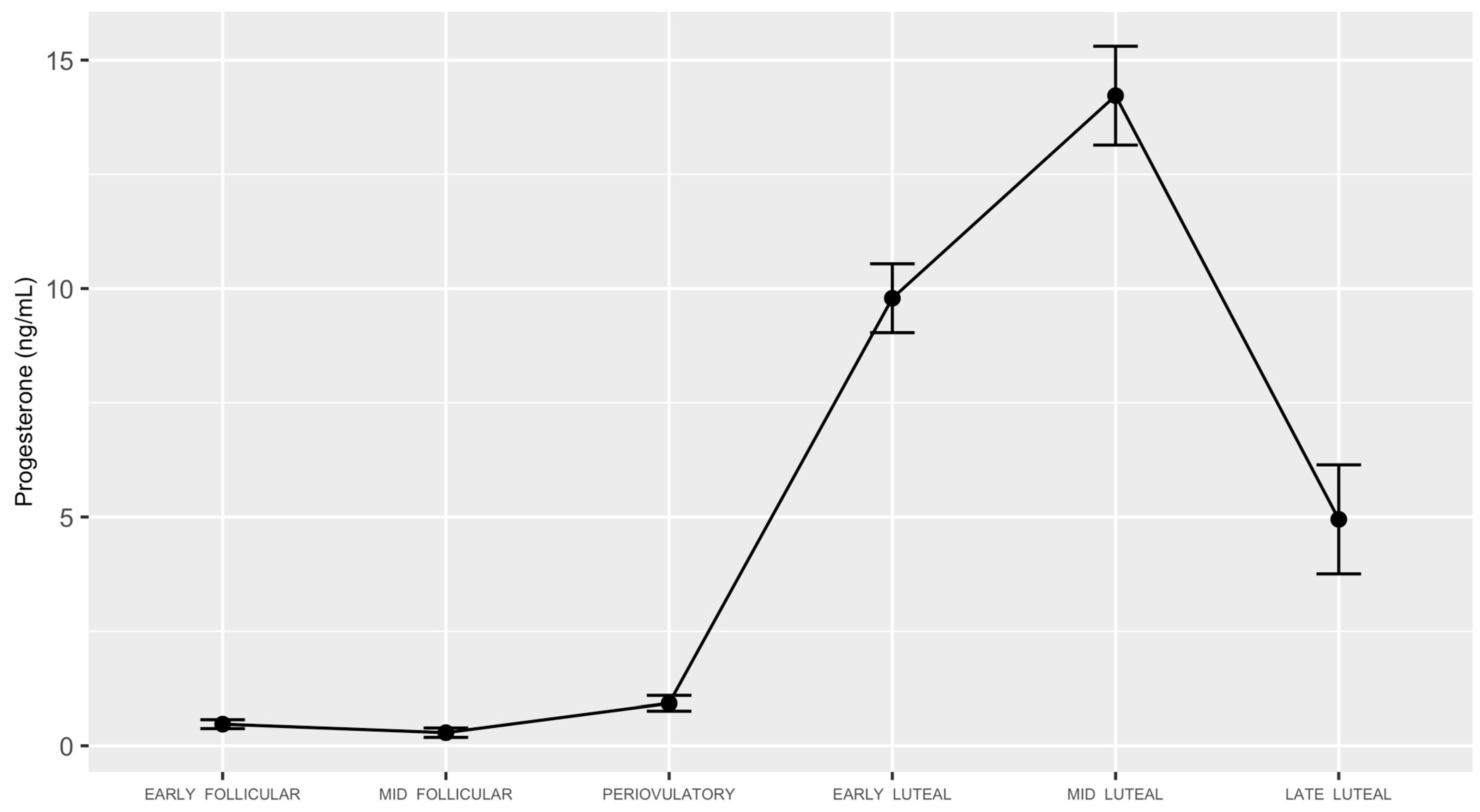
3. Results
3.1. Study Participants and Menstrual Cycle Characteristics
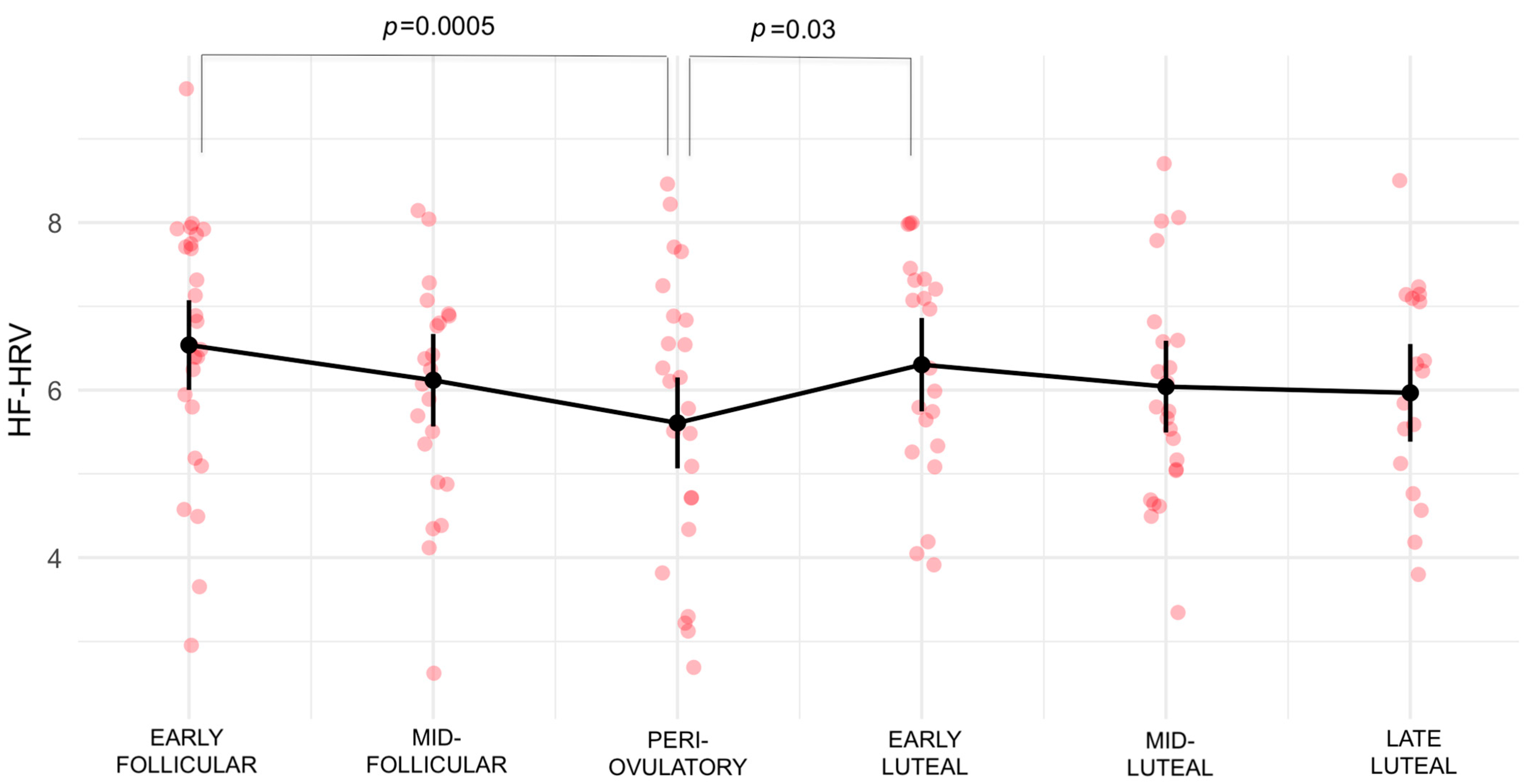
3.2. High Frequency-Heart Rate Variability
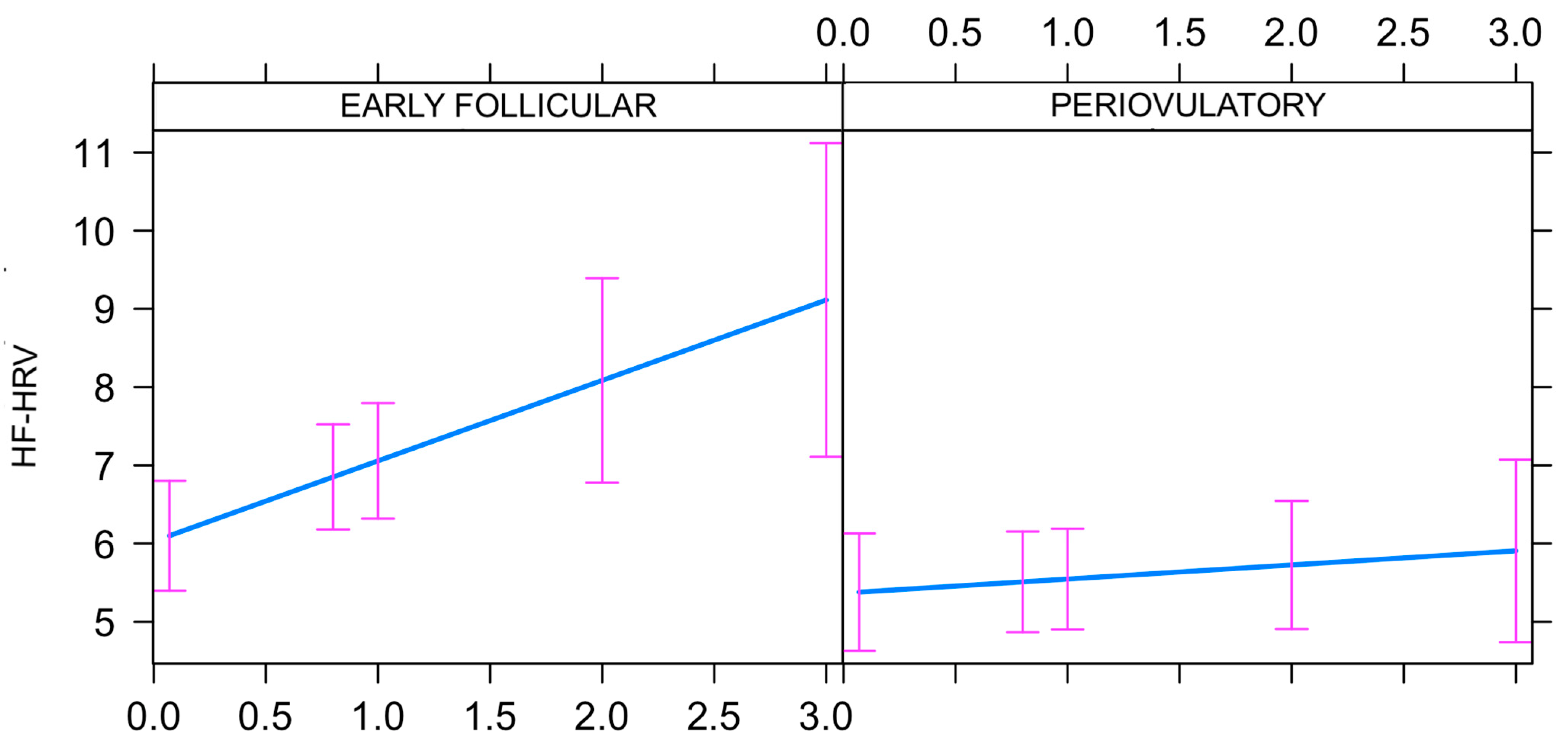
3.3. Progesterone by High Frequency-Heart Rate Variability Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buhusi, C.V.; Meck, W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005, 6, 755–765. [Google Scholar] [CrossRef]
- Golombek, D.A.; Bussi, I.L.; Agostino, P.V. Minutes, Days and years: Molecular interactions among different scales of biological timing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20120465. [Google Scholar] [CrossRef] [PubMed]
- Laje, R.; Agostino, P.V.; Golombek, D.A. The Times of Our Lives: Interaction Among Different Biological Periodicities. Front. Integr. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- De Mouzon, J.; Testart, J.; Lefevre, B.; Pouly, J.-L.; Frydman, R. Time relationships between basal body temperature and ovulation or plasma progestins. Fertil. Steril. 1984, 41, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Halbrecht, I. Ovarian function and body temperature. Lancet 1945, 2, 668. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. Thermal Changes in the Normal Menstrual Cycle. Br. Med. J. 1963, 1, 102–104. [Google Scholar] [CrossRef]
- Baker, F.C.; Driver, H.S. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef]
- Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [CrossRef]
- Thayer, J.F.; Sternberg, E. Beyond heart rate variability: Vagal regulation of allostatic systems. Ann. N. Y. Acad. Sci. 2006, 1088, 361–372. [Google Scholar] [CrossRef]
- Zahn, D.; Adams, J.; Krohn, J.; Wenzel, M.; Mann, C.G.; Gomille, L.K.; Jacobi-Scherbening, V.; Kubiak, T. Heart rate variability and self-control—A meta-analysis. Biol. Psychol. 2016, 115, 9–26. [Google Scholar] [CrossRef]
- Lewis, M.J.; Phillips, J.E. Older people’s cardiac responses as indicators of stress in familiar and unfamiliar environments. Psychophysiology 2012, 49, 478–483. [Google Scholar] [CrossRef]
