Apoptotic Changes, Oxidative Stress and Immunomodulatory Effects in the Liver of Japanese Seabass (Lateolabrax japonicus) Induced by Ammonia-Nitrogen Stress during Keep-Live Transport
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experiment to Determine LC50 and Experimental Procedure:
2.3. Sample Collection and Processing
2.4. Biochemical, Immunological and Oxidation Parameters Analysis
2.5. Apoptosis Detection
2.6. Histological Examination
2.7. Real-Time PCR
3. Results
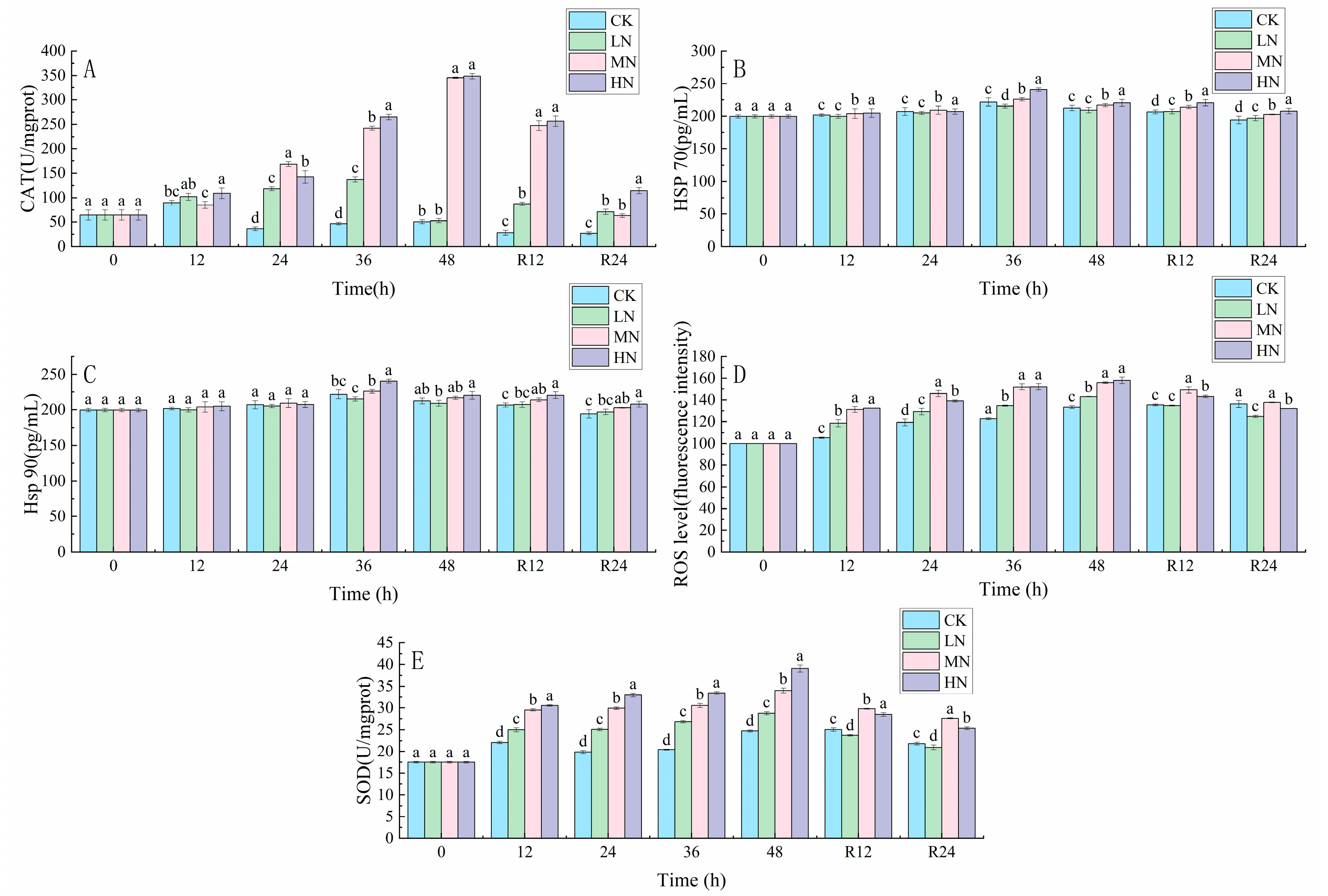
3.1. Oxidative Stress Response
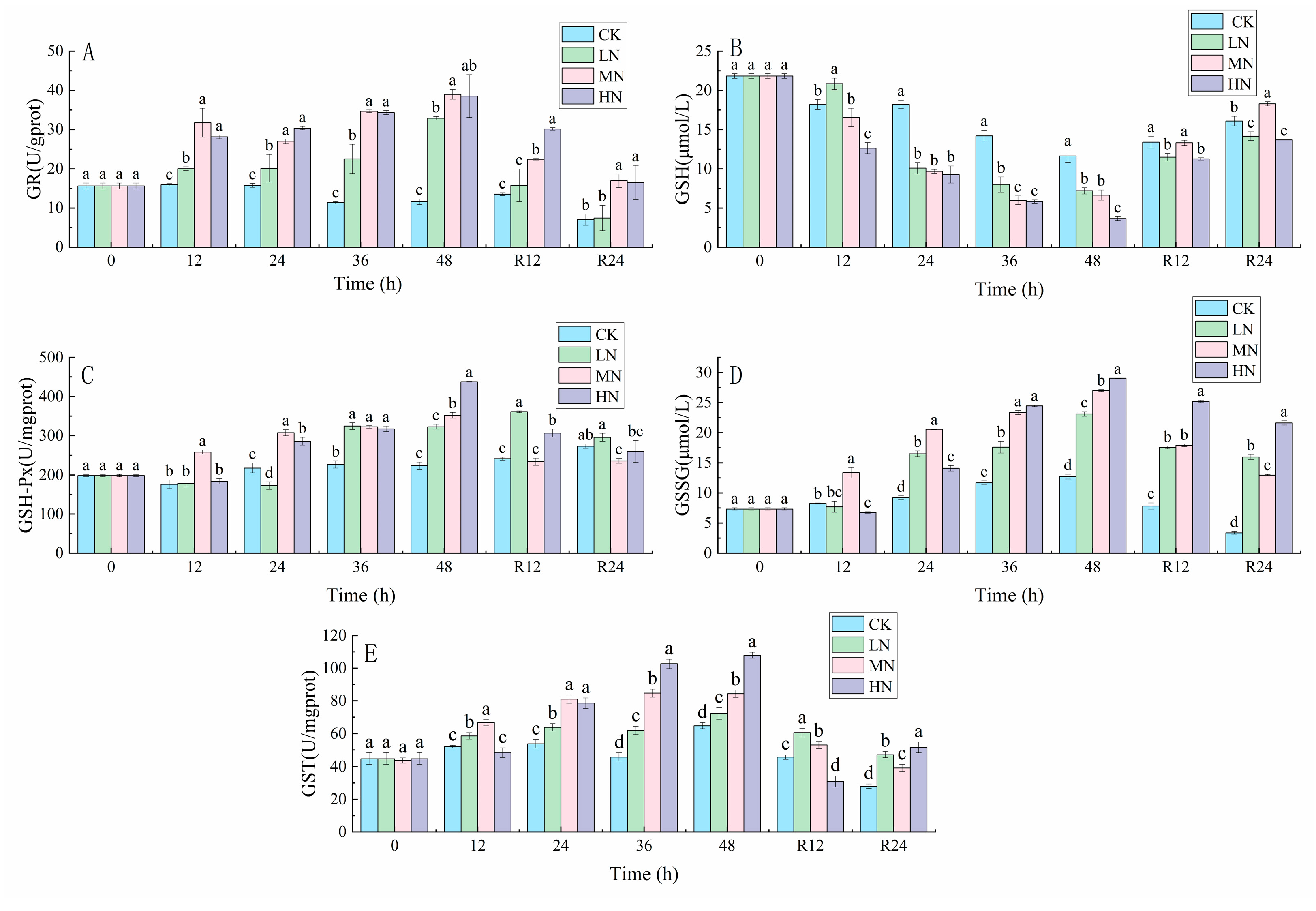
3.2. Glutathione-Related Reactions
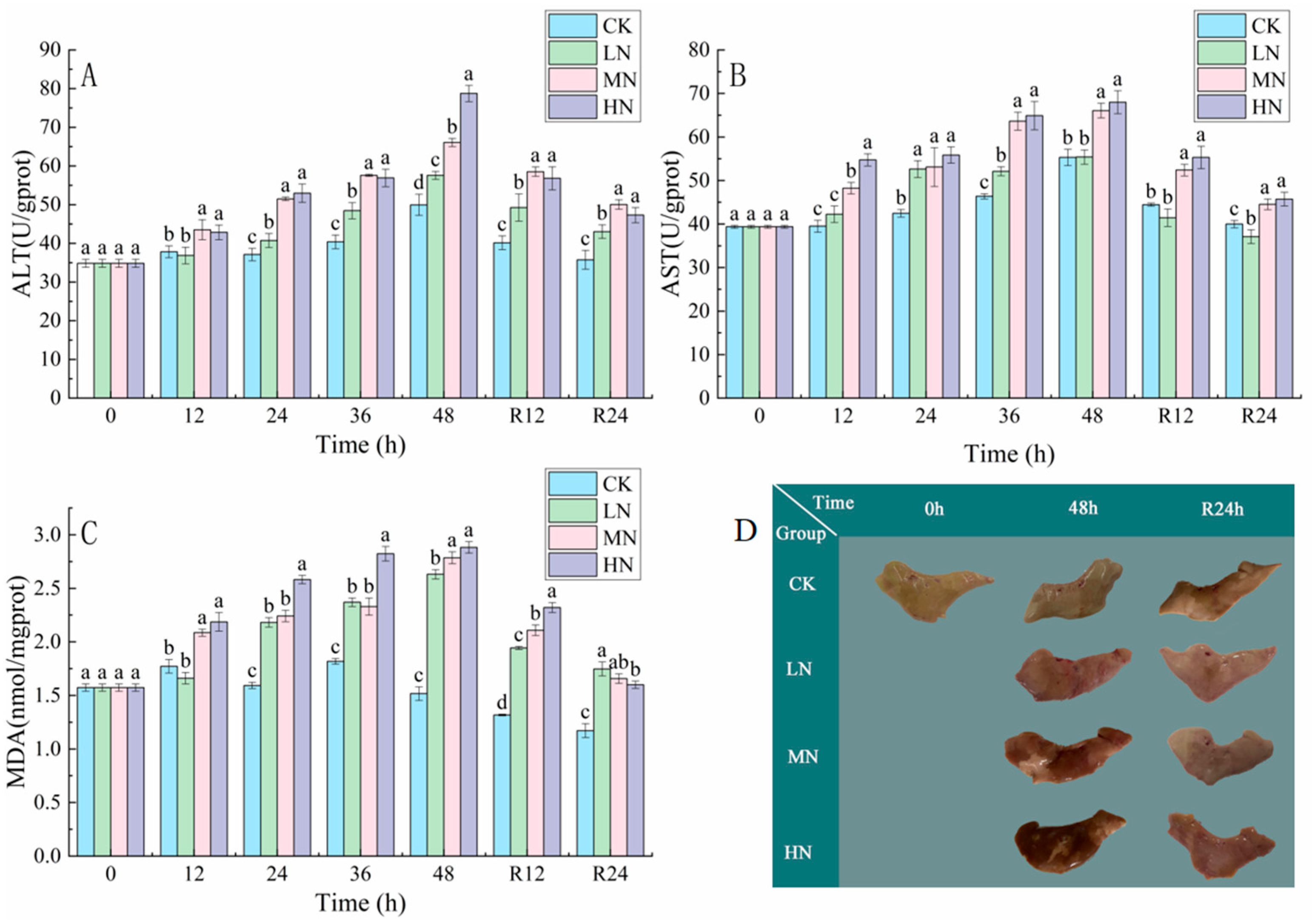
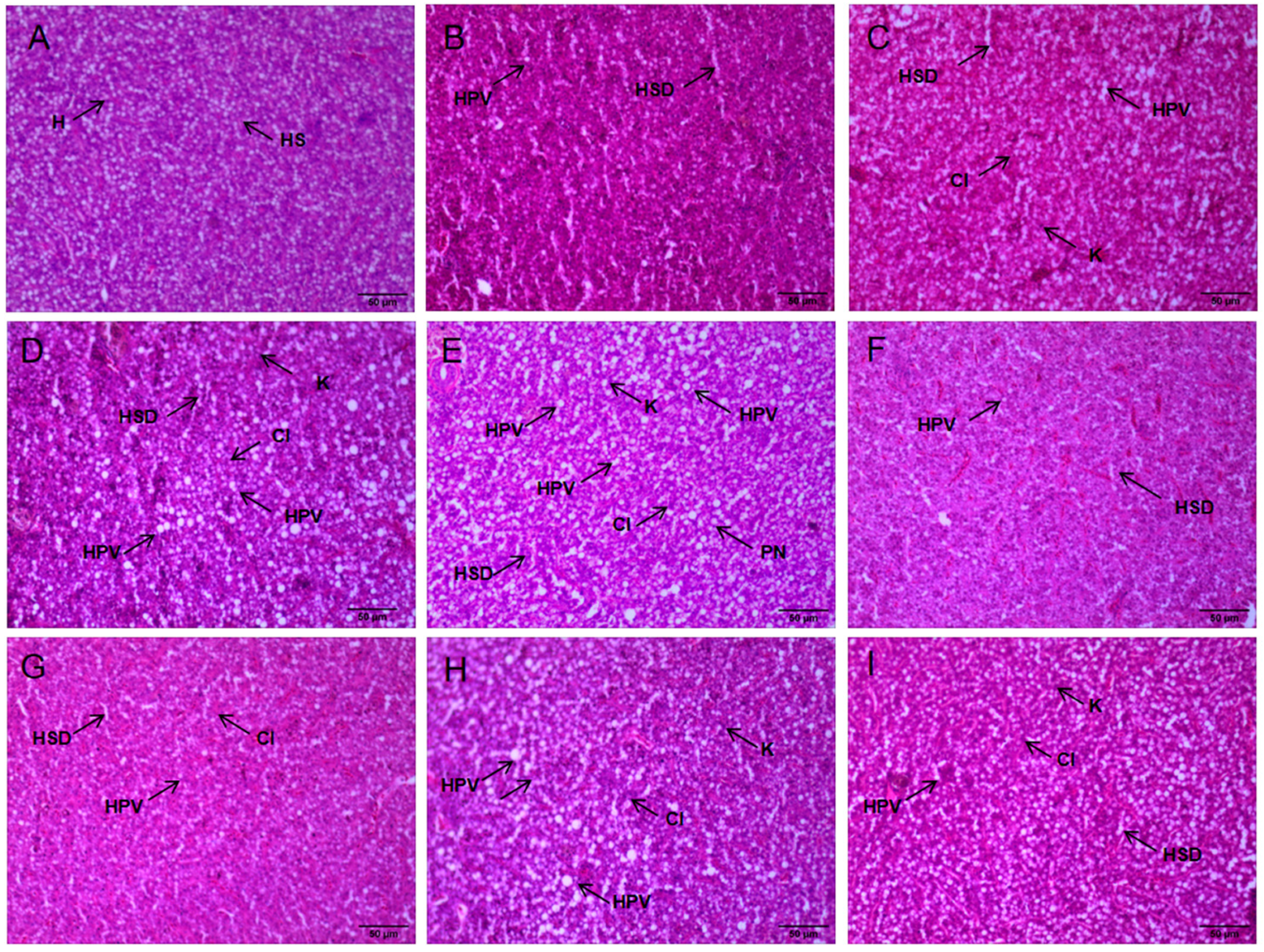
3.3. Tissue Damage and Liver Pathology
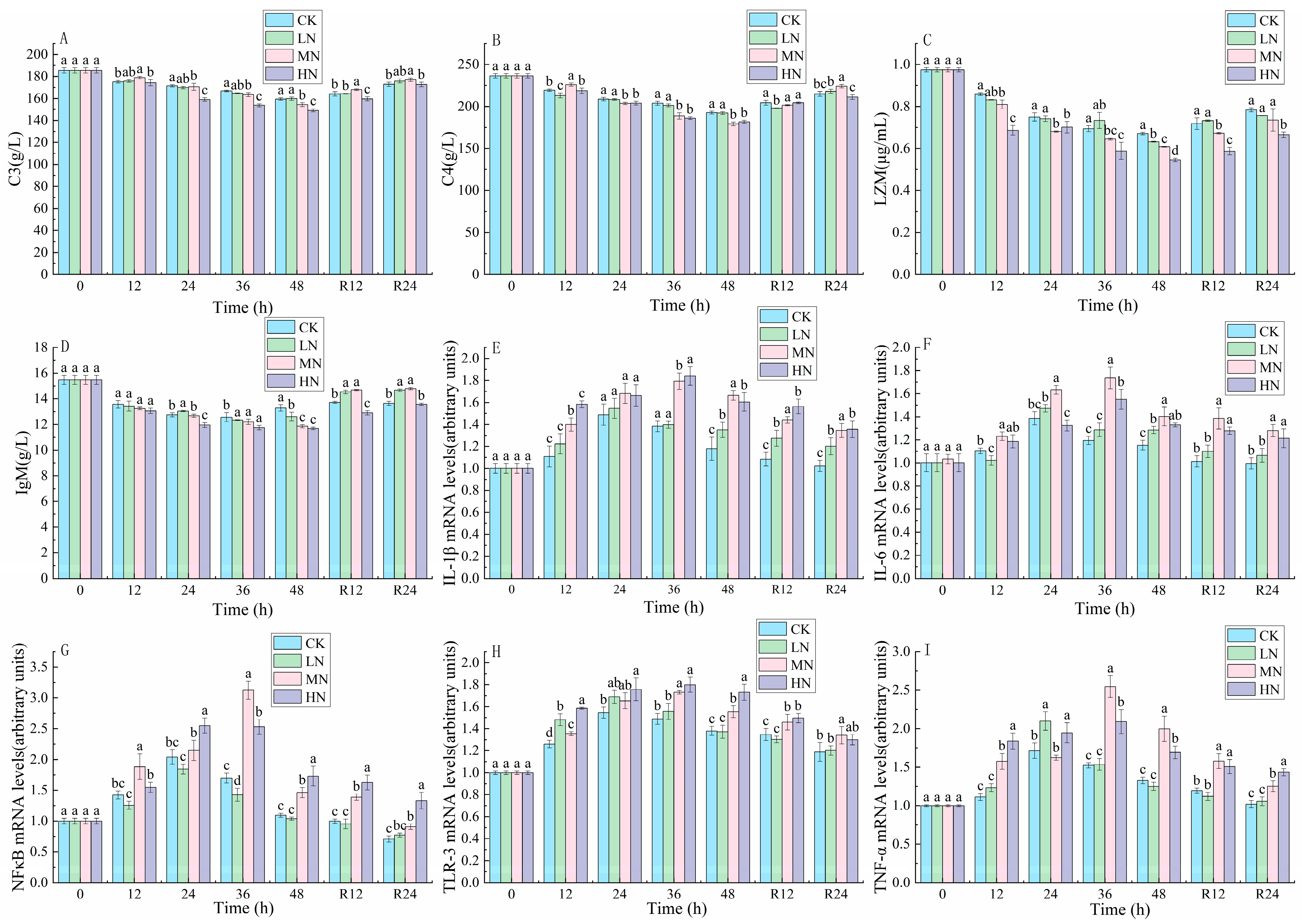
3.4. Immune Response and Inflammatory Factors
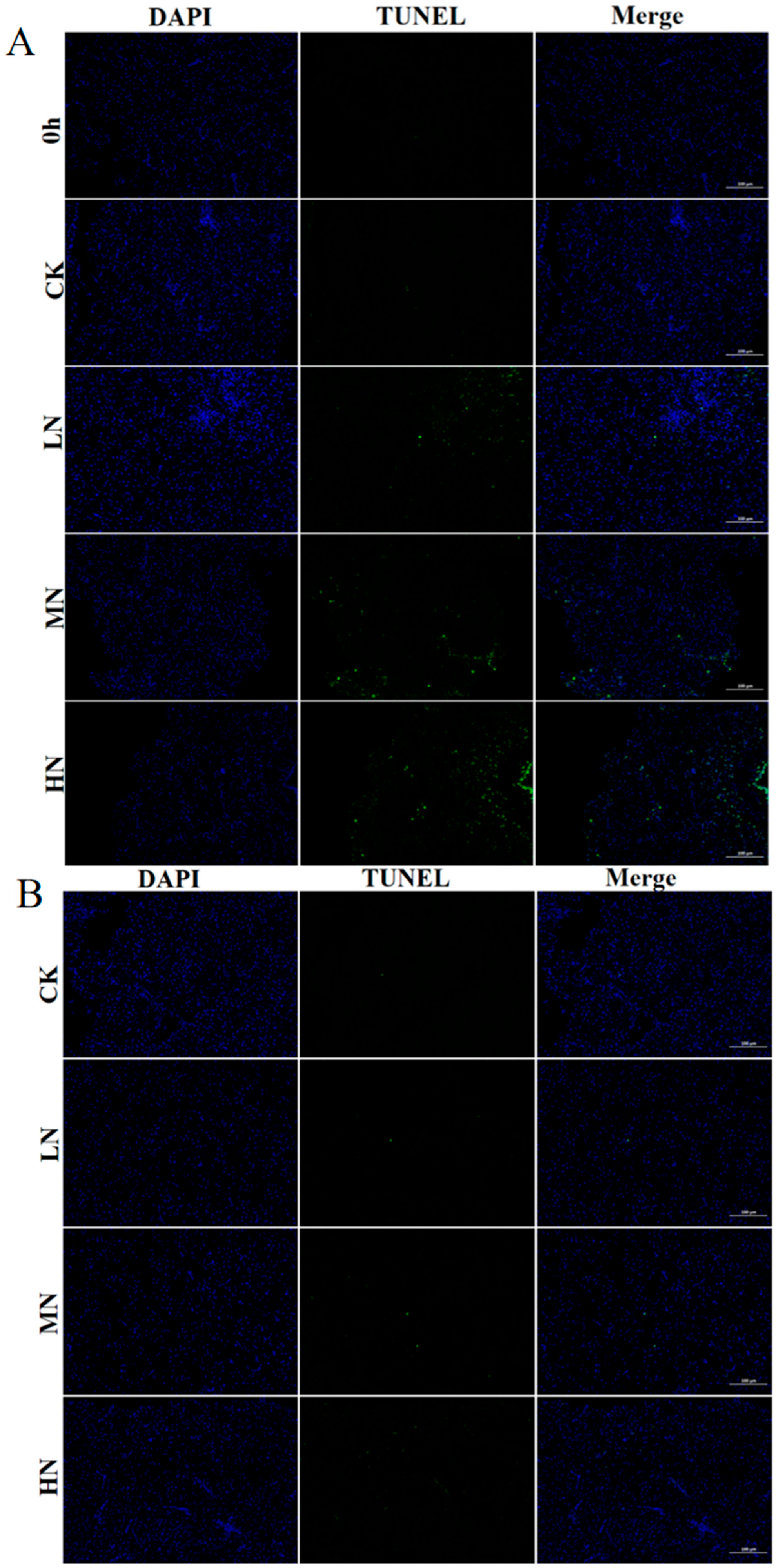
3.5. Apoptosis
4. Discussion
4.1. The impact of NH3-N Stress Transport on the Antioxidant Defense System of the Liver of Japanese Seabass
4.2. The Impact of NH3-N Stress Transport on Liver Tissue Damage in Japanese Seabass
4.3. The Impact of NH3-N Stress Transport on Immunological and Inflammatory Responses in Japanese Seabass
4.4. The Impact of NH3-N Stress Transport on Apoptosis in Japanese Seabass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Liu, M.; Jiang, K.; Wang, M.; Wang, L. Aflatoxin B1 (AFB1) induced dysregulation of intestinal microbiota and damage of antioxidant system in pacific white shrimp (Litopenaeus vannamei). Aquaculture 2018, 495, 940–947. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Chmelova, L.; Sehonova, P.; Blahova, J.; Doubkova, V.; Plhalova, L.; Fiorino, E.; Vojtek, L.; Vicenova, M.; Siroka, Z.; et al. The effects of a therapeutic formalin bath on selected immunological and oxidative stress parameters in common carp (Cyprinus carpio). Sci. Total Environ. 