Gonad Ontogeny and Sex Differentiation in a Poeciliid, Gambusia holbrooki: Transition from a Bi- to a Mono-Lobed Organ
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection and Processing
2.2. Embryonic Staging and Gonad Histology
2.3. Genetic Sexing of Embryos and Neonates
2.4. Cloning and Characterisation of Key Gonadosoma Markers
2.5. RNA Extraction, Reverse Transcription and Quantitative PCR (qPCR)
2.6. qPCR Data Normalisation and Statistical Analysis
2.7. Whole-Mount In Situ Hybridisation (WM-ISH)
3. Results
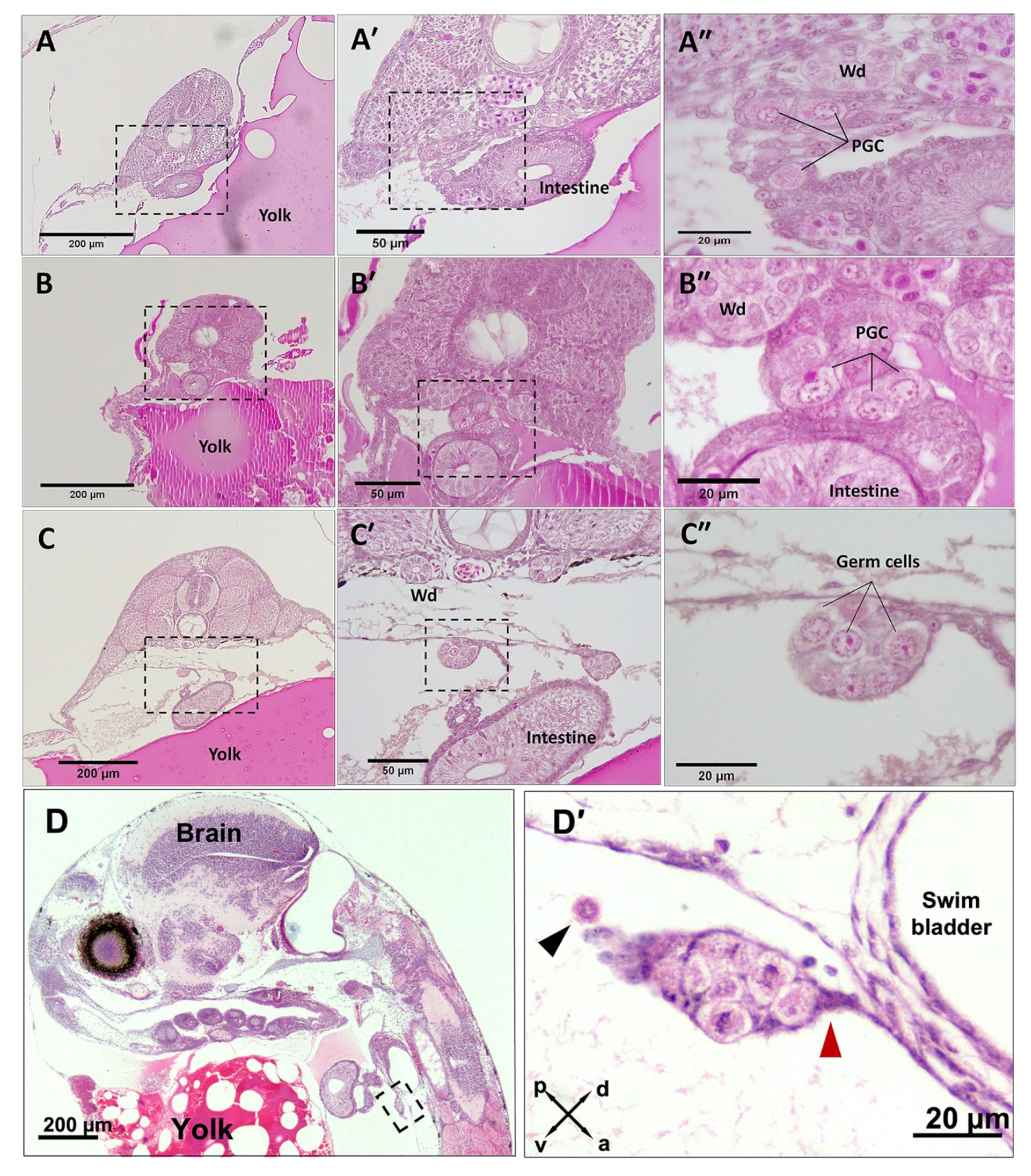
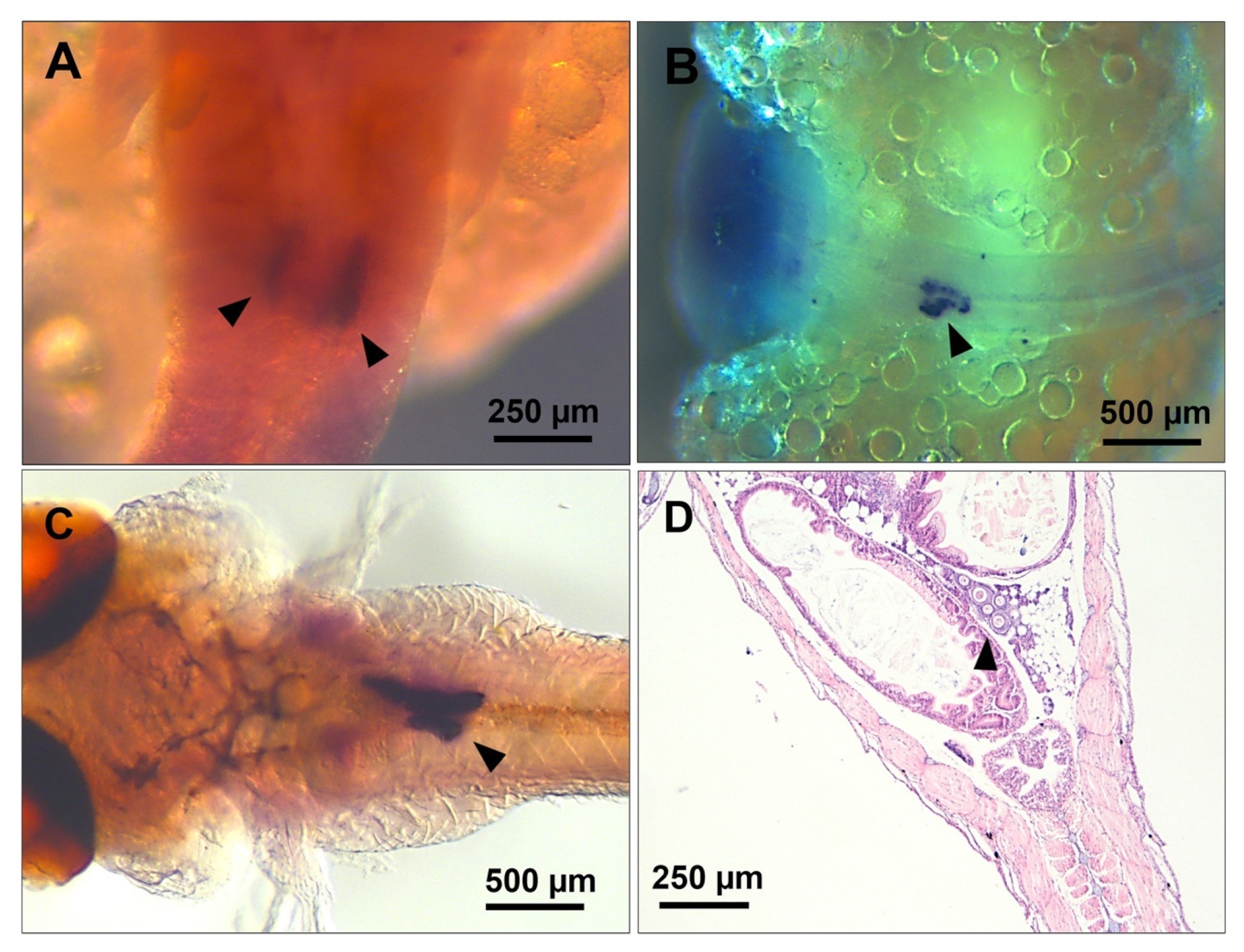
3.1. PGC Colonisation Occurs before Complete Somitogenesis
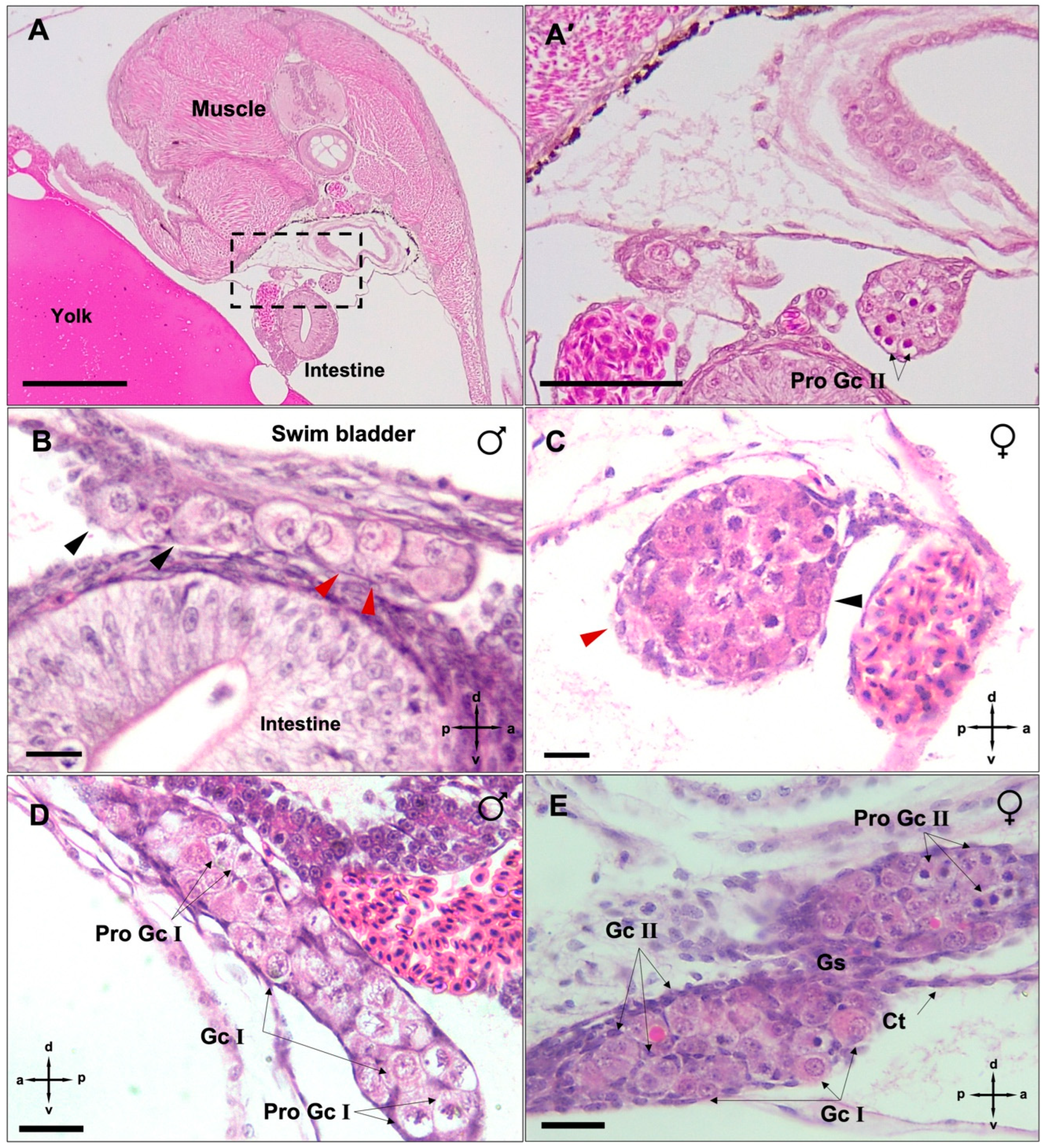
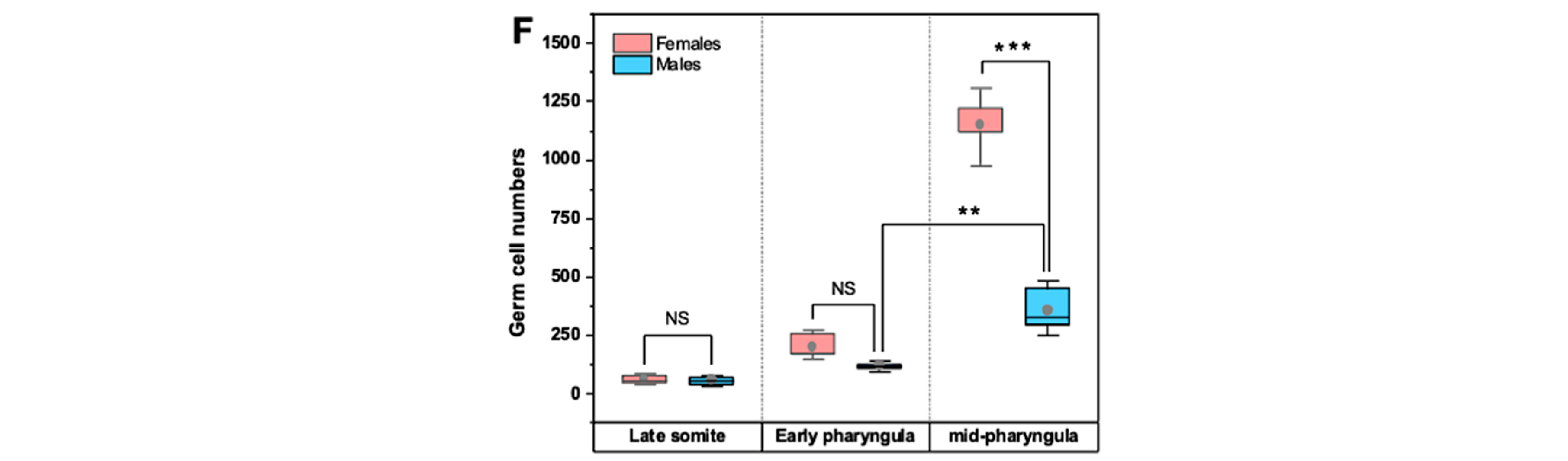
3.2. Mitotic Proliferation of Germ Cells
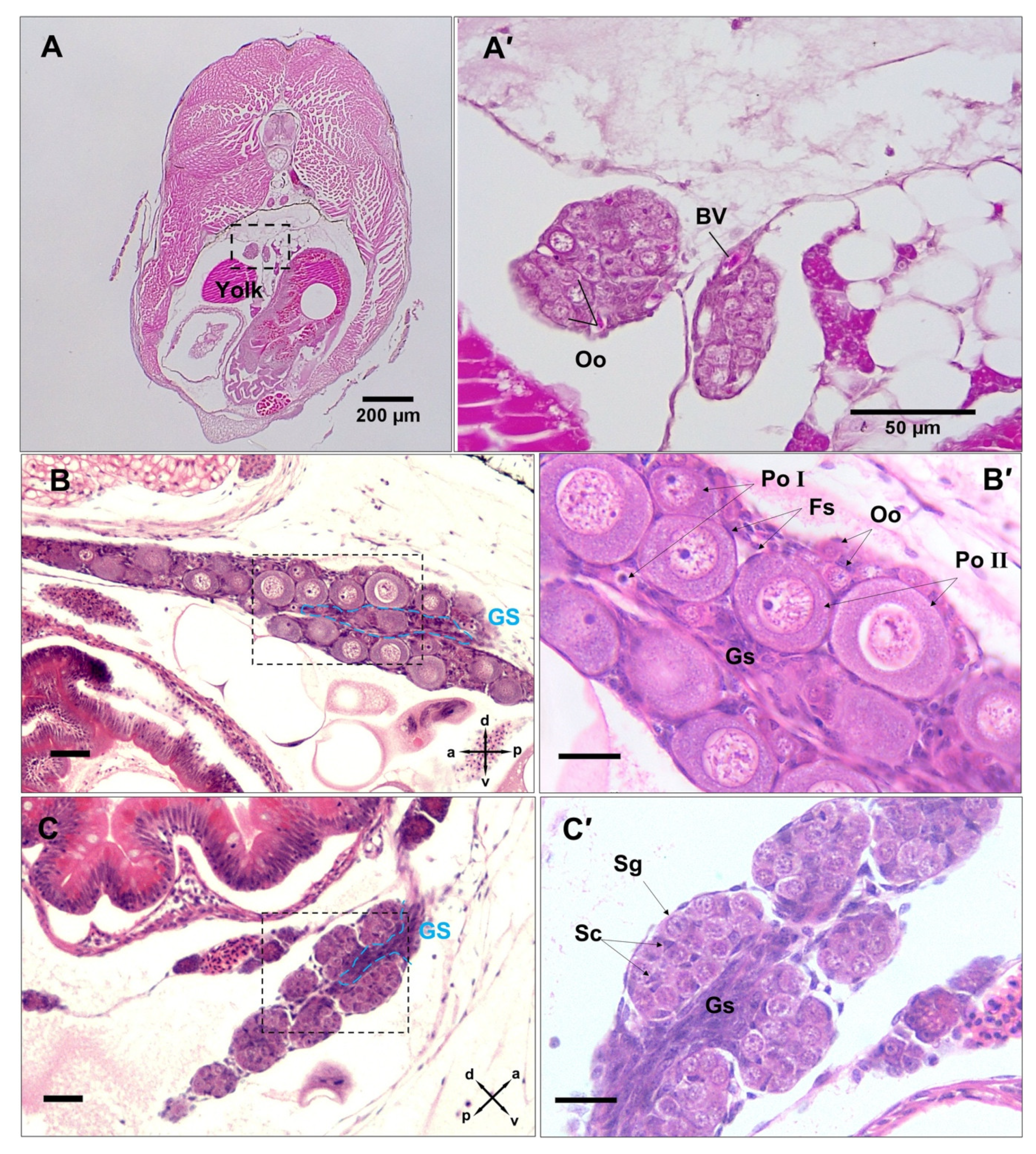
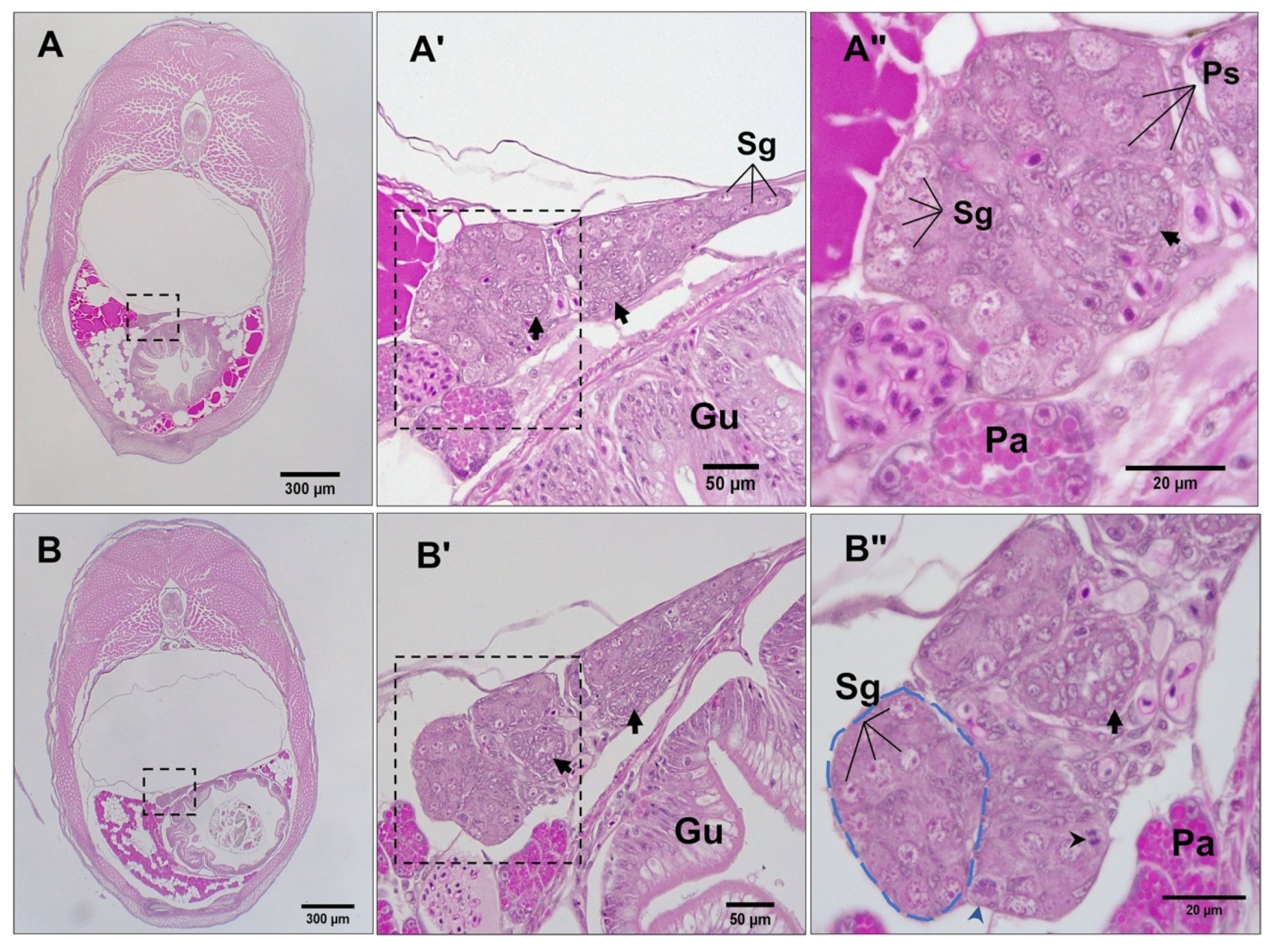
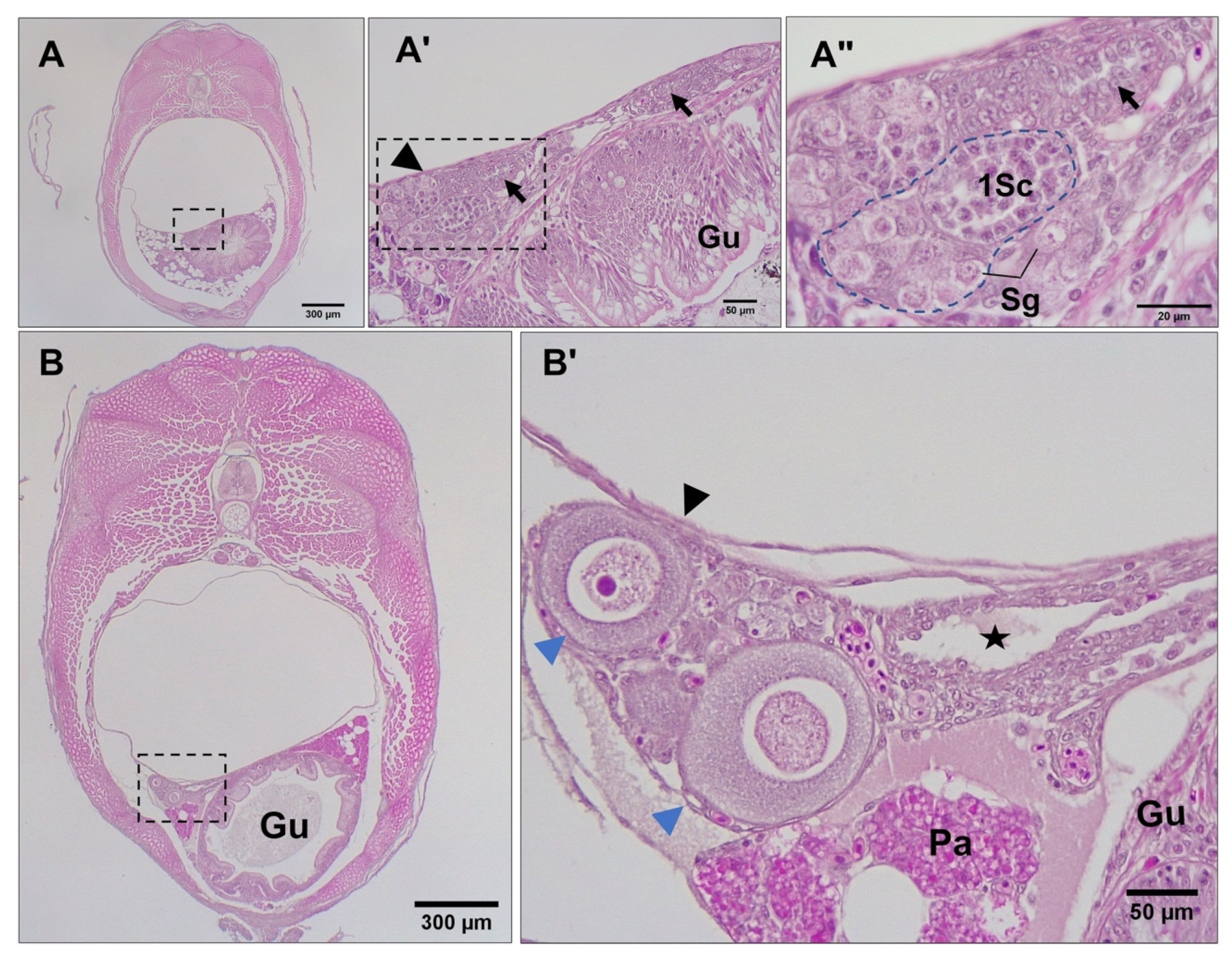
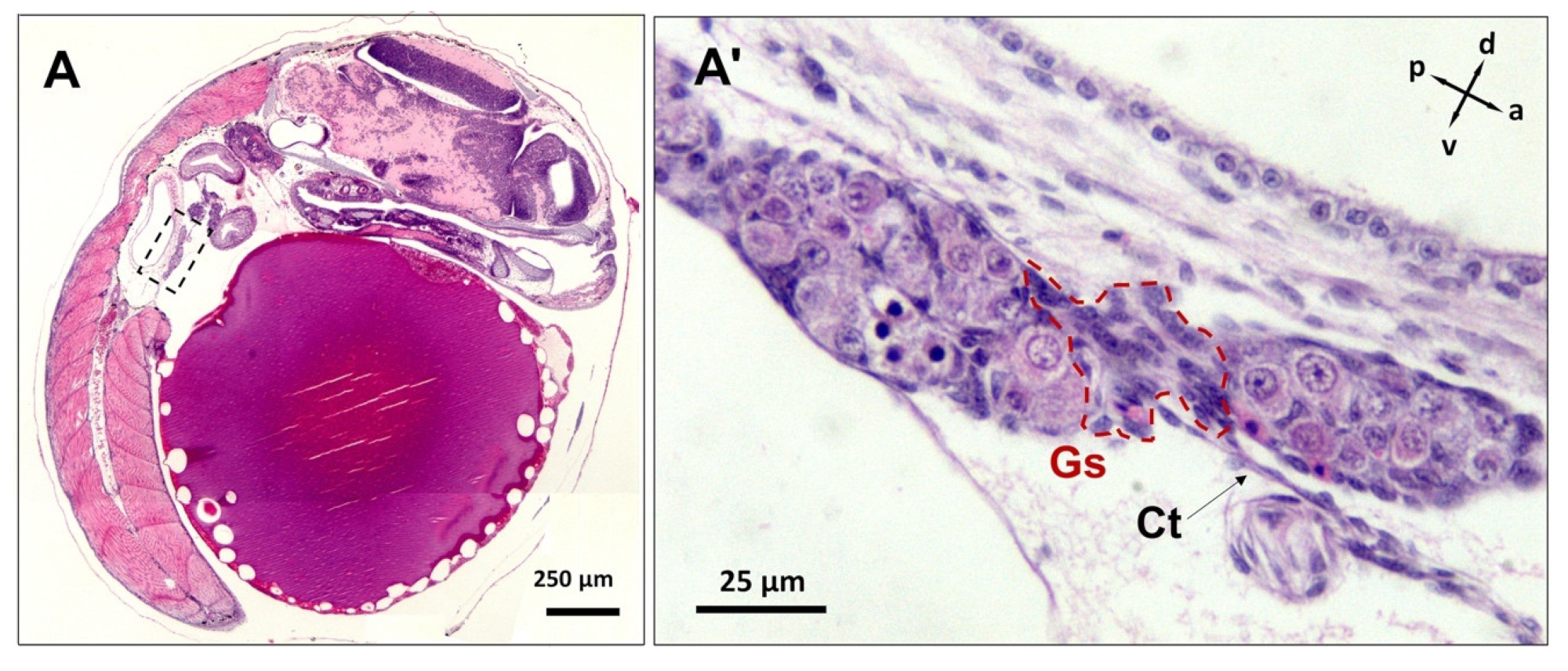
3.3. Differentiation of Gonads
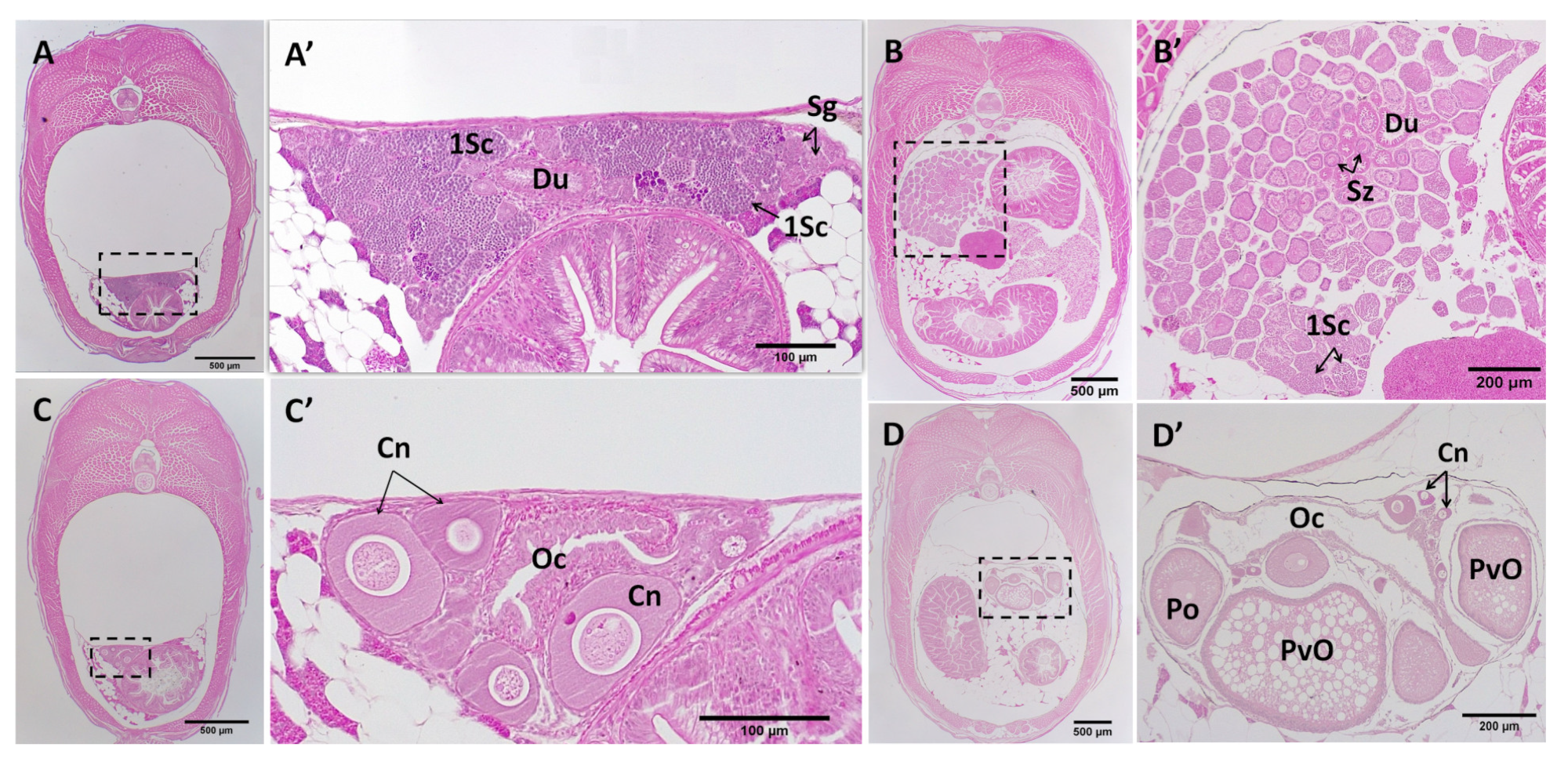
3.4. Post-Natal Gonad Development
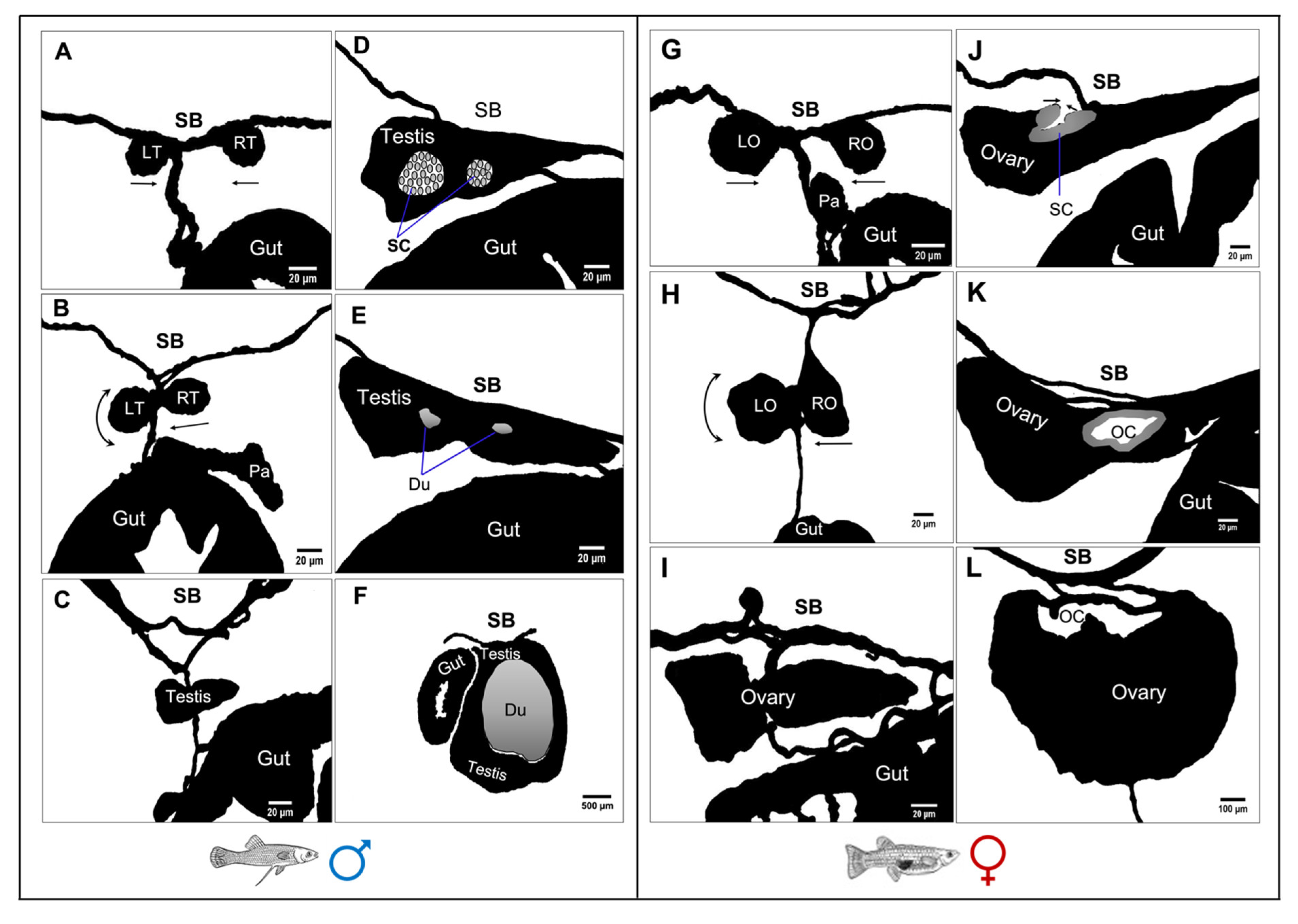
3.5. Gross Morphological Fusion of the Gonad Lobes
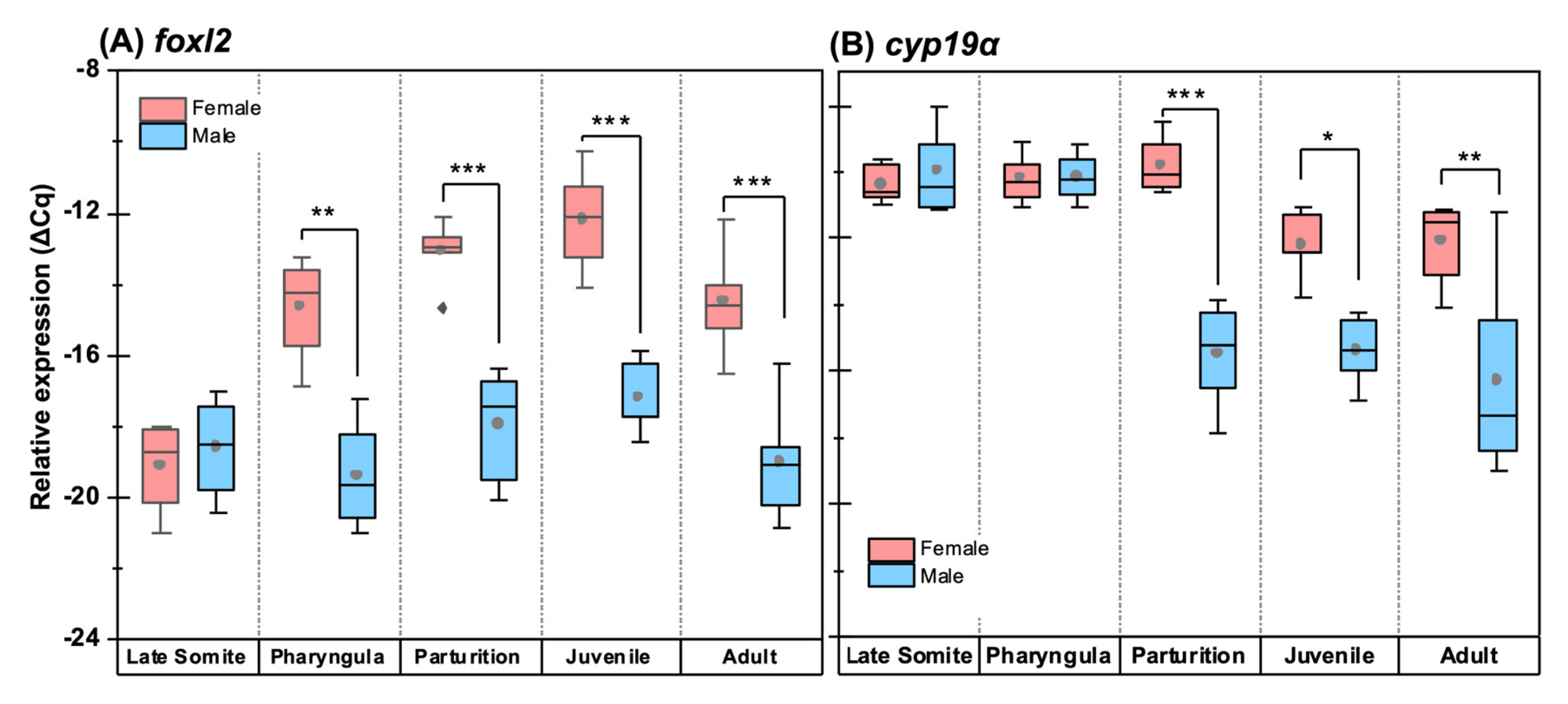
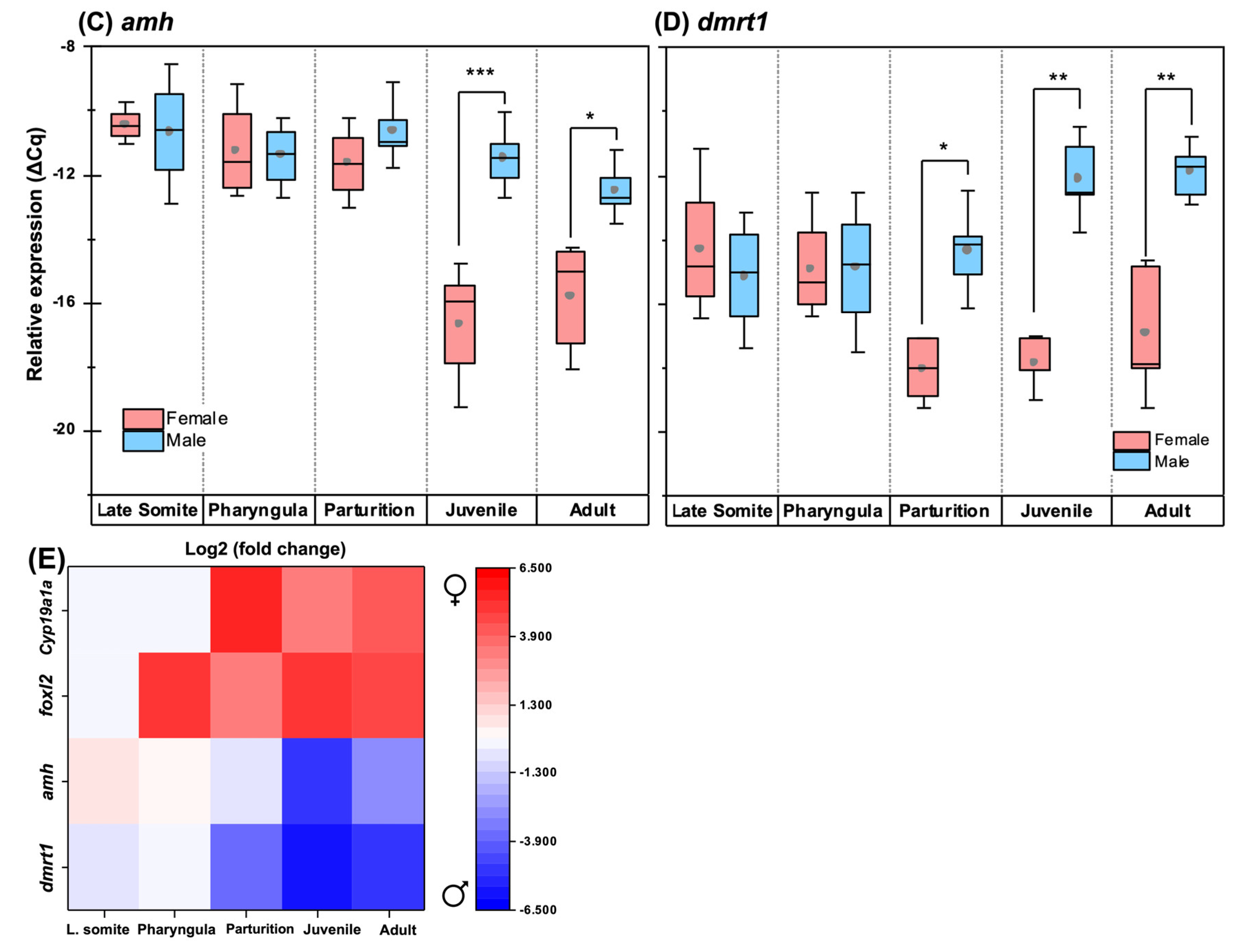
3.6. Quantitative Expression Patterns of Gonadosoma Markers
4. Discussion
4.1. Morphology and Clustering of Germ Cells Is Sex-Dimorphic in Undifferentiated Gonads
4.2. Ovarian Differentiation Precedes Testis Differentiation and Occurs Earlier than in Most Teleosts
4.3. Gonadosoma Markers Forecast the Timing of Sex Differentiation
4.4. Embryonic Recapitulation of the Bi-Lobed Gonad
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raz, E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Herpin, A.; Fischer, P.; Liedtke, D.; Kluever, N.; Neuner, C.; Raz, E.; Schartl, M. Sequential SDF1a and b-induced mobility guides Medaka PGC migration. Dev. Biol. 2008, 320, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Yamaji, M. Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 2012, 4, a008375. [Google Scholar] [CrossRef] [PubMed]
- Razmi, K.; Patil, J.G. Primordial Germ Cell Development in the Poeciliid, Gambusia holbrooki, Reveals Shared Features Between Lecithotrophs and Matrotrophs. Front. Cell Dev. Biol. 2022, 10, 793498. [Google Scholar] [CrossRef]
