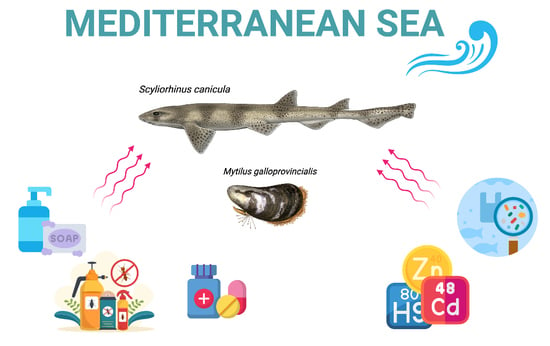
Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Bivalve Molluscs and Elasmobranchs as Suitable Indicators of Emerging Pollutants
3.1. Bivalve Molluscs
3.2. Elasmobranchs
4. An Overview of Emerging Contaminants Toxicity
4.1. Microplastics
4.2. Personal Care Products
4.3. Pharmaceuticals
4.4. Pesticides
4.5. Heavy Metals
5. Research Gaps and Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zicarelli, G.; Multisanti, C.R.; Falco, F.; Faggio, C. Evaluation of Toxicity of Personal Care Products (PCPs) in Freshwaters: Zebrafish as a Model. Environ. Toxicol. Pharmacol. 2022, 94, 103923. [Google Scholar] [CrossRef] [PubMed]
- Monique, M.; Giuseppe, P.; Francesca, F.; Davide, D.P.; Savoca, S.; Gioele, C.; Teresa, R.; Giovanni, P.; Eleonora, G.; Nunziacarla, S.; et al. Investigating the Effects of Microplastic Ingestion in Scyliorhinus canicula from the South of Sicily. Sci. Total Environ. 2022, 850, 157875. [Google Scholar] [CrossRef]
- Spada, L.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; DI Leo, A. Heavy Metals Monitoring in Mussels Mytilus galloprovincialis from the Apulian Coasts (Southern Italy). Mediterr. Mar. Sci. 2013, 14, 99. [Google Scholar] [CrossRef]
- Boldrocchi, G.; Spanu, D.; Polesello, S.; Valsecchi, S.; Garibaldi, F.; Lanteri, L.; Ferrario, C.; Monticelli, D.; Bettinetti, R. Legacy and Emerging Contaminants in the Endangered Filter Feeder Basking Shark Cetorhinus maximus. Mar. Pollut. Bull. 2022, 176, 113466. [Google Scholar] [CrossRef] [PubMed]
- Brumovský, M.; Bečanová, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of Emerging Concern in the Open Sea Waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef]
- López-Serna, R.; Postigo, C.; Blanco, J.; Pérez, S.; Ginebreda, A.; de Alda, M.L.; Petrović, M.; Munné, A.; Barceló, D. Assessing the Effects of Tertiary Treated Wastewater Reuse on the Presence Emerging Contaminants in a Mediterranean River (Llobregat, NE Spain). Environ. Sci. Pollut. Res. 2012, 19, 1000–1012. [Google Scholar] [CrossRef]
- Munschy, C.; Marchand, P.; Venisseau, A.; Veyrand, B.; Zendong, Z. Levels and Trends of the Emerging Contaminants HBCDs (Hexabromocyclododecanes) and PFCs (Perfluorinated Compounds) in Marine Shellfish along French Coasts. Chemosphere 2013, 91, 233–240. [Google Scholar] [CrossRef]
- Dachs, J.; Méjanelle, L. Organic Pollutants in Coastal Waters, Sediments, and Biota: A Relevant Driver for Ecosystems during the Anthropocene? Estuaries Coasts 2010, 33, 1–14. [Google Scholar] [CrossRef]
- Impellitteri, F.; Curpăn, A.-S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public Health 2022, 19, 16830. [Google Scholar] [CrossRef]
- Pagano, M.; Fabrello, J.; Multisanti, C.R.; Zicarelli, G.; Ciscato, M.; Boldrin, F.; Giacobbe, S.; Matozzo, V.; Faggio, C. A First Insight into Haemocytes of Pinctada imbricata radiata: A Morpho-functional Characterization. Microsc. Res. Tech. 2023, 86, 368–377. [Google Scholar] [CrossRef]
- Pagano, M.; Savoca, S.; Impellitteri, F.; Albano, M.; Capillo, G.; Faggio, C. Toxicological Evaluation of Acetylsalicylic Acid in Non-Target Organisms: Chronic Exposure on Mytilus galloprovincialis (Lamarck, 1819). Front. Physiol. 2022, 13, 1165. [Google Scholar] [CrossRef]
- Wehner, F.; Olsen, H.; Tinel, H.; Kinne-Saffran, E.; Kinne, R.K.H. Cell Volume Regulation: Osmolytes, Osmolyte Transport, and Signal Transduction. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–80. [Google Scholar]
- Tresnakova, N.; Famulari, S.; Zicarelli, G.; Impellitteri, F.; Pagano, M.; Presti, G.; Filice, M.; Caferro, A.; Gulotta, E.; Salvatore, G.; et al. Multi-Characteristic Toxicity of Enantioselective Chiral Fungicide Tebuconazole to a Model Organism Mediterranean Mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalve: Mytilidae). Sci. Total Environ. 2023, 862, 160874. [Google Scholar] [CrossRef]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Hollerova, A.; Svobodova, Z.; Faldyna, M.; Faggio, C. Effects of Plastic Particles on Aquatic Invertebrates and Fish—A Review. Environ. Toxicol. Pharmacol. 2022, 96, 104013. [Google Scholar] [CrossRef]