2019, 653, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.K.; Hur, Y.B. Temperature-mediated changes in stress responses, acetylcholinesterase, and immune responses of juvenile olive flounder Paralichthys olivaceus in a bio-floc environment. Aquaculture 2019, 506, 453–458. [Google Scholar] [CrossRef]
- Liu, B.; Jia, R.; Huang, B.; Lei, J. Interactive effect of ammonia and crowding stress on ion-regulation and expression of immune-related genes in juvenile turbot (Scophthalmus maximus). Mar. Freshw. Behav. Physiol. 2017, 50, 179–194. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Tissue Structure in Fish Exposed to Ammonia Nitrogen: A Review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef]
- Zheng, T.; Song, Z.; Tao, Y.; Qiang, J.; Ma, J.; Lu, S.; Xu, P. Transport stress induces innate immunity responses through TLR and NLR signaling pathways and increases mucus cell number in gills of hybrid yellow catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂). Fish Shellfish Immunol. 2022, 127, 166–175. [Google Scholar] [CrossRef]
- Wu, Y.; You, X.; Sun, W.; Xiong, G.; Shi, L.; Qiao, Y.; Wu, W.; Li, X.; Wang, J.; Ding, A.; et al. Insight into acute heat stress on meat qualities of rainbow trout (Oncorhynchus mykiss) during short-time transportation. Aquaculture 2021, 543, 737013. [Google Scholar] [CrossRef]
- Molayemraftar, T.; Peyghan, R.; Razi Jalali, M.; Shahriari, A. Single and combined effects of ammonia and nitrite on common carp, Cyprinus carpio: Toxicity, hematological parameters, antioxidant defenses, acetylcholinesterase, and acid phosphatase activities. Aquaculture 2022, 548, 737676. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Luo, X.; Wen, M.; Wang, C.; Qin, C.; Shao, J.; Gan, L.; Dong, R.; Jiang, H. Effect of acute ammonia toxicity on inflammation, oxidative stress and apoptosis in head kidney macrophage of Pelteobagrus fulvidraco and the alleviation of curcumin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109098. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Liu, B.; Guo, L.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat. Toxicol. 2021, 240, 105969. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tan, P.; Dong, X.; Xu, H.; Mai, K.; Ai, Q. Dietary vegetable oil suppressed non-specific immunity and liver antioxidant capacity but induced inflammatory response in Japanese sea bass (Lateolabrax japonicus). Fish Shellfish Immunol. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Liang, X.; Wu, X.; Gu, X.; Han, J.; Xue, M. N-carbamoylglutamate improves lipid metabolism, inflammation, and apoptosis responses in visceral adipocytes of Japanese seabass (Lateolabrax japonicus), in vivo and in vitro. Anim. Nutr. 2021, 7, 707–715. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Wang, L.; Song, K.; Lu, K.; Zhang, L.; Ma, X.; Zhang, C. Effects of dietary vitamin E levels on growth, antioxidant capacity and immune response of spotted seabass (Lateolabrax maculatus) reared at different water temperatures. Aquaculture 2023, 565, 739141. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Wen, H.; Wang, K.; Qi, X.; Li, J.; He, F.; Li, Y. Genome-wide identification and characterization of toll-like receptor genes in spotted sea bass (Lateolabrax maculatus) and their involvement in the host immune response to Vibrio harveyi infection. Fish Shellfish Immunol. 2019, 92, 782–791. [Google Scholar] [CrossRef]
- Xiang, Y.; Jia, P.; Liu, W.; Yi, M.; Jia, K. Comparative transcriptome analysis reveals the role of p53 signalling pathway during red-spotted grouper nervous necrosis virus infection in Lateolabrax japonicus brain cells. J. Fish Dis. 2019, 42, 585–595. [Google Scholar] [CrossRef]
