Reduction in mRNA Expression of the Neutrophil Chemoattract Factor CXCL1 in Pseudomonas aeruginosa Treated Barth Syndrome B Lymphoblasts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Bacterial Stimulation
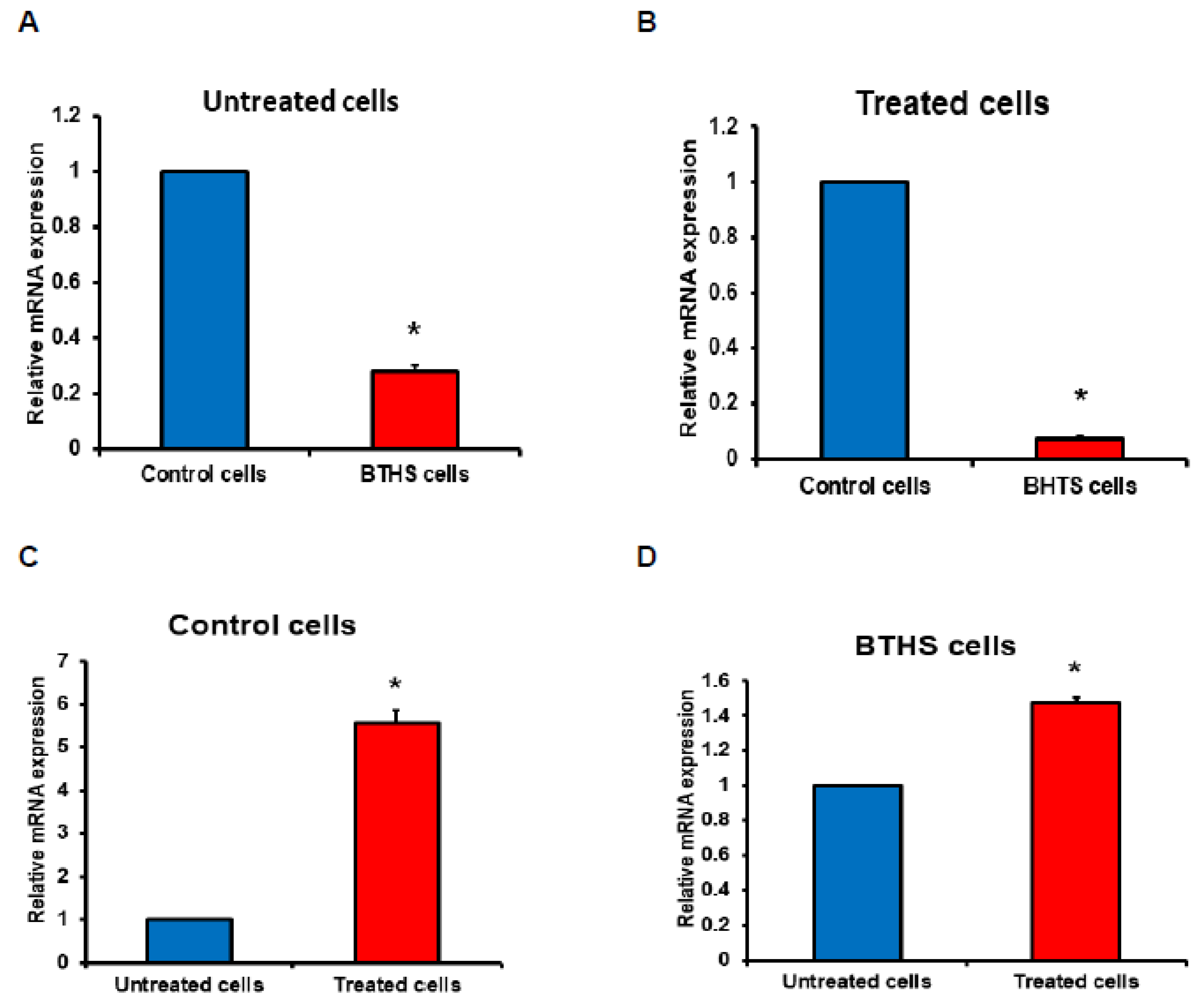
2.3. Relative Gene Expression
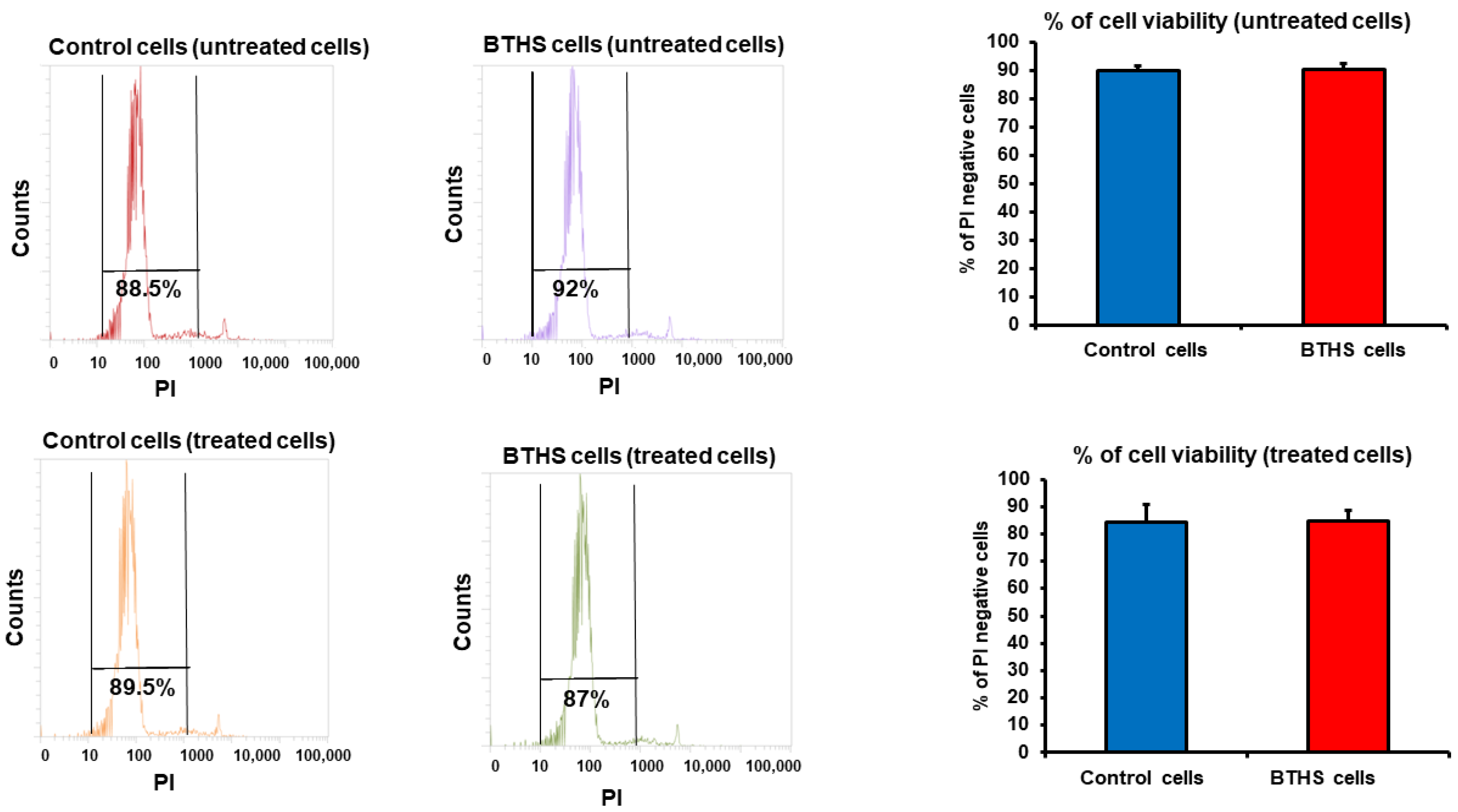
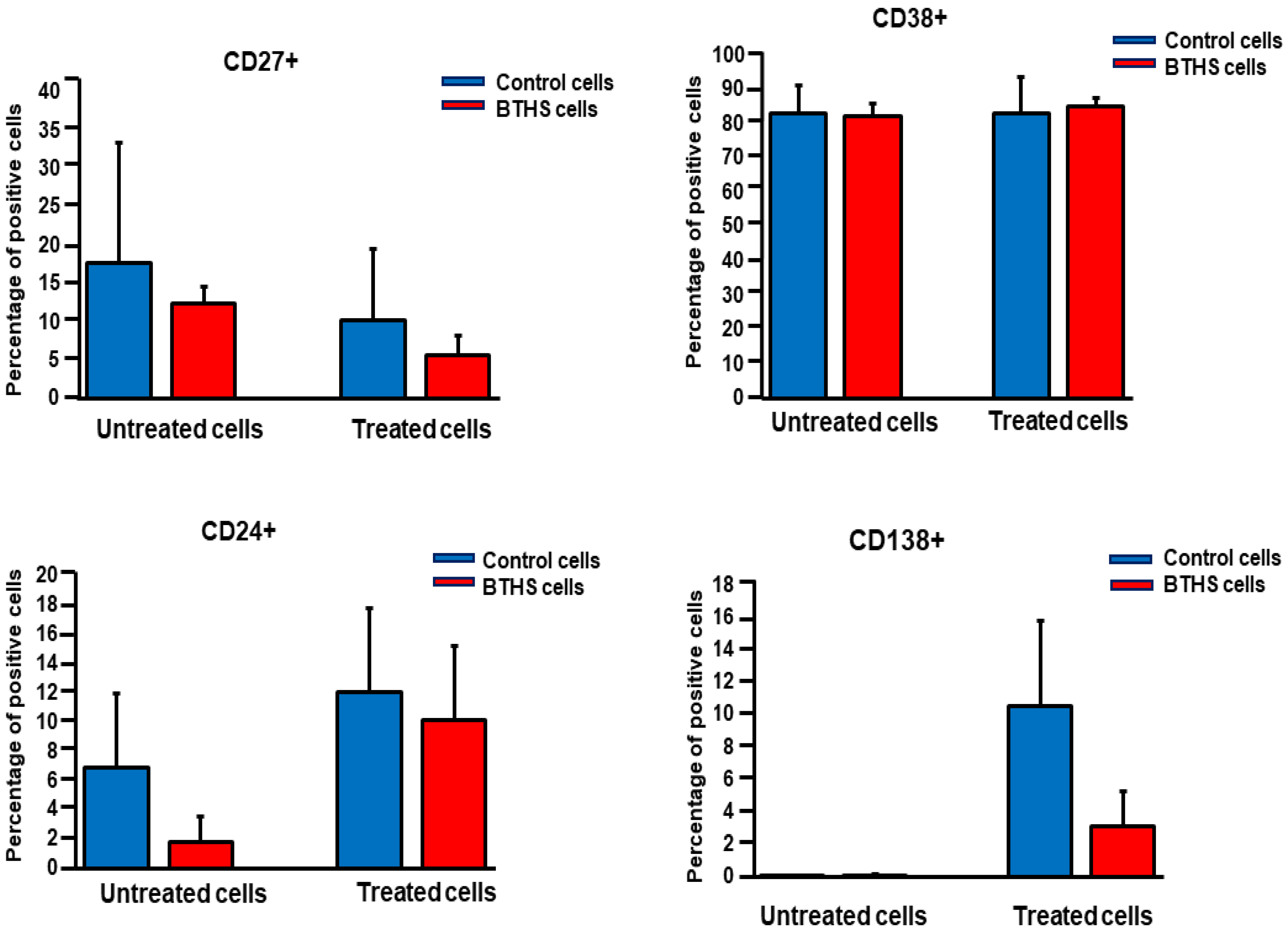
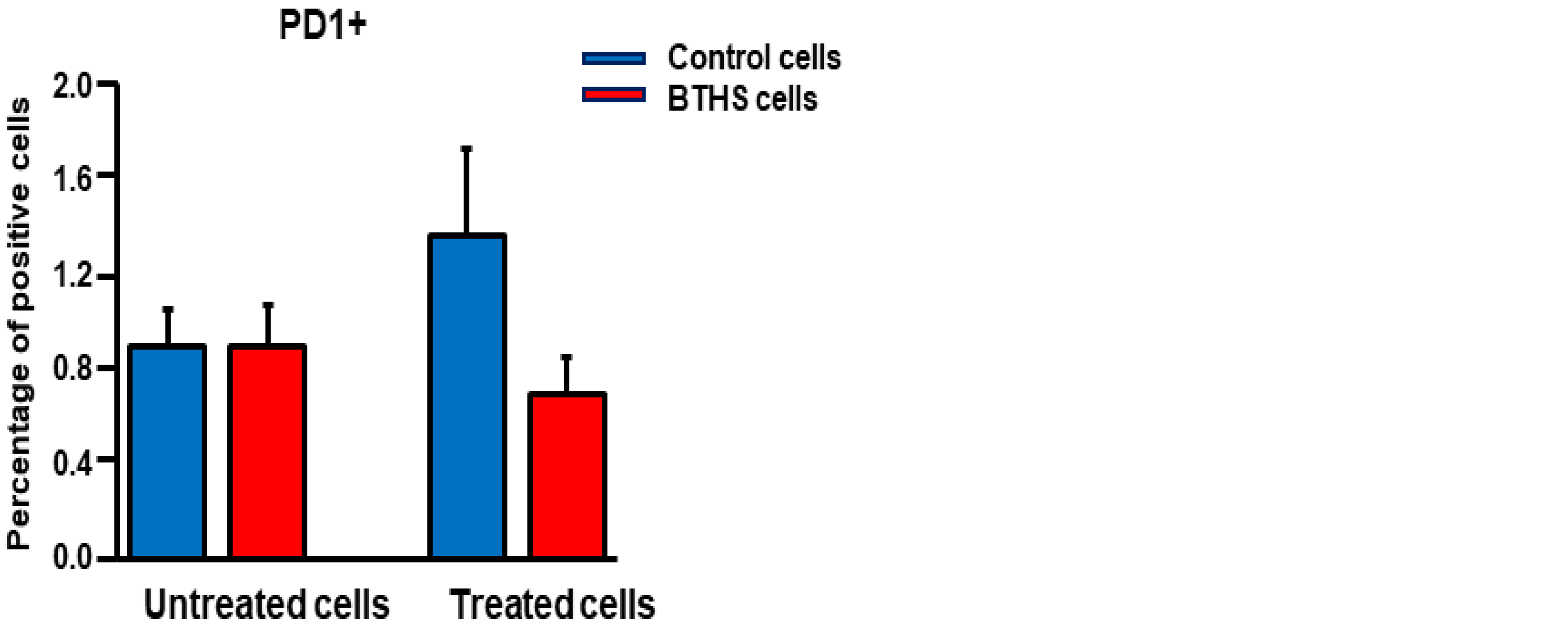
Cell Viability and Surface Marker Expression Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Van der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.M.; Veer-Korthof, E.T.V.T.; Van der Harten, J.J.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.I.; Cheatham, J.P.; Clark, B.J.; Nigro, M.A.; Powell, B.R.; Sherwood, G.W.; Sladky, J.T.; Swisher, W.P. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J. Pediatr. 1991, 119, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth Syndrome. Orphanet J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Hauff, K.D.; Hatch, G.M. Cardiolipin metabolism and Barth Syndrome. Prog. Lipid Res. 2006, 45, 91–101. [Google Scholar] [CrossRef]