- Piazzese, D.; Bonanno, A.; Bongiorno, D.; Falco, F.; Indelicato, S.; Milisenda, G.; Vazzana, I.; Cammarata, M. Co-Inertia Multivariate Approach for the Evaluation of Anthropogenic Impact on Two Commercial Fish along Tyrrhenian Coasts. Ecotoxicol. Environ. Saf. 2019, 182, 109435. [Google Scholar] [CrossRef]
- Ravi, R.; Athisuyambulingam, M.; Kanagaraj, S.; Tresnakova, N.; Impellitteri, F.; Viswambaran, G.; Faggio, C. Impact of Chlorpyrifos on Cytopathological Indices in Mangrove Crab, Episesarma tetragonum (Fabricius). Vet. Sci. 2023, 10, 53. [Google Scholar] [CrossRef]
- Moreira, S.M.; Guilhermino, L. The Use of Mytilus galloprovincialis Acetylcholinesterase and Glutathione S-Transferases Activities as Biomarkers of Environmental Contamination along the Northwest Portuguese Coast. Environ. Monit. Assess. 2005, 105, 309–325. [Google Scholar] [CrossRef]
- Lam, P.K.S. Use of Biomarkers in Environmental Monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- González-Fernández, C.; Albentosa, M.; Campillo, J.A.; Viñas, L.; Romero, D.; Franco, A.; Bellas, J. Effect of Nutritive Status on Mytilus galloprovincialis Pollution Biomarkers: Implications for Large-Scale Monitoring Programs. Aquat. Toxicol. 2015, 167, 90–105. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue Mussels (Mytilus edulis Spp.) as Sentinel Organisms in Coastal Pollution Monitoring: A Review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Merola, C.; Perugini, M.; Aliko, V.; Faggio, C. Sentinel Species Selection for Monitoring Microplastic Pollution: A Review on One Health Approach. Ecol. Indic. 2022, 145, 109587. [Google Scholar] [CrossRef]
- Curpan, A.-S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Review: Mytilus galloprovincialis: An Essential, Low-Cost Model Organism for the Impact of Xenobiotics on Oxidative Stress and Public Health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ruiz, R.; Picó, Y.; Campo, J. Bioaccumulation of Emerging Contaminants in Mussel (Mytilus galloprovincialis): Influence of Microplastics. Sci. Total Environ. 2021, 796, 149006. [Google Scholar] [CrossRef] [PubMed]
- Tiktak, G.P.; Butcher, D.; Lawrence, P.J.; Norrey, J.; Bradley, L.; Shaw, K.; Preziosi, R.; Megson, D. Are Concentrations of Pollutants in Sharks, Rays and Skates (Elasmobranchii) a Cause for Concern? A Systematic Review. Mar. Pollut. Bull. 2020, 160, 111701. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Falco, F.; Bono, G.; Cammarata, M.; Cavalca, J.; Vazzana, I.; Dara, M.; Scannella, D.; Guicciardi, S.; Faggio, C.; Ragonese, S. Stress Related Blood Values in Scyliorhinus canicula as Live-Indicators of Physiological Status after Bottom Trawling Capture Activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023, 263, 110802. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Sánchez, F.; Fernández, A.; Olaso, I. Is the Lesser Spotted Dogfish (Scyliorhinus canicula) Population from the Cantabrian Sea a Unique Stock? Fish. Res. 2004, 69, 57–71. [Google Scholar] [CrossRef]
- Esposito, G.; Prearo, M.; Renzi, M.; Anselmi, S.; Cesarani, A.; Barcelò, D.; Dondo, A.; Pastorino, P. Occurrence of Microplastics in the Gastrointestinal Tract of Benthic by–Catches from an Eastern Mediterranean Deep–Sea Environment. Mar. Pollut. Bull. 2022, 174, 113231. [Google Scholar] [CrossRef]
- Barbieri, M.; Maltagliati, F.; Roldán, M.I.; Castelli, A. Molecular Contribution to Stock Identification in the Small-Spotted Catshark, Scyliorhinus canicula (Chondrichthyes, Scyliorhinidae). Fish. Res. 2014, 154, 11–16. [Google Scholar] [CrossRef]
- Kousteni, V.; Karachle, P.K.; Megalofonou, P. Diet of the Small-Spotted Catshark Scyliorhinus canicula in the Aegean Sea (Eastern Mediterranean). Mar. Biol. Res. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Wosnick, N.; Niella, Y.; Hammerschlag, N.; Chaves, A.P.; Hauser-Davis, R.A.; da Rocha, R.C.C.; Jorge, M.B.; de Oliveira, R.W.S.; Nunes, J.L.S. Negative Metal Bioaccumulation Impacts on Systemic Shark Health and Homeostatic Balance. Mar. Pollut. Bull. 2021, 168, 112398. [Google Scholar] [CrossRef]
- Smith, L.E. Plastic Ingestion by Scyliorhinus canicula Trawl Captured in the North Sea. Mar. Pollut. Bull. 2018, 130, 6–7. [Google Scholar] [CrossRef]