- Le, Y.; Jia, P.; Jin, Y.; Liu, W.; Jia, K.; Yi, M. The antiviral role of heat shock protein 27 against red spotted grouper nervous necrosis virus infection in sea perch. Fish Shellfish Immunol. 2017, 70, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; Dong, Y.; Mei, J.; Xie, J. Effects of tricaine methanesulphonate (MS-222) on physiological stress and fresh quality of sea bass (Lateolabrax maculatus) under simulated high-density and long-distance transport stress. Biology 2023, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Miao, L.-H.; Pan, W.-J.; Huang, X.; Dengu, J.M.; Zhang, W.-X.; Ge, X.-P.; Liu, B.; Ren, M.-C.; Zhou, Q.-L.; et al. Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish Shellfish Immunol. 2018, 76, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Shukry, M.; El Euony, O.I.; Mohamed Soliman, M.; Noreldin, A.E.; Ghetas, H.A.; Dawood, M.A.O.; Khallaf, M.A. Hazardous Effects of SiO2 Nanoparticles on Liver and Kidney Functions, Histopathology Characteristics, and Transcriptomic Responses in Nile Tilapia (Oreochromis niloticus) Juveniles. Biology 2021, 10, 183. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Niu, C. Diverse defense responses to ammonia stress in three freshwater turtles. Aquaculture 2022, 546, 737302. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, N.; Wang, C.; Long, J.; Chen, Y.; Deng, Z.; Zhang, Z.; Zhao, R.; Sun, J.; Wang, Z.; et al. Acute ammonia stress-induced oxidative and heat shock responses modulated by transcription factors in Litopenaeus vannamei. Fish Shellfish Immunol. 2022, 128, 181–187. [Google Scholar] [CrossRef]
- Sinha, A.K.; Zinta, G.; AbdElgawad, H.; Asard, H.; Blust, R.; De Boeck, G. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 174, 21–31. [Google Scholar] [CrossRef]