- Reichman-Fried, M.; Minina, S.; Raz, E. Autonomous modes of behavior in primordial germ cell migration. Dev. Cell 2004, 6, 589–596. [Google Scholar] [CrossRef]
- Minina, S.; Reichman-Fried, M.; Raz, E. Control of receptor internalization, signaling level, and precise arrival at the target in guided cell migration. Curr. Biol. 2007, 17, 1164–1172. [Google Scholar] [CrossRef]
- Paksa, A.; Raz, E. Zebrafish germ cells: Motility and guided migration. Curr. Opin. Cell Biol. 2015, 36, 80–85. [Google Scholar] [CrossRef]
- Pares, G.; Ricardo, S. FGF control of E-cadherin targeting in the Drosophila midgut impacts on primordial germ cell motility. J. Cell Sci. 2016, 129, 354–366. [Google Scholar] [CrossRef]
- Huss, D.J.; Saias, S.; Hamamah, S.; Singh, J.M.; Wang, J.; Dave, M.; Kim, J.; Eberwine, J.; Lansford, R. Avian Primordial Germ Cells Contribute to and Interact With the Extracellular Matrix During Early Migration. Front. Cell Dev. Biol. 2019, 7, 35. [Google Scholar] [CrossRef]
- Di Carlo, A.; De Felici, M. A role for E-cadherin in mouse primordial germ cell development. Dev. Biol. 2000, 226, 209–219. [Google Scholar] [CrossRef]
- Jenkins, A.B.; McCaffery, J.M.; Van Doren, M. Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development 2003, 130, 4417–4426. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M. Sex determination in the teleost medaka, Oryzias latipes. Annu. Rev. Genet. 2005, 39, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, K.R.; Nusslein-Volhard, C. Germ line control of female sex determination in zebrafish. Dev. Biol. 2008, 324, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental Effects on Fish Sex Determination and Differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef]
- Godwin, J. Social determination of sex in reef fishes. Semin. Cell Dev. Biol. 2009, 20, 264–270. [Google Scholar] [CrossRef]
- Nagahama, Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol. Biochem. 2005, 31, 105–109. [Google Scholar] [CrossRef]
- Kwan, T.N.; Patil, J.G. Sex biased expression of anti-Mullerian hormone (amh) gene in a live bearing fish, Gambusia holbrooki: Evolutionary implications and potential role in sex differentiation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 231, 59–66. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Patil, J.G. Stages of embryonic development in the live-bearing fish, Gambusia holbrooki. Dev. Dyn. 2021, 251, 287–320. [Google Scholar] [CrossRef]