- Muñoz-Baquero, M.; Marco-Jiménez, F.; García-Domínguez, X.; Ros-Santaella, J.L.; Pintus, E.; Jiménez-Movilla, M.; García-Párraga, D.; García-Vazquez, F.A. Comparative Study of Semen Parameters and Hormone Profile in Small-Spotted Catshark (Scyliorhinus canicula): Aquarium-Housed vs. Wild-Captured. Animals 2021, 11, 2884. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Santamaria, N.; Marcotrigiano, G.O. Residue Levels of DDTs and Toxic Evaluation of Polychlorinated Biphenyls (PCBs) in Scyliorhinus canicula Liver from the Mediterranean Sea (Italy). Mar. Pollut. Bull. 2006, 52, 696–700. [Google Scholar] [CrossRef]
- Avio, C.G.; Pittura, L.; d’Errico, G.; Abel, S.; Amorello, S.; Marino, G.; Gorbi, S.; Regoli, F. Distribution and Characterization of Microplastic Particles and Textile Microfibers in Adriatic Food Webs: General Insights for Biomonitoring Strategies. Environ. Pollut. 2020, 258, 113766. [Google Scholar] [CrossRef]
- Baldwin, W.S.; Bain, L.J.; Di Giulio, R.; Kullman, S.; Rice, C.D.; Ringwood, A.H.; van den Hurk, P. 20th Pollutant Responses in Marine Organisms (PRIMO 20): Global Issues and Fundamental Mechanisms Caused by Pollutant Stress in Marine and Freshwater Organisms. Aquat. Toxicol. 2020, 227, 105620. [Google Scholar] [CrossRef]
- Lipej, L.; Cumani, F.; Acquavita, A.; Bettoso, N. Plastic Impact on Sharks and Rays. In Plastic Pollution and Marine Conservation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 153–185. [Google Scholar]
- Aiguo, Z.; Di, S.; Chong, W.; Yuliang, C.; Shaolin, X.; Peiqin, L.; Guohuan, X.; Huijuan, T.; Jixing, Z. Characteristics and Differences of Microplastics Ingestion for Farmed Fish with Different Water Depths, Feeding Habits and Diets. J. Environ. Chem. Eng. 2022, 10, 107189. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in Cosmetics: Environmental Issues and Needs for Global Bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of Emerging Concern: A Review of New Approach in AOP Technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of Selected Tricyclic Antidepressants on Early-Life Stages of Common Carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Chromcova, L.; Blahova, J.; Zivna, D.; Plhalova, L.; Casuscelli, F.; Tocco, D.; Divisova, L.; Prokes, M.; Faggio, C.; Tichy, F.; et al. NeemAzal T/S—Toxicity to Early-Life Stages of Common Carp (Cyprinus carpio L.). Vet. Med. 2015, 60, 23–30. [Google Scholar] [CrossRef]
- Cappello, T.; De Marco, G.; Oliveri Conti, G.; Giannetto, A.; Ferrante, M.; Mauceri, A.; Maisano, M. Time-Dependent Metabolic Disorders Induced by Short-Term Exposure to Polystyrene Microplastics in the Mediterranean Mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2021, 209, 111780. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and Transcriptional Effects of Polystyrene Microplastics in Larval Stages of the Mediterranean Mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1047. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R. Microplastic Pollution Profile of Mediterranean Mussels (Mytilus galloprovincialis) Collected along the Turkish Coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Valente, T.; Sbrana, A.; Scacco, U.; Jacomini, C.; Bianchi, J.; Palazzo, L.; de Lucia, G.A.; Silvestri, C.; Matiddi, M. Exploring Microplastic Ingestion by Three Deep-Water Elasmobranch Species: A Case Study from the Tyrrhenian Sea. Environ. Pollut. 2019, 253, 342–350. [Google Scholar] [CrossRef]
- López-López, L.; Preciado, I.; González-Irusta, J.M.; Arroyo, N.L.; Muñoz, I.; Punzón, A.; Serrano, A. Incidental Ingestion of Meso- and Macro-Plastic Debris by Benthic and Demersal Fish. Food Webs 2018, 14, 1–4. [Google Scholar] [CrossRef]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of Microplastics by Demersal Fish from the Spanish Atlantic and Mediterranean Coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S.; Compa, M.; Guijarro, B. Exploring the Relation between Plastic Ingestion in Species and Its Presence in Seafloor Bottoms. Mar. Pollut. Bull. 2020, 160, 111641. [Google Scholar] [CrossRef]