- Ou, H.; Liang, J.; Liu, J. Effects of acute ammonia exposure on oxidative stress, endoplasmic reticulum stress and apoptosis in the kuruma shrimp (Marsupenaeus japonicus). Aquac. Rep. 2022, 27, 101383. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, Y.; Lu, W.; Zhang, Q.; Cheng, J. Genomic and Transcriptomic Landscape and Evolutionary Dynamics of Heat Shock Proteins in Spotted Sea Bass (Lateolabrax maculatus) under Salinity Change and Alkalinity Stress. Biology 2022, 11, 353. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Mahboub, H.H.; Sheraiba, N.I.; Abduljabbar, M.H.; Mahmoud, Y.K.; Abomughaid, M.M.; Ismail, A.K. Ammonia toxicity in Nile tilapia: Potential role of dietary baicalin on biochemical profile, antioxidant status and inflammatory gene expression. Aquac. Rep. 2023, 28, 101434. [Google Scholar] [CrossRef]
- Bian, C.; Yu, H.; Yang, K.; Mei, J.; Xie, J. Effects of single-, dual-, and multi-frequency ultrasound-assisted freezing on the muscle quality and myofibrillar protein structure in large yellow croaker (Larimichthys crocea). Food Chem. X 2022, 15, 100362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, J.; Qi, Q.; Xu, M.; Xu, R. Effects of ammonia exposure on anxiety behavior, oxidative stress and inflammation in guppy (Poecilia reticulate). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 265, 109539. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Ma, H.; Shi, X.; Zhou, W.; Pan, W.; Song, Y.; Chen, Q.; Yu, X.; Niu, C.; Yang, Y.; et al. Effects of microbe-derived antioxidants on growth, digestive and aminotransferase activities, and antioxidant capacities in the hepatopancreas of Eriocheir sinensis under ammonia nitrogen stress. Aquac. Fish. 2023, in press. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Paknejad, H.; Mirzargar, S.S. Hepatoprotective effects of dietary Artemisia (Artemisia annua) leaf extract on common carp (Cyprinus carpio) exposed to ambient ammonia. Aquaculture 2020, 527, 735443. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, K.; Wang, G.; Mo, W.; Huang, Y.; Cao, J. Metabolic changes, antioxidant status, immune response and resistance to ammonia stress in juvenile yellow catfish (Pelteobagrus fulvidraco) fed diet supplemented with sodium butyrate. Aquaculture 2021, 536, 736441. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Zhang, M.; Jiang, H.; Li, M. Chronic toxicity study of ammonia exposure in juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2023, 567, 739266. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Sun, Z.; Jiang, H.; Qian, Y.; Wang, R.; Li, M. The effects of acute and chronic ammonia exposure on growth, survival, and free amino acid abundance in juvenile Japanese sea perch Lateolabrax japonicus. Aquaculture 2022, 560, 738512. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Huang, Y.; Chang, K.; Zhao, X. Addition of Chlorella sorokiniana meal in the diet of juvenile rainbow trout (Oncorhynchus mykiss): Influence on fish growth, gut histology, oxidative stress, immune response, and disease resistance against Aeromonas salmonicida. Fish Shellfish Immunol. 2022, 129, 243–250. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Wang, X.; Fang, Y.; Cao, S.; Huang, B.; Chen, H.; Xing, R.; Liu, B. Toxicity in Takifugu rubripes exposed to acute ammonia: Effects on immune responses, brain neurotransmitter levels, and thyroid endocrine hormones. Ecotoxicol. Environ. Saf. 2022, 244, 114050. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huang, B.; Meng, X.S.; Zhang, T.; Zhao, K.-F.; Chen, H.-B.; Xing, R.; Liu, B.-L. Alterations in hematological and biochemical parameters, oxidative stress, and immune response in Takifugu rubripes under acute ammonia exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 243, 108978. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Q.; Dong, B.; Han, D.; Zhu, X.; Liu, H.; Yang, Y.; Xie, S.; Jin, J. Resveratrol attenuated oxidative stress and inflammatory and mitochondrial dysfunction induced by acute ammonia exposure in gibel carp (Carassius gibelio). Ecotoxicol. Environ. Saf. 2023, 251, 114544. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Qian, Y.; Shi, G.; Wang, R. Ammonia toxicity in the yellow catfish (Pelteobagrus fulvidraco): The mechanistic insight from physiological detoxification to poisoning. Fish Shellfish Immunol. 2020, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group | Actual NH3-N Concentration (mg/L) |
|---|---|
| Control group (CK) | 0.3 |
| Low NH3-N group (LA, 6.4 mg/L) | 6.5 |
| Medium NH3-N group (MA, 12.8 mg/L) | 11.5 |
| High NH3-N group (HA, 19.2 mg/L) | 19.0 |
| Stage | Temperature and Time | Process |
|---|---|---|
| Stage 1 | 95 °C, 30 s | predenaturation |
| Stage 2 | 95 °C, 15 s | denature |
| 60 °C, 30 s (40 cycles) | annealing/extension | |
| Stage 3 | 65 °C → 95 °C | melting curve: every time the temperature rises by 0.5 °C, the fluorescence signal is collected |
| Gene | Primer Sequence (5′–3′) | Product Length (bp) | Temperature (°C) | Efficiency (%) | References |
|---|---|---|---|---|---|
| Apoptosis gene | |||||
| Actin | F: CAACTGGGATGACATGGAGAAG | 200 | 60 | 101 | [16] |
| R: TTGGCTTTGGGGTTCAGG | |||||
| TNF-α | F: GACTCCATAGGCAGCAAAGC | 205 | 60 | 103.2 | [17] |
| R: AGAAAGTCTTGCCCTCGTCA | |||||
| IL-1β | F: CTGAACATCAAGGGCACAGA | 192 | 60 | 92.8 | [17] |
| R: GTTGAAGGGGACAGACCTGA | |||||
| IL-6 | F: TACAATGTCCTCCTCAAGCACG | 129 | 60 | 98.4 | [18] |
| R: GCCTTTGACCTCCTCCATCAG | |||||
| TLR-3 | F: GGCCTGGATCAAATTCAAGA | 139 | 60 | 103 | [19] |
| R: GACAGGGGACTGAATGGAGA | |||||
| NF-κB | F: GAAGGTATGGGAGGAGGAGTTT | 189 | 60 | 102 | [20] |
| R: AACCACAGGGTCCAGAGGAAA | |||||
| Pro-inflammatory factor | |||||
| P53 | F: ACCATCCTGCTGAGCTTCAT | 178 | 60 | 95.3 | [20] |
| R: GCCCAAAACAAGTCCCTCTG | |||||
| Caspase 3 | F: ATCACAGCAACTACGCCTCATTCG | 176 | 60 | 98.9 | [17] |
| R: GCCTCTGCAAGCCTGGATGAAG | |||||
| Caspase 9 | F: TGCGGAGGAGGTGAACGAGAC | 138 | 60 | 90.5 | [17] |