- Zegallai, H.M.; Hatch, G.M. Barth syndrome: Cardiolipin, cellular pathophysiology, management, and novel therapeutic targets. Mol. Cell. Biochem. 2021, 476, 1605–1629. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Rao, E.S.; Pierre, G.; Chronopoulou, E.; Hornby, B.; Heyman, A.; Vernon, H.J. Clinical presentation and natural history of Barth Syndrome: An overview. J. Inherit. Metab. Dis. 2022, 45, 7–16. [Google Scholar] [CrossRef]
- Thompson, R.; Jefferies, J.; Wang, S.; Pu, W.T.; Takemoto, C.; Hornby, B.; Heyman, A.; Chin, M.T.; Vernon, H.J. Current and future treatment approaches for Barth syndrome. J. Inherit. Metab. Dis. 2022, 45, 17–28. [Google Scholar] [CrossRef]
- Xu, Y.; Malhotra, A.; Ren, M.; Schlame, M. The enzymatic function of tafazzin. J. Biol. Chem. 2006, 281, 39217–39224. [Google Scholar] [CrossRef]
- Steward, C.G.; Groves, S.J.; Taylor, C.T.; Maisenbacher, M.K.; Versluys, B.; Newbury-Ecob, R.A.; Ozsahin, H.; Damin, M.K.; Bowen, V.M.; McCurdy, K.R.; et al. Neutropenia in Barth syndrome: Characteristics, risks, and management. Curr. Opin. Hematol. 2019, 26, 6–15. [Google Scholar] [CrossRef]
- Kuijpers, T.W.; Maianski, N.A.; Tool, A.T.; Becker, K.; Plecko, B.; Valianpour, F.; Wanders, R.J.; Pereira, R.; Van Hove, J.; Verhoeven, A.J.; et al. Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood 2000, 103, 3915–3923. [Google Scholar] [CrossRef]
- Dai, Y.; Tang, Y.; He, F.; Zhang, Y.; Cheng, A.; Gan, R.; Wu, Y. Screening and functional analysis of differentially expressed genes in EBV-transformed lymphoblasts. Virol. J. 2012, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Zegallai, H.M.; Abu-El-Rub, E.; Olayinka-Adefemi, F.; Cole, L.K.; Sparagna, G.C.; Marshall, A.J.; Hatch, G.M. Tafazzin deficiency in mouse mesenchymal stem