- Madgett, A.S.; Yates, K.; Webster, L.; McKenzie, C.; Brownlow, A.; Moffat, C.F. The Concentration and Biomagnification of PCBs and PBDEs across Four Trophic Levels in a Marine Food Web. Environ. Pollut. 2022, 309, 119752. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, K.; Badis, A.; Moulai-Mostefa, N. Study of Heavy Metal Bioaccumulation in Mytilus galloprovincialis (Lamark 1819) from Heavy Metal Mixture Using the CCF Design. Environ. Technol. Innov. 2022, 25, 102202. [Google Scholar] [CrossRef]
- Mille, T.; Cresson, P.; Chouvelon, T.; Bustamante, P.; Brach-Papa, C.; Bruzac, S.; Rozuel, E.; Bouchoucha, M. Trace Metal Concentrations in the Muscle of Seven Marine Species: Comparison between the Gulf of Lions (North-West Mediterranean Sea) and the Bay of Biscay (North-East Atlantic Ocean). Mar. Pollut. Bull. 2018, 135, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chouvelon, T.; Cresson, P.; Bouchoucha, M.; Brach-Papa, C.; Bustamante, P.; Crochet, S.; Marco-Miralles, F.; Thomas, B.; Knoery, J. Oligotrophy as a Major Driver of Mercury Bioaccumulation in Medium-to High-Trophic Level Consumers: A Marine Ecosystem-Comparative Study. Environ. Pollut. 2018, 233, 844–854. [Google Scholar] [CrossRef]
- Marques, A.F.S.; Alves, L.M.F.; Moutinho, A.; Lemos, M.F.L.; Novais, S.C. Scyliorhinus canicula (Linnaeus, 1758) Metal Accumulation: A Public Health Concern for Atlantic Fish Consumers? Mar. Pollut. Bull. 2021, 169, 112477. [Google Scholar] [CrossRef]
- Antelo, L.T.; Ordóñez-del Pazo, T.; Lopes, C.; Franco-Uría, A.; Pérez-Martín, R.I.; Alonso, A.A. Pollutant Levels in Discarded Fish Species by Spanish Trawlers Operating in the Great Sole Bank and the Atlantic Coast of the Iberian Peninsula. Mar. Pollut. Bull. 2016, 108, 303–310. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Chekri, R.; Leufroy, A.; Jitaru, P.; Millour, S.; Marchond, N.; Chafey, C.; Testu, C.; Zinck, J.; Cresson, P.; et al. Trace Element Contamination in Fish Impacted by Bauxite Red Mud Disposal in the Cassidaigne Canyon (NW French Mediterranean). Sci. Total Environ. 2019, 690, 16–26. [Google Scholar] [CrossRef]
- Veron, A.; Dell’Anno, A.; Angelidis, M.O.; Aloupi, M.; Danovaro, R.; Radakovitch, O.; Poirier, A.; Heussner, S. Pollutant Pb Burden in Mediterranean Centroscymnus Coelolepis Deep-Sea Sharks. Mar. Pollut. Bull. 2022, 174, 113245. [Google Scholar] [CrossRef]
- Ibor, O.R.; Andem, A.B.; Eni, G.; Arong, G.A.; Adeougn, A.O.; Arukwe, A. Contaminant Levels and Endocrine Disruptive Effects in Clarias Gariepinus Exposed to Simulated Leachate from a Solid Waste Dumpsite in Calabar, Nigeria. Aquat. Toxicol. 2020, 219, 105375. [Google Scholar] [CrossRef]
- Aliko, V.; Multisanti, C.R.; Turani, B.; Faggio, C. Get Rid of Marine Pollution: Bioremediation an Innovative, Attractive, and Successful Cleaning Strategy. Sustainability 2022, 14, 11784. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T.; Guerranti, C.; Scopetani, C.; Chelazzi, D.; Giarrizzo, T. A Potpourri of Microplastics in the Sea Surface and Water Column of the Mediterranean Sea. Trends Anal. Chem. 2019, 110, 321–326. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and Its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Filella, M. Questions of Size and Numbers in Environmental Research on Microplastics: Methodological and Conceptual Aspects. Environ. Chem. 2015, 12, 527. [Google Scholar] [CrossRef]
- Llorca, M.; Álvarez-Muñoz, D.; Ábalos, M.; Rodríguez-Mozaz, S.; Santos, L.H.M.L.M.; León, V.M.; Campillo, J.A.; Martínez-Gómez, C.; Abad, E.; Farré, M. Microplastics in Mediterranean Coastal Area: Toxicity and Impact for the Environment and Human Health. Trends Environ. Anal. Chem. 2020, 27, e00090. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of Microplastic Size on the Adsorption Behavior and Mechanism of Triclosan on Polyvinyl Chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and Biomagnification of Microplastics in Marine Organisms: A Review and Meta-Analysis of Current Data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Bråte, I.L.N.; Blázquez, M.; Brooks, S.J.; Thomas, K.V. Weathering Impacts the Uptake of Polyethylene Microparticles from Toothpaste in Mediterranean Mussels (M. galloprovincialis). Sci. Total Environ. 2018, 626, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics Alter Digestive Enzyme Activities in the Marine Bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef] [PubMed]