- Koya, Y.; Fujita, A.; Niki, F.; Ishihara, E.; Miyama, H. Sex differentiation and pubertal development of gonads in the viviparous mosquitofish, Gambusia affinis. Zool. Sci. 2003, 20, 1231–1242. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Torres, L.; Tiersch, T.R. Activation of free sperm and dissociation of sperm bundles (spermatozeugmata) of an endangered viviparous fish, Xenotoca eiseni. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 218, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Zane, L.; Francescato, S.; Pilastro, A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 2003, 421, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, C.; Daymond, E.; Evans, J.P. Extreme fertilization bias towards freshly inseminated sperm in a species exhibiting prolonged female sperm storage. R. Soc. Open Sci. 2018, 5, 172195. [Google Scholar] [CrossRef] [PubMed]
- Norazmi-Lokman, N.H.; Purser, G.J.; Patil, J.G. Gravid Spot Predicts Developmental Progress and Reproductive Output in a Livebearing Fish, Gambusia holbrooki. PLoS ONE 2016, 11, e0147711. [Google Scholar] [CrossRef]
- Norazmi-Lokman, N.H.; Purser, G.J.; Patil, J.G. Efficacy of estradiol in feminising the eastern mosquitofish, Gambusia holbrooki: Advance towards developing a genetic control option. Mar. Freshw. Res. 2021, 72, 1657. [Google Scholar] [CrossRef]
- Pyke, G.H. A Review of the Biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fish. 2005, 15, 339–365. [Google Scholar] [CrossRef]
- Norazmi-Lokman, N.H. Hormonal Feminization and Associated Reproductive Impacts in the Eastern Mosquitofish Gambusia holbrooki. Ph.D. Thesis, University of Tasmania, Hobart, TAS, Australia, 2016. [Google Scholar]
- Meffe, G.K. Plasticity of life-history characters in eastern mosquitofish (Gambusia holbrooki: Poeciliidae) in response to thermal stress. Copeia 1992, 1992, 94–102. [Google Scholar] [CrossRef]
- Nguyen, H.; Bell, J.D.; Patil, J.G. Daily ageing to delineate population dynamics of the invasive fish Gambusia holbrooki: Implications for management and control. Biol. Invasions 2021, 23, 2261–2270. [Google Scholar] [CrossRef]
- Lloyd, L.N.; Arthington, A.H.; Milton, D.A. The mosquitofish—A valuable mosquito-control agent or a pest? In The Ecology of Exotic Animals and Plants Some Australian Case Histories; Kitching, R.L., Ed.; John Wiley & Sons: Brisbane, QLD, Australia, 1986. [Google Scholar]