- Denver, J.W.; Reed, S.F.; Porges, S.W. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol. Psychol. 2007, 74, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Thayer, J.F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol. 2015, 98 Pt 2, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Soumpasis, I.; Grace, B.; Johnson, S. Real-life insights on menstrual cycles and ovulation using big data. Hum. Reprod. Open 2020, 2020, hoaa011. [Google Scholar] [CrossRef]
- Harlow, S.D.; Ephross, S.A. Epidemiology of menstruation and its relevance to women’s health. Epidemiol. Rev. 1995, 17, 265–286. [Google Scholar] [CrossRef]
- Mumford, S.; Schisterman, E.F.; Gaskins, A.J.; Pollack, A.Z.; Perkins, N.J.; Whitcomb, B.W.; Ye, A.; Wactawski-Wende, J. Realign ment and multiple imputation of longitudinal data: An application to menstrual cycle data. Paediatr. Perinat. Epidemiol. 2011, 25, 448–459. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Würth, L.; Schneider, E.; Thayer, J.F.; Ditzen, B.; Jarczok, M.N. A Systematic Review and Meta-Analysis of Within-Person Changes in Cardiac Vagal Activity across the Menstrual Cycle: Implications for Female Health and Future Studies. J. Clin. Med. 2019, 8, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Pollard, T.M.; Unwin, N. The optimal timing of blood collection during the menstrual cycle for the assessment of endogenous sex hormones: Can interindividual differences in levels over the whole cycle be assessed on a single day? Cancer Epidemiol. Biomark. Prev. 2002, 11, 147–151. [Google Scholar]
- Tworoger, S.S.; Sluss, P.; Hankinson, S.E. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006, 66, 2476–2482. [Google Scholar] [CrossRef]
- Fehring, R.J.; Schneider, M.; Raviele, K. Variability in the phases of the menstrual cycle. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 376–384. [Google Scholar] [CrossRef]
- Windham, G.C.; Elkin, E.; Fenster, L.; Waller, K.; Anderson, M.; Mitchell, P.R.; Lasley, B.; Swan, S.H. Ovarian hormones in premenopausal women: Variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology 2002, 13, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; Manatunga, A.K.; Marcus, M. Validity of self-reported menstrual cycle length. Ann. Epidemiol. 2007, 17, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Voronova, N.V.; Meigal, A.Y.; E Yelaeva, L.; I Kuzmina, G. Heart Rate Variability in Women duirng Various Seasons and Phases of the Menstrual Cycle. Ekol. Cheloveka Hum. Ecol. 2015, 22, 20–26. [Google Scholar]