- Picot-Groz, M.; Fenet, H.; Martinez Bueno, M.J.; Rosain, D.; Gomez, E. Diurnal Variations in Personal Care Products in Seawater and Mussels at Three Mediterranean Coastal Sites. Environ. Sci. Pollut. Res. 2018, 25, 9051–9059. [Google Scholar] [CrossRef] [PubMed]
- Montes-Grajales, D.; Fennix-Agudelo, M.; Miranda-Castro, W. Occurrence of Personal Care Products as Emerging Chemicals of Concern in Water Resources: A Review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef]
- Sadutto, D.; Andreu, V.; Ilo, T.; Akkanen, J.; Picó, Y. Pharmaceuticals and Personal Care Products in a Mediterranean Coastal Wetland: Impact of Anthropogenic and Spatial Factors and Environmental Risk Assessment. Environ. Pollut. 2021, 271, 116353. [Google Scholar] [CrossRef]
- Pinheiro, M.; Martins, I.; Raimundo, J.; Caetano, M.; Neuparth, T.; Santos, M.M. Stressors of Emerging Concern in Deep-Sea Environments: Microplastics, Pharmaceuticals, Personal Care Products and Deep-Sea Mining. Sci. Total Environ. 2023, 876, 162557. [Google Scholar] [CrossRef]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and Spatial Distribution of Pharmaceuticals and Personal Care Products (PPCPs) in the Aquatic Environment, Their Characteristics, and Adopted Legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A Review of Personal Care Products in the Aquatic Environment: Environmental Concentrations and Toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Fisher, M.; MacPherson, S.; Braun, J.M.; Hauser, R.; Walker, M.; Feeley, M.; Mallick, R.; Bérubé, R.; Arbuckle, T.E. Paraben Concentrations in Maternal Urine and Breast Milk and Its Association with Personal Care Product Use. Environ. Sci. Technol. 2017, 51, 4009–4017. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Costa, S.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Toxic Impacts Induced by Sodium Lauryl Sulfate in Mytilus galloprovincialis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 242, 110656. [Google Scholar] [CrossRef]
- Yeltekin, A.Ç.; Oğuz, A.R. Toxic Effects of Sodium Lauryl Sulfate on Antioxidant Defense System and DNA Damage in Fish Primary Hepatocyte Cultures. Maced. Vet Rev. 2022, 45, 169–175. [Google Scholar] [CrossRef]
- Walker, C.H.; Sibly, R.M.; Sibly, R.M.; Peakall, D.B. Principles of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429109515. [Google Scholar]
- Martins, M.F.; Costa, P.G.; Bianchini, A. Maternal Transfer of Pharmaceuticals and Personal Care Products in the Brazilian Guitarfish Pseudobatos horkelii. Environ. Adv. 2022, 8, 100228. [Google Scholar] [CrossRef]
- Martins, M.F.; Costa, P.G.; Bianchini, A. Maternal Transfer of Polycyclic Aromatic Hydrocarbons in an Endangered Elasmobranch, the Brazilian Guitarfish. Chemosphere 2021, 263, 128275. [Google Scholar] [CrossRef]
- Martins, M.F.; Costa, P.G.; da Guerreiro, A.S.; Bianchini, A. Consequences of Prenatal Exposure to Contaminants in Elasmobranchs: Biochemical Outcomes during the Embryonic Development of Pseudobatos horkelii. Environ. Pollut. 2023, 323, 121276. [Google Scholar] [CrossRef]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and Ecotoxicological Assessment of Pharmaceuticals: Is There a Risk for the Mediterranean Aquatic Environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef]
- Feo, M.L.; Bagnati, R.; Passoni, A.; Riva, F.; Salvagio Manta, D.; Sprovieri, M.; Traina, A.; Zuccato, E.; Castiglioni, S. Pharmaceuticals and Other Contaminants in Waters and Sediments from Augusta Bay (Southern Italy). Sci. Total Environ. 2020, 739, 139827. [Google Scholar] [CrossRef]
- Yuan, M.; Faggio, C.; Perugini, M.; Aliko, V.; Wang, Y. Editorial: Pharmaceuticals, Personal Care Products and Endocrine Disrupting Chemicals: The Physiological Consequences of Exposure to Pollutants in Aquatic Animals. Front. Physiol. 2023, 14, 122. [Google Scholar] [CrossRef]
- Gelsleichter, J.; Szabo, N.J. Uptake of Human Pharmaceuticals in Bull Sharks (Carcharhinus leucas) Inhabiting a Wastewater-Impacted River. Sci. Total Environ. 2013, 456–457, 196–201. [Google Scholar] [CrossRef]
