Generation of Herbicide-Resistant Soybean by Base Editing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector Construction and Soybean Transformation
2.2. Mutation and Transgene-Free Detection
2.3. Off-Target Detection
2.4. Herbicide Tolerance Assay
2.5. Development of Allele-Specific PCR Markers
3. Results
3.1. Targeted Base Editing of GmAHAS Genes
3.2. Identification of GmAHAS4 P180S Mutants
3.3. Herbicide Tolerance of GmAHAS4 P180S Mutants
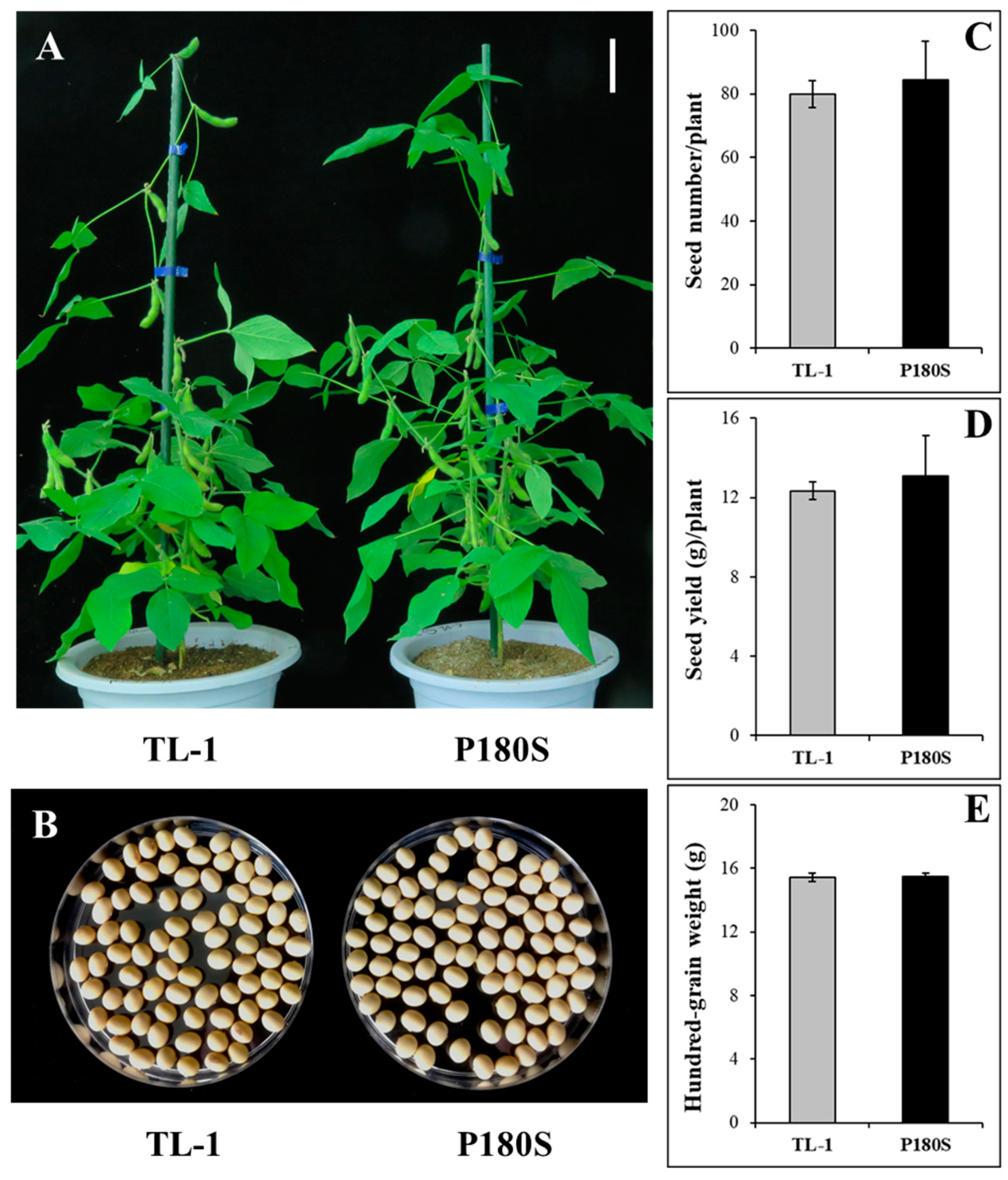
3.4. Agronomic Traits of GmAHAS4 P180S Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colbach, N.; Chauvel, B.; Darmency, H.; Délye, C.; Corre, V.L. Choosing the best cropping systems to target pleiotropic effects when managing single-gene herbicide resistance in grass weeds. A blackgrass simulation study. Pest Manag. Sci. 2016, 72, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, J.; Chai, Z.; Chen, S.; Bai, Y.; Zong, Y.; Chen, K.; Li, J.; Jiang, L.; Gao, C. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat. Plants 2019, 5, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Mackelprang, R.; Lemaux, P.G. Genetic engineering and editing of plants: An analysis of new and persisting questions. Annu. Rev. Plant Biol. 2020, 71, 659–687. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.; Li, Y.; Cheng, J.; Wu, C.; Cao, J.; Jin, S. Herbicide Resistance: Another Hot Agronomic Trait for Plant Genome Editing. Plants 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Chung, J.H.; Lee, K.J.; Kwon, J.; Kim, J.W.; Im, J.H.; Kim, D.S. Herbicide-based weed management for soybean production in the far eastern region of Russia. Agronomy 2020, 10, 1823. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.; Papastylianou, P.; Gazoulis, I.; Kakabouki, I.; Tsekoura, A. Yield, quality and weed control in soybean crop as affected by several cultural and weed management practices. Not. Bot. Horti Agrobot. 2020, 48, 329–341. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Komatsu, A.; Ohtake, M.; Shimatani, Z.; Nishida, K. Production of herbicide-sensitive strain to prevent volunteer rice infestation using a CRISPR-Cas9 cytidine deaminase fusion. Front. Plant Sci. 2021, 11, 925. [Google Scholar] [CrossRef]
- Lonhienne, T.; Low, Y.S.; Garcia, M.D.; Croll, T.; Gao, Y.; Wang, Q.; Brillault, L.; Williams, C.M.; Fraser, J.A.; McGeary, R.P.; et al. Structures of fungal and plant acetohydroxyacid synthases. Nature 2020, 586, 317–321. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Wu, H.; Liu, C.; Huang, C.; Lan, J.; Zhao, Y.; Xie, C. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. Crop. J. 2020, 8, 449–456. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, Y.; Du, L.; Zhang, X.; Liu, W.; Wang, J. Unravelling the effect of two herbicide resistance mutations on acetolactate synthase kinetics and growth traits. J. Exp. Bot. 2020, 71, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Tranel, P.J.; Wright, T.R.; Heap, I.M. Mutations in Herbicide-Resistant Weeds to ALS Inhibitors. 2023. Available online: http://www.weedscience.com (accessed on 1 June 2022).