- Patil, J.G.; Norazmi-Lokman, N.H.; Kwan, T.N. Reproductive viability of paradoxically masculinised Gambusia holbrooki generated following diethylstilbestrol (DES) treatment. Comp. Biochem. Physiol. Part B 2020, 248, 110468. [Google Scholar] [CrossRef]
- Patil, J.G. An Adaptive Genetic Management Plan for Eradication of Gambusia holbrooki from Tasmania Australia; Inland Fisheries Service: New Norfolk, TAS, Australia, 2012; p. 30.
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Day, C., Ed.; Methods in Molecular Biology (Methods and Protocols); Humana Press: New York, NY, USA, 2014; Volume 1180. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Mateizel, I.; Kemp, C.; Cauffman, G.; Sermon, K.; Leyns, L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int. J. Dev. Biol. 2006, 50, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T.M. Validation of Common Housekeeping Genes as Reference for qPCR Gene Expression Analysis During iPS Reprogramming Process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 34. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.; Moorman, A.F. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Hamaguchi, S. A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res. 1982, 227, 139–151. [Google Scholar] [CrossRef]
- Ye, D.; Zhu, L.; Zhang, Q.; Xiong, F.; Wang, H.; Wang, X.; He, M.; Zhu, Z.; Sun, Y. Abundance of Early Embryonic Primordial Germ Cells Promotes Zebrafish Female Differentiation as Revealed by Lifetime Labeling of Germline. Mar. Biotechnol. 2019, 21, 217–228. [Google Scholar] [CrossRef]
- Nishimura, T.; Sato, T.; Yamamoto, Y.; Watakabe, I.; Ohkawa, Y.; Suyama, M.; Kobayashi, S.; Tanaka, M. foxl3 is a germ cell–intrinsic factor involved in sperm-egg fate decision in medaka. Science 2015, 349, 328–331. [Google Scholar] [CrossRef]
- Pan, Z.J.; Zhu, C.K.; Wang, H.; Zhou, F.J.; Qiang, X.G. Gonadal morphogenesis and sex differentiation in cultured Ussuri catfish Tachysurus ussuriensis. J. Fish Biol. 2017, 91, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Billard, R.; Jalabert, B.; Escaffre, A.-M.; Cauty, C. Changes in the number of germ cells in the gonads of the rainbow trout (Salmo gairdneri) during the first 10 post-hatching weeks. Reprod. Nutr. Dev. 1982, 22, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Tanaka, M. Gonadal development in fish. Sex. Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuda, M.; Kajiura-Kobayashi, H.; Suzuki, A.; Saito, N.; Nakamoto, M.; Shibata, N.; Nagahama, Y. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev. Dyn. 2004, 231, 518–526. [Google Scholar] [CrossRef]