- Corsolini, S.; Guerranti, C.; Perra, G.; Focardi, S. Polybrominated Diphenyl Ethers, Perfluorinated Compounds and Chlorinated Pesticides in Swordfish (Xiphias gladius) from the Mediterranean Sea. Environ. Sci. Technol. 2008, 42, 4344–4349. [Google Scholar] [CrossRef]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological Risk and Estimates of Organophosphate Pesticides Loads into the Central Mediterranean Sea from Volturno River, the River of the “Land of Fires” Area, Southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef]
- Comoretto, L.; Chiron, S. Comparing Pharmaceutical and Pesticide Loads into a Small Mediterranean River. Sci. Total Environ. 2005, 349, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Stara, A.; Kubec, J.; Zuskova, E.; Buric, M.; Faggio, C.; Kouba, A.; Velisek, J. Effects of S-Metolachlor and Its Degradation Product Metolachlor OA on Marbled Crayfish (Procambarus virginalis). Chemosphere 2019, 224, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F. Insecticides Mode of Action in Relation to Their Toxicity to Non-Target Organisms. J. Environ. Anal. Toxicol. 2012, s4-002. [Google Scholar] [CrossRef]
- Bado-Nilles, A.; Gagnaire, B.; Thomas-Guyon, H.; Le Floch, S.; Renault, T. Effects of 16 Pure Hydrocarbons and Two Oils on Haemocyte and Haemolymphatic Parameters in the Pacific Oyster, Crassostrea gigas (Thunberg). Toxicol. Vitr. 2008, 22, 1610–1617. [Google Scholar] [CrossRef]
- Matozzo, V.; Zampieri, C.; Munari, M.; Marin, M.G. Glyphosate Affects Haemocyte Parameters in the Clam Ruditapes philippinarum. Mar. Environ. Res. 2019, 146, 66–70. [Google Scholar] [CrossRef]
- Del Giudice, G.; Prisco, M.; Agnese, M.; Verderame, M.; Rosati, L.; Limatola, E.; Andreuccetti, P. Effects of Nonylphenol on Vitellogenin Synthesis in Adult Males of the Spotted Ray Torpedo Marmorata. J. Fish. Biol. 2012, 80, 2112–2121. [Google Scholar] [CrossRef]
- Marsili, L.; Coppola, D.; Giannetti, M.; Casini, S.; Fossi, M.C.; Van Wyk, J.H.; Sperone, E.; Tripepi, S.; Micarelli, P.; Rizzuto, S. Skin Biopsies as a Sensitive Non-Lethal Technique for the Ecotoxicological Studies of Great White Shark (Carcharodon carcharias) Sampled in South Africa. Expert Opin. Environ. Biol. 2016, 4, 2. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Bioindicator Organisms: Heavy Metal Pollution Evaluation in the Ionian Sea (Mediterranean Sea—Italy). Environ. Monit. Assess. 2005, 102, 159–166. [Google Scholar] [CrossRef]
- Salvo, A.; Potortì, A.G.; Cicero, N.; Bruno, M.; Lo Turco, V.; Di Bella, G.; Dugo, G. Statistical Characterisation of Heavy Metal Contents in Paracentrotus lividus from Mediterranean Sea. Nat. Prod. Res. 2014, 28, 718–726. [Google Scholar] [CrossRef]
- Kemp, M.; Wepener, V.; de Kock, K.N.; Wolmarans, C.T. Metallothionein Induction as Indicator of Low Level Metal Exposure to Aquatic Macroinvertebrates from a Relatively Unimpacted River System in South Africa. Bull. Environ. Contam. Toxicol. 2017, 99, 662–667. [Google Scholar] [CrossRef]
- Riani, E.; Cordova, M.R.; Arifin, Z. Heavy Metal Pollution and Its Relation to the Malformation of Green Mussels Cultured in Muara Kamal Waters, Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2018, 133, 664–670. [Google Scholar] [CrossRef]
- Kwon, Y.-K.; Jung, Y.-S.; Park, J.-C.; Seo, J.; Choi, M.-S.; Hwang, G.-S. Characterizing the Effect of Heavy Metal Contamination on Marine Mussels Using Metabolomics. Mar. Pollut. Bull. 2012, 64, 1874–1879. [Google Scholar] [CrossRef]
- Vlahogianni, T.H.; Valavanidis, A. Heavy-Metal Effects on Lipid Peroxidation and Antioxidant Defence Enzymes in Mussels Mytilus galloprovincialis. Chem. Ecol. 2007, 23, 361–371. [Google Scholar] [CrossRef]
- Bonsignore, M.; Salvagio Manta, D.; Oliveri, E.; Sprovieri, M.; Basilone, G.; Bonanno, A.; Falco, F.; Traina, A.; Mazzola, S. Mercury in Fishes from Augusta Bay (Southern Italy): Risk Assessment and Health Implication. Food Chem. Toxicol. 2013, 56, 184–194. [Google Scholar] [CrossRef]
- Reinero, F.R.; Milazzo, C.; Minervino, M.; Marchio, C.; Filice, M.; Bevacqua, L.; Giglio, G.; Leonetti, F.L.; Micarelli, P.; Tripepi, S.; et al. Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology 2022, 11, 663. [Google Scholar] [CrossRef]
- Humbert, J.-F.; Dorigo, U. Biodiversity and Aquatic Ecosystem Functioning: A Mini-Review. Aquat. Ecosyst. Health Manag. 2005, 8, 367–374. [Google Scholar] [CrossRef]