- Ghio, C.; Ramos, M.L.; Altieri, E.; Bulos, M.; Sala, C.A. Molecular characterization of Als1, an acetohydroxyacid synthase mutation conferring resistance to sulfonylurea herbicides in soybean. Theor. Appl. Genet. 2013, 126, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2020, 18, 1384–1395. [Google Scholar] [CrossRef]
- Shi, Q.; Lu, M.; Tian, L. An efficient and specific CRISPR-Cas9 genome editing system targeting soybean phytoene desaturase genes. BMC Biotechnol. 2022, 22, 7. [Google Scholar]
- Wang, F.; Xu, Y.; Li, W.; Chen, Z.; Wang, J.; Fan, F.; Tao, Y.; Jiang, Y.; Zhu, Q.-H.; Yang, J. Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. Crop. J. 2021, 9, 305–312. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Gosavi, G.; Ren, B.; Cao, Y.; Kuang, Y.; Zhou, C.; Spetz, C.; Yan, F.; Zhou, X.; et al. Targeted base editing in rice with CRISPR/ScCas9 system. Plant Biotechnol. J. 2020, 18, 1645–1647. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Li, J. The CRISPR/Cas9 revolution continues: From base editing to prime editing in plant science. J. Genet. Genom. 2021, 48, 661–670. [Google Scholar] [CrossRef]
- Zhang, D.; Hussain, A.; Manghwar, H.; Xie, K.; Xie, S.; Zhao, S.; Larkin, R.M.; Qing, P.; Jin, S.; Ding, F. Genome editing with the CRISPR-Cas system: An art, ethicsand global regulatory perspective. Plant Biotechnol. J. 2020, 18, 1651–1669. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, Y.; Zhu, J.K.; Botella, J.R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 2021, 1, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-Editing-Mediated Artificial Evolution of OsALS1 In Planta to Develop Novel Herbicide-Tolerant Rice Germplasms. Mol. Plant 2020, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kuang, Y.; Yan, F.; Li, S.; Ren, B.; Gosavi, G.; Spetz, C.; Li, X.; Wang, X.; Zhou, X.; et al. Developing a novel artificial rice germplasm for dinitroaniline herbicide resistance by base editing of OsTubA2. Plant Biotechnol. J. 2020, 19, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol. Plant 2017, 10, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef]
- Wu, J.; Chen, C.; Xian, G.; Liu, D.; Lin, L.; Yin, S.; Sun, Q.; Fang, Y.; Zhang, H.; Wang, Y. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol. J. 2020, 18, 1857–1859. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, S.; Meng, X.; Chai, Z.; Wang, D.; Yuan, Y.; Chen, K.; Jiang, L.; Li, J.; Gao, C. Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci. China Life Sci. 2021, 64, 1624–1633. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, M.; Ding, B.; Mei, D.; Wang, W.; Wang, H.; Zhou, R.; Liu, J.; Li, C.; Hu, Q. Base editing with high efficiency in allotetraploid oilseed rape by A3A-PBE base editing system. Plant Biotechnol. J. 2020, 19, 87–97. [Google Scholar] [CrossRef]
- Michno, J.M.; Virdi, K.; Stec, A.O.; Liu, J.; Wang, X.; Xiong, Y.; Stupar, R.M. Integration, abundance, and transmission of mutations and transgenes in a series of CRISPR/Cas9 soybean lines. BMC Biotechnol. 2020, 20, 10. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Q.; Gan, Z.; Hou, Z.; Zhang, Y.; Li, Y.; Li, H.; Nan, H.; Yang, C.; Chen, L.; et al. Multiplex CRISPR/Cas9-mediatedknockoutofsoybean LNK2 advances flowering time. Crop. J. 2021, 9, 767–776. [Google Scholar] [CrossRef]
- Bai, M.; Yuan, J.; Kuang, H.; Gong, P.; Li, S.; Zhang, Z.; Liu, B.; Sun, J.; Yang, M.; Yang, L.; et al. Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol. J. 2010, 18, 721–731. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.; Jiang, L.; You, X.; Ma, P.; Xi, Z.; Wang, N.N. Generation of Herbicide-Resistant Soybean by Base Editing. Biology 2023, 12, 741. https://doi.org/10.3390/biology12050741
Wei T, Jiang L, You X, Ma P, Xi Z, Wang NN. Generation of Herbicide-Resistant Soybean by Base Editing. Biology. 2023; 12(5):741. https://doi.org/10.3390/biology12050741
Chicago/Turabian StyleWei, Tao, Linjian Jiang, Xiang You, Pengyu Ma, Zhen Xi, and Ning Ning Wang. 2023. "Generation of Herbicide-Resistant Soybean by Base Editing" Biology 12, no. 5: 741. https://doi.org/10.3390/biology12050741
APA StyleWei, T., Jiang, L., You, X., Ma, P., Xi, Z., & Wang, N. N. (2023). Generation of Herbicide-Resistant Soybean by Base Editing. Biology, 12(5), 741. https://doi.org/10.3390/biology12050741