- Saito, D.; Morinaga, C.; Aoki, Y.; Nakamura, S.; Mitani, H.; Furutani-Seiki, M.; Kondoh, H.; Tanaka, M. Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai. Dev. Biol. 2007, 310, 280–290. [Google Scholar] [CrossRef]
- Morinaga, C.; Tomonaga, T.; Sasado, T.; Suwa, H.; Niwa, K.; Yasuoka, A.; Henrich, T.; Watanabe, T.; Deguchi, T.; Yoda, H.; et al. Mutations affecting gonadal development in Medaka, Oryzias latipes. Mech. Dev. 2004, 121, 829–839. [Google Scholar] [CrossRef]
- Lewis, Z.R.; McClellan, M.C.; Postlethwait, J.H.; Cresko, W.A.; Kaplan, R.H. Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus). J. Morphol. 2008, 269, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Egami, N. Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J. Embryol. Exp. Morphol. 1972, 28, 385–395. [Google Scholar] [PubMed]
- Van den Hurk, R.; Slof, G.A. A Morphological and Experimental Study of Gonadal Sex Differentiation in the Rainbow Trout, Salmo gairdneri. Cell Tissue Res. 1981, 218, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.; Yamashita, M.; Nagahama, Y. Testicular Development Induced by a Recessive Mutation during Gonadal Differentiation of Female Common Carp (Cyprinus carpio, L.). Dev. Growth Differ. 1992, 34, 535–544. [Google Scholar] [CrossRef]
- Rasmussen, T.H.; Jespersen, A.; Korsgaard, B. Gonadal morphogenesis and sex differentiation in intraovarian embryos of the viviparous fish Zoarces viviparus (Teleostei, Perciformes, Zoarcidae): A histological and ultrastructural study. J. Morphol. 2006, 267, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Process of functional sex reversal of the gonad in the female guppy, Poecilia reticulata, treated with androgen before birth. Dev. Growth Differ. 1975, 17, 167–175. [Google Scholar] [CrossRef]
- Goodrich, H.B.; Dee, J.E.; Flynn, C.M.; Mercer, R.N. Germ cells and Sex differentiation in Lebistes reticulatus. Biol. Bull. 1934, 67, 83–96. [Google Scholar] [CrossRef]
- Dildine, G.C. Studies in teleostean reproduction. I. Embryonic hermaphroditism in Lebistes reticulatus. J. Morphol. 1936, 60, 261–277. [Google Scholar] [CrossRef]