| Type of Pollutant | Impact on Aquatic Organisms | References |
|---|---|---|
| DDTs and toxic evaluation of polychlorinated biphenyls (PCBs) | Accumulation profile analysis of individual PCB congeners and the levels of DDT and its metabolites in the liver of S. canicula, from the Mediterranean Sea. | Storelli et al. [35] |
| Microplastic particles and textile microfibers | Occurrence of MPs in Adriatic food webs in different species (several fish and invertebrates including M. galloprovincialis) | Avio et al. [36] |
| MPs | MPs influence on marine organisms | Baldwin et al. [37] |
| MPs | Plastic impact on sharks and rays. | Lipej et al. [38] |
| MPs | Analysis of the different influence of water depth, feeding habits and diets on microplastics accumulation. | Aiguo et al. [39] |
| MPs and NPs | Toxic effects and bioaccumulation on several aquatic species identified as suitable bioindicators of microplastic pollution. | Multisanti et al. [22] |
| MPs in cosmetics | Statistical data on MPs in cosmetics. | Guerranti et al. [40] |
| Pharmaceuticals and Personal care products | Deep explanation about different chemical compositions: pharmaceuticals and personal care products. | Salimi et al. [41] |
| Pharmaceuticals | Analysis on the impact of tricyclic antidepressants on non-target organisms showed hearth, brain, and cranial and caudal kidney damage; oxidative damage of lipids and also a significant increase in mortality. | Sehonova et al. [42] |
| Pesticide (fungicide) | Study of the potential risks of a fungicide in the model organism M. galloprovincialis, mainly through its bioaccumulation, and the alteration of fundamental physiological processes. | Tresnakova et al. [13] |
| Pesticide (insecticide) | Evaluation of toxicity due to NeemAzal T/S exposure on early-life stages of common carp (Cyprinus carpio L.) showed gills histophatological changes linked to a significant increase in glutathione oxidase, glutathione S-transferase activity and also an increase in oxidised lipids. Moreover, the exposure also induced slow hatching and an increase in mortality. | Chromcova et al. [43] |
| Geographical Location | Kind of Pollutant | Species | Influence | References |
|---|---|---|---|---|
| Strait of Messina, central Mediterranean Sea | Polystyrene microplastics | Mytilus galloprovincialis | Insights into early mechanisms of toxicity of polystyrene MPs in mussels. Disorders in osmoregulation, energy and protein metabolism, and oxidative stress were detected. | Cappello et al. [44] |
| North Adriatic Sea | 3 μm polystyrene microplastics | Mytilus galloprovincialis (larvae) | Gene expression of genes involved in growth and adaptation mechanisms, due to exposure to polystyrene MPs, was altered. | Capolupo et al. [45] |
| Black Sea, Aegean, and the Marmara Sea | Microplastic particles | Mytilus galloprovincialis | Occurrence of MPs in all the samples from each geographic area analysed. | Gedik and Eryaşar [46] |
| Southern region of the central Mediterranean Sea. | Plastics injection | Scyliorhinus canicula | The presence of plastics, especially macroplastics in the gastrointestinal (GI) tract, was correlated with an increase in the hepatosomatic index and an increased expression of 3 essential immune system genes. It is hypothesized that these effects are induced by additives that are leaching from the ingested plastics. Moreover, plastic particles also act as endocrine disruptors. | Mancia et al. [47] |
| Portuguese coast | Plastic injection/ particles and fibers | Scyliorhinus canicula | Depending on the size of the animal, ingested plastic fragments can be small enough to be expelled from the organism through faeces, but larger fragments may be retained in GI tract, causing a false sense of satiety. This paper showed that pelagic species ingest more particles, whereas benthic species ingest more fibres (in relationship to the presence of high quantities of fibres on the seabed). | Neves et al. [48] |
| Tyrrhenian Sea | Plastic injection | Scyliorhinus canicula | MPs occurrence in sharks’ GI tract. The main part of particles detected were dark-colored fibers (blue or black) in the size range of 100 μm–330 μm and in high percentages recorded in all three examined species, in high percentages. | Valente et al. [49] |