- Teixeira, A.L.; Ramos, P.S.; Vianna, L.; Ricardo, D.R. Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiology 2015, 52, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Tousignant-Laflamme, Y.; Marchand, S. Autonomic reactivity to pain throughout the menstrual cycle in healthy women. Clin. Auton. Res. 2009, 19, 167–173. [Google Scholar] [CrossRef]
- Saeki, Y.; Atogami, F.; Takahashi, K.; Yoshizawa, T. Reflex control of autonomic function induced by posture change during the menstrual cycle. J. Auton. Nerv. Syst. 1997, 66, 69–74. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, R.; Oei, T.P.; Wang, Q.; Zhao, Y.; Liu, Y. Variation in the stress response between high- and low-neuroticism female undergraduates across the menstrual cycle. Stress 2013, 16, 503–509. [Google Scholar] [CrossRef]
- Leicht, A.S.; Hirning, D.A.; Allen, G.D. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp. Physiol. 2003, 88, 441–446. [Google Scholar] [CrossRef]
- Seebauer, M.; Frühwirth, M.; Moser, M. Changes of respiratory sinus arrhythmia during the menstrual cycle depend on average heart rate. Eur. J. Appl. Physiol. 2002, 87, 309–314. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Jarczok, M.N.; Eckstein, M.; Schneider, E.; Brenner, I.G.; Duffy, K.; Schweizer, S.; Kiesner, J.; Thayer, J.F.; et al. Menstrual Cycle Changes in Vagally-Mediated Heart Rate Variability are Associated with Progesterone: Evidence from Two Within-Person Studies. J. Clin. Med. 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Howards, P.P.; Schisterman, E.F.; Wactawski-Wende, J.; Reschke, J.E.; Frazer, A.A.; Hovey, K.M. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: The BioCycle Study. Am. J. Epidemiol. 2009, 169, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Ware, L.; Capodilupo, E.R. Patterns of endogenous and exogenous ovarian hormone modulation on recovery metrics across the menstrual cycle. BMJ Open Sport Exerc. Med. 2021, 7, e001047. [Google Scholar] [CrossRef] [PubMed]