- Kottler, V.A.; Feron, R.; Nanda, I.; Klopp, C.; Du, K.; Kneitz, S.; Helmprobst, F.; Lamatsch, D.K.; Lopez-Roques, C.; Lluch, J.; et al. Independent Origin of XY and ZW Sex Determination Mechanisms in Mosquitofish Sister Species. Genetics 2020, 214, 193–209. [Google Scholar] [CrossRef]
- Charlesworth, D. The Guppy Sex Chromosome System and the Sexually Antagonistic Polymorphism Hypothesis for Y Chromosome Recombination Suppression. Genes 2018, 9, 264. [Google Scholar] [CrossRef]
- Baron, D.; Cocquet, J.; Xia, X.; Fellous, M.; Guiguen, Y.; Veitia, R.A. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J. Mol. Endocrinol. 2004, 33, 705–715. [Google Scholar] [CrossRef]
- Nakamoto, M.; Matsuda, M.; Wang, D.S.; Nagahama, Y.; Shibata, N. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2006, 344, 353–361. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Ma, H.; Liu, X.; Shi, H.; Li, M.; Wang, D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology 2017, 158, 2634–2647. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.G.; Gunasekera, R.M. Tissue and sexually dimorphic expression of ovarian and brain aromatase mRNA in the Japanese medaka (Oryzias latipes): Implications for their preferential roles in ovarian and neural differentiation and development. Gen. Comp. Endocrinol. 2008, 158, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Zhang, Z.; Qin, M.; Ge, W. Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 Leads to All-male Offspring Due to Failed Ovarian Differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cheng, H.; Huang, X.; Gao, S.; Yu, H.; Zhou, R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem. Biophys. Res. Commun. 2005, 330, 950–957. [Google Scholar] [CrossRef]
- Webster, K.A.; Schach, U.; Ordaz, A.; Steinfeld, J.S.; Draper, B.W.; Siegfried, K.R. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017, 422, 33–46. [Google Scholar] [CrossRef]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A.; et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef]
- Winkler, C.; Hornung, U.; Kondo, M.; Neuner, C.; Duschl, J.; Shima, A.; Schartl, M. Developmentally regulated and non-sex-specific expression of autosomal dmrt genes in embryos of the Medaka fish (Oryzias latipes). Mech. Dev. 2004, 121, 997–1005. [Google Scholar] [CrossRef]