| R: CGGTTCGTCGGACATGCTCAG | |||||
| Bax | F: GCTCCAAAGGATGATAAACGAC | 182 | 60 | 96.7 | [20] |
| R: AACAGTGCAACCACCCGAC | |||||
| Bcl2 | F: GTGGGGCTCTTCGCTTTTG | 191 | 60 | 95.1 | [21] |
| R: CCATCCTCCTTGGCTCTGGA | |||||
| Time | Groups | HPV | HSD | PN | K | CI |
|---|---|---|---|---|---|---|
| 0 h | CK | − | − | − | − | − |
| 48 h | CK | + | + | + | + | + |
| LN | ++ | ++ | + | + | + | |
| MN | +++ | ++ | + | + | + | |
| HN | +++ | +++ | ++ | ++ | ++ | |
| R24 h | CK | + | + | + | − | − |
| LN | + | + | + | − | − | |
| MN | ++ | + | + | + | + | |
| HN | ++ | ++ | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Yan, Q.; Dong, Y.; Ding, Z.; Mei, J.; Xie, J. Apoptotic Changes, Oxidative Stress and Immunomodulatory Effects in the Liver of Japanese Seabass (Lateolabrax japonicus) Induced by Ammonia-Nitrogen Stress during Keep-Live Transport. Biology 2023, 12, 769. https://doi.org/10.3390/biology12060769
Guo M, Yan Q, Dong Y, Ding Z, Mei J, Xie J. Apoptotic Changes, Oxidative Stress and Immunomodulatory Effects in the Liver of Japanese Seabass (Lateolabrax japonicus) Induced by Ammonia-Nitrogen Stress during Keep-Live Transport. Biology. 2023; 12(6):769. https://doi.org/10.3390/biology12060769
Chicago/Turabian StyleGuo, Meijie, Qi Yan, Yixuan Dong, Zhaoyang Ding, Jun Mei, and Jing Xie. 2023. "Apoptotic Changes, Oxidative Stress and Immunomodulatory Effects in the Liver of Japanese Seabass (Lateolabrax japonicus) Induced by Ammonia-Nitrogen Stress during Keep-Live Transport" Biology 12, no. 6: 769. https://doi.org/10.3390/biology12060769
APA StyleGuo, M., Yan, Q., Dong, Y., Ding, Z., Mei, J., & Xie, J. (2023). Apoptotic Changes, Oxidative Stress and Immunomodulatory Effects in the Liver of Japanese Seabass (Lateolabrax japonicus) Induced by Ammonia-Nitrogen Stress during Keep-Live Transport. Biology, 12(6), 769. https://doi.org/10.3390/biology12060769