| Bay Biskay | Plastic injection | Scyliorhinus canicula | High percentage of MPs occurrence. | López-López et al. [50] |
| Spanish Atlantic and Mediterranean coasts | Plastic injection | Scyliorhinus canicula | 17% of sharks analysed were found to contain MPs in their stomach. | Bellas et al. [51] |
| The west coast of Mallorca and in the Mallorca Channel | Plastic injection | Scyliorhinus canicula | This paper demonstrated that increasing plastic pollution is related to the increase in water depth, which possibly indicates that plastic pollution is more dependent on depth than spatial coverage. | Alomar et al. [52] |
| Scotland | Concentration and biomagnification of PCBs and PBDEs | Scyliorhinus canicula | All marine mammals, demersal, and pelagic fish had detectable PCBs in their tissues. | Madgett et al. [53] |
| Tipaza, Algeria | Mixture of Cd, Zn, Cu | Mytilus galloprovincialis | Short-term sublethal effects of cadmium (Cd), zinc (Zn), and copper (Cu), carried out via the bioaccumulation. The results revealed a high mortality in mussels exposed to the lowest concentrations of Cu. However, Cd and Zn exposure did not induce a high mortality. | Boudjema et al. [54] |
| NW Mediterranean Sea & N.E Atlantic Ocean | Metals | Scyliorhinus canicula | The shark S. canicula had the highest Zn concentrations, and this aspect has a connection because bioaccumulation capacity of Zn from seawater was particularly pronounced in this species. Thus, the peculiar metabolism of S. canicula regarding Zn may explain the high values measured excluding local contamination. | Mille et al. [55] |
| NW Mediterranean Sea&NE Atlantic Ocean | Metals | Scyliorhinus canicula | Analysis of Hg concentrations in sharks. Particularly, S. canicula presents the highest Hg concentrations | Chouvelon et al. [56] |
| Portugal | Metals | Scyliorhinus canicula | Atlantic lesser-spotted dogfish accumulate high levels of As, Zn, Fe, and Al, which were found to accumulate more in the skin than in sharks’ muscles. As levels in muscle reveal this fish unfit for feed production in the EU. Indeed, guideline limits for human consumption were overcome for Hg and As. Risk assessment of meHg and iAs levels indicate a potential risk for human health. | Marques et al. [57] |
| Great Sole Bank and the Atlantic coast of the Iberian Peninsula | Metals | Scyliorhinus canicula | Analysis of pollutant levels in discarded fish species by coast of the Iberian Peninsula trawlers showed Hg, Cd, and Pb concentrations in different sampled tissues. | Antelo et al. [58] |
| NW French Mediterranean | Trace elements | Scyliorhinus canicula | S. canicula presents the highest Al, As, Cd, Co, and Hg concentrations. | Bouchoucha et al. [59] |
| Mediterranean | Pollutant Pb burden | Centroscymnus coelolepis | Pb content of Mediterranean deep-sea C. coelolepis is among the lowest encountered in sharks from various habitats. Pb isotope imprints reveal that Pb is mainly from anthropogenic origin in C. coelolepis tissue | Veron et al. [60] |
| Nigeria | Pollutant from a solid waste dumpsite | Clarias gariepinus | These pollutants were found to, possibly, induce endocrine disruption. | Ibor et al. [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Impellitteri, F.; Multisanti, C.R.; Rusanova, P.; Piccione, G.; Falco, F.; Faggio, C. Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea. Biology 2023, 12, 767. https://doi.org/10.3390/biology12060767
Impellitteri F, Multisanti CR, Rusanova P, Piccione G, Falco F, Faggio C. Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea. Biology. 2023; 12(6):767. https://doi.org/10.3390/biology12060767
Chicago/Turabian StyleImpellitteri, Federica, Cristiana Roberta Multisanti, Polina Rusanova, Giuseppe Piccione, Francesca Falco, and Caterina Faggio. 2023. "Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea" Biology 12, no. 6: 767. https://doi.org/10.3390/biology12060767
APA StyleImpellitteri, F., Multisanti, C. R., Rusanova, P., Piccione, G., Falco, F., & Faggio, C. (2023). Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea. Biology, 12(6), 767. https://doi.org/10.3390/biology12060767