- Masuyama, H.; Yamada, M.; Kamei, Y.; Fujiwara-Ishikawa, T.; Todo, T.; Nagahama, Y.; Matsuda, M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosom. Res. 2012, 20, 163–176. [Google Scholar] [CrossRef]
- Morohashi, K.I.; Omura, T. Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J. 1996, 10, 1569–1577. [Google Scholar] [CrossRef]
- Wang, D.-S.; Kobayashi, T.; Zhou, L.-Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.-I.; Nagahama, Y. Foxl2 Up-Regulates Aromatase Gene Transcription in a Female-Specific Manner by Binding to the Promoter as Well as Interacting with Ad4 Binding Protein/Steroidogenic Factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef]
- Georges, A.; Auguste, A.; Bessiere, L.; Vanet, A.; Todeschini, A.L.; Veitia, R.A. FOXL2: A central transcription factor of the ovary. J. Mol. Endocrinol. 2014, 52, R17–R33. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ye, L.; Chen, H. Sex determination and maintenance: The role of DMRT1 and FOXL2. Asian J. Androl. 2017, 19, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 139, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Kilcoyne, K.R.; Sharpe, R.M.; Kavanagh, Á.; Anderson, R.A.; Brown, P.; Smith, L.B.; Jørgensen, A.; Mitchell, R.T. DMRT1 repression using a novel approach to genetic manipulation induces testicular dysgenesis in human fetal gonads. Hum. Reprod. 2018, 33, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Baron, B.; Buckle, F.; Espina, S. Environmental factors and sexual differentiation in Poecilia sphenops Valenciennes (Pisces: Poeciliidae). Aquac. Res. 2002, 33, 615–619. [Google Scholar] [CrossRef]
- Greven, H. Part1: Reproductive biology and life history. In Ecology and Evolution of Poeciliid Fishes; Evans, J.P., Pilastro, A., Ingo, I., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 2–58. [Google Scholar]
- Torres-Martinez, A.; Ruiz de Dios, L.; Hernandez-Franyutti, A.; Uribe, M.C.; Sanchez, W.C. Structure of the testis and spermatogenesis of the viviparous teleost Poecilia mexicana (Poeciliidae) from an active sulfur spring cave in Southern Mexico. J. Morphol. 2019, 280, 1537–1547. [Google Scholar] [CrossRef]
- McLeod, R. Counting the Cost: Impact of Invasive Animals in Australia, 2004; Norris, A., Ed.; Cooperative Research Centre for Pest Animal Control: Canberra, NSW, Australia, 2004. [Google Scholar]