- Kayacan, Y.; Makaracı, Y.; Ozgocer, T.; Ucar, C.; Yıldız, S. Cortisol Awakening Response and Heart Rate Variability in the Menstrual Cycle of Sportswomen. Res. Q. Exerc. Sport 2021, 92, 760–769. [Google Scholar] [CrossRef]
- Ramesh, S.; James, M.T.; Holroyd-Leduc, J.M.; Wilton, S.B.; Sola, D.Y.; Ahmed, S.B. Heart rate variability as a function of menopausal status, menstrual cycle phase, and estradiol level. Physiol. Rep. 2022, 10, e15298. [Google Scholar] [CrossRef]
- Grant, A.D.; Newman, M.; Kriegsfeld, L.J. Ultradian rhythms in heart rate variability and distal body temperature anticipate onset of the luteinizing hormone surge. Sci. Rep. 2020, 10, 20378. [Google Scholar] [CrossRef]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J.; Sun, J.; Dang, N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites 2022, 12, 941. [Google Scholar] [CrossRef]
- Endicott, J.; Nee, J.; Harrison, W. Daily Record of Severity of Problems (DRSP): Reliability and validity. Arch. Womens Ment. Health 2006, 9, 41–49. [Google Scholar] [CrossRef]
- Hamidovic, A.; Davis, J.; Soumare, F.; Naveed, A.; Ghani, Y.; Semiz, S.; Khalil, D.; Wardle, M. Allopregnanolone Is Associated with a Stress-Induced Reduction of Heart Rate Variability in Premenstrual Dysphoric Disorder. J. Clin. Med. 2023, 12, 1553. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- ARUP Laboratories. Luteinizing Hormone, Serum: ARUP Laboratories Test Directory. Available online: https://ltd.aruplab.com/Tests/Pub/0070093 (accessed on 15 September 2022).
- Roche Diagnostics GmbH. Elecsys LH Assay; Roche Diagnostics GmbH: Indianapolis, IN, USA, 2020. [Google Scholar]
- Hamidovic, A.; Davis, J.; Soumare, F.; Datta, A.; Naveed, A. Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle. Biomolecules 2023, 13, 652. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. Beck depression inventory (BDI). Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Golden, S.H.; Folsom, A.R.; Haskell, W.; Liao, D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: The Atherosclerosis Risk In Communities study, 1987–1998. Circulation 2003, 107, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Cai, J.; Barnes, R.W.; Tyroler, H.A.; Rautaharju, P.; Holme, I.; Heiss, G. Association of cardiac autonomic function and the development of hypertension: The ARIC study. Am. J. Hypertens. 1996, 9 Pt 1, 1147–1156. [Google Scholar] [CrossRef]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart rate variability for risk assessment of myocardial ischemia in patients without known coronary artery disease: The HRV-DETECT (heart rate variability for the detection of myocardial ischemia) study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Felmingham, K.L.; Nichols, D. Reproducibility of saliva progesterone measured by immunoassay compared to liquid chromatography mass spectrometry. Anal. Biochem. 2020, 610, 113984. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Iriguchi, M.; Koda, H.; Koyama, T.; Masataka, N. Modulation of visual attention assessed using the Stroop task during the menstrual cycle: Comparison between the menstrual and ovulation phases. Color Res. Appl. 2019, 44, 1024–1033. [Google Scholar] [CrossRef]
- Nashiro, K.; Yoo, H.J.; Cho, C.; Min, J.; Feng, T.; Nasseri, P.; Bachman, S.L.; Lehrer, P.; Thayer, J.F.; Mather, M. Effects of a Randomised Trial of 5-Week Heart Rate Variability Biofeedback Intervention on Cognitive Function: Possible Benefits for Inhibitory Control. Appl. Psychophysiol. Biofeedback 2023, 48, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
| Category | Mean (SD) or N |
|---|---|
| AGE | |
| 26.53 (5.01) | |
| RACE | |
| White | 10 |
| African American | 7 |
| American Indian/Alaska Native | 1 |
| Asian | 5 |
| Native Hawaiian or Other Pacific Islander | 0 |
| More than 1 race | 1 |
| Unknown/Do not want to specify | 4 |
| ETHNICITY | |
| Hispanic | 9 |
| Non-Hispanic | 17 |
| Unknown/Do not want to specify | 2 |
| STUDENT STATUS | |
| Yes | 15 |
| No | 13 |
| MARITAL STATUS | |
| Single/Never married | 25 |
| Married | 2 |
| Divorced | 1 |
| INCOME | |
| Less than USD 20,000 | 13 |
| USD 20,000–34,999 | 5 |
| USD 35,000–49,999 | 4 |
| USD 50,000–74,999 | 3 |
| USD 75,000 or more | 3 |
| MENARCHE AGE | |
| 11.89 (1.44) | |
| BMI * | |
| 25.55 (4.75) | |
| BDI ** | |
| 2.60 (3.10) | |
| Contrast | Estimate | SE | df | t-Ratio | p-Value |
|---|---|---|---|---|---|
| Early follicular–Mid-follicular | 0.4203 | 0.221 | 101 | 1.898 | 0.4093 |
| Early follicular–Periovulatory | 0.9302 | 0.215 | 100 | 4.324 | 0.0005 *** |
| Early follicular–Early luteal | 0.2347 | 0.225 | 101 | 1.044 | 0.9019 |
| Early follicular–Mid-luteal | 0.4969 | 0.219 | 101 | 2.265 | 0.2182 |
| Early follicular–Late luteal | 0.5709 | 0.242 | 101 | 2.361 | 0.18 |
| Mid-follicular–Periovulatory | 0.5098 | 0.228 | 101 | 2.236 | 0.231 |
| Mid-follicular–Early luteal | −0.1857 | 0.234 | 101 | −0.794 | 0.9679 |
| Mid-follicular–Mid-luteal | 0.0765 | 0.23 | 101 | 0.333 | 0.9994 |
| Mid-follicular–Late luteal | 0.1506 | 0.254 | 102 | 0.592 | 0.9914 |
| Periovulatory–Early luteal | −0.6955 | 0.23 | 101 | −3.02 | 0.0367 * |
| Periovulatory–Mid-luteal | −0.4333 | 0.224 | 101 | −1.93 | 0.3901 |
| Periovulatory–Late luteal | −0.3593 | 0.247 | 101 | −1.455 | 0.6931 |
| Early luteal–Mid-luteal | 0.2622 | 0.235 | 101 | 1.118 | 0.8728 |
| Early luteal–Late luteal | 0.3362 | 0.258 | 102 | 1.304 | 0.7824 |
| Mid-luteal–Late luteal | 0.074 | 0.249 | 101 | 0.298 | 0.9997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Davis, J.; Wardle, M.; Naveed, A.; Soumare, F. Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability. Biology 2023, 12, 785. https://doi.org/10.3390/biology12060785
Hamidovic A, Davis J, Wardle M, Naveed A, Soumare F. Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability. Biology. 2023; 12(6):785. https://doi.org/10.3390/biology12060785
Chicago/Turabian StyleHamidovic, Ajna, John Davis, Margaret Wardle, Aamina Naveed, and Fatimata Soumare. 2023. "Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability" Biology 12, no. 6: 785. https://doi.org/10.3390/biology12060785
APA StyleHamidovic, A., Davis, J., Wardle, M., Naveed, A., & Soumare, F. (2023). Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability. Biology, 12(6), 785. https://doi.org/10.3390/biology12060785