- Reznick, D.; Bryant, M.; Holmes, D. The evolution of senescence and post-reproductive lifespan in guppies (Poecilia reticulata). PLoS Biol. 2006, 4, e7. [Google Scholar] [CrossRef]
- Croft, D.P.; Brent, L.J.; Franks, D.W.; Cant, M.A. The evolution of prolonged life after reproduction. Trends Ecol. Evol. 2015, 30, 407–416. [Google Scholar] [CrossRef]
| Gene | Primer Sequence 5′–3′ * | Amplicon Size (bp) | Annealing Temperature (°C) | Reference/ Accession ID | |
|---|---|---|---|---|---|
| House keeping | beta-actin | F—CGGCAGGACTTCACCTACAGACACCT | 99 | 68 | [4] |
| R—CTTGCACAAACCGGAGCCGTTGTCA | |||||
| gapdh | F—AGCCAAGGCTGTTGGCAAGGTCATC | 133 | 67.5 | [18] | |
| R—GTCATCATACTTGGCTGGTTTCTCC | |||||
| pgk1 | F—GATGATCATCGGTGGCGGCATGG | 96 | 66.5 | OL988673 | |
| R—ATACAGCGCCTTCCTCGTCGAACA | |||||
| rps18 | F—GGAGAGGCTGAAGAAGATCAGGGCTC | 109 | 66.5 | OL988674 | |
| ACCGACAGTGCGACCACGACG | |||||
| Male biased | dmrt1 | F—CACCCTTCGTCAGCCTGGAGGAGA | 85 | 67.0 | OL988671 |
| R—ATGGTCGAGTCGTAGCTGGTAGGTGAA | |||||
| amh | F—CCCCTGCAGATGGAGAGCTGGGCGTCATTT | 88 | 64 | [18] | |
| AACGTCGTCCCTGAARTGCAAGCAGA | |||||
| Female biased | cyp19a1a | F—GCTTGTGGAGGAGATGAGCACGGTT | 97 | 63.5 | (Patil, personal collection) |
| R—CATCACTTTCAGTCTTTCATAACTGACG | |||||
| foxl2 | F—GCAAAGGGAGAGGCAGAGGAGGA | 108 | 66.0 | OL988672 | |
| R—CTCTACCGCCTCTCCCACTGAAACCA | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razmi, K.; Tran, N.K.; Patil, J.G. Gonad Ontogeny and Sex Differentiation in a Poeciliid, Gambusia holbrooki: Transition from a Bi- to a Mono-Lobed Organ. Biology 2023, 12, 731. https://doi.org/10.3390/biology12050731
Razmi K, Tran NK, Patil JG. Gonad Ontogeny and Sex Differentiation in a Poeciliid, Gambusia holbrooki: Transition from a Bi- to a Mono-Lobed Organ. Biology. 2023; 12(5):731. https://doi.org/10.3390/biology12050731
Chicago/Turabian StyleRazmi, Komeil, Ngoc Kim Tran, and Jawahar G. Patil. 2023. "Gonad Ontogeny and Sex Differentiation in a Poeciliid, Gambusia holbrooki: Transition from a Bi- to a Mono-Lobed Organ" Biology 12, no. 5: 731. https://doi.org/10.3390/biology12050731
APA StyleRazmi, K., Tran, N. K., & Patil, J. G. (2023). Gonad Ontogeny and Sex Differentiation in a Poeciliid, Gambusia holbrooki: Transition from a Bi- to a Mono-Lobed Organ. Biology, 12(5), 731. https://doi.org/10.3390/biology12050731