cells promote reprogramming of activated B lymphocytes toward immunosuppressive phenotypes. FASEB J. 2022, 36, e22443. [Google Scholar] [CrossRef] [PubMed]
- Zegallai, H.M.; Abu-El-Rub, E.; Cole, L.K.; Field, J.; Mejia, E.M.; Gordon, J.W.; Marshall, A.J.; Hatch, G.M. Tafazzin deficiency impairs mitochondrial metabolism and function of lipopolysaccharide activated B lymphocytes in mice. FASEB J. 2021, 35, e22023. [Google Scholar] [CrossRef]
- Corrado, M.; Edwards-Hicks, J.; Villa, M.; Flachsmann, L.J.; Sanin, D.E.; Jacobs, M.; Baixauli, F.; Stanczak, M.; Anderson, E.; Azuma, M.; et al. Dynamic cardiolipin synthesis is required for CD8(+) T Cell immunity. Cell Metab. 2020, 32, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.R.R.; Crozier, R.W.E.; Hunter, K.D.; Claypool, S.M.; Fajardo, V.A.; LeBlanc, P.J.; MacNeil, A.J. Tafazzin modulates allergen-induced mast cell inflammatory mediator secretion. ImmunoHorizons 2021, 5, 182–192. [Google Scholar] [CrossRef]
- Karmakar, U.; Vermeren, S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology 2021, 164, 689–700. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Daniel, S.; Noël, L.-H.; Mouthon, L. Neutrophils and B lymphocytes in ANCA-associated vasculitis. Apmis 2009, 117, 27–31. [Google Scholar] [CrossRef]
- Moser, B.; Clark-Lewis, I.; Zwahlen, R.; Baggiolini, M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J. Exp. Med. 1990, 171, 1797–1802. [Google Scholar] [CrossRef]
- Schumacher, C.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc. Natl. Acad. Sci. USA 1992, 89, 10542–10546. [Google Scholar] [CrossRef]
- Sohn, J.; Milosevic, J.; Brouse, T.; Aziz, N.; Elkhoury, J.; Wang, S.; Hauschild, A.; Van Gastel, N.; Cetinbas, M.; Tufa, S.F.; et al. A new murine model of Barth syndrome neutropenia links TAFAZZIN deficiency to increased ER stress-induced apoptosis. Blood Adv. 2022, 6, 2557–2577. [Google Scholar] [CrossRef]
- Lee, B.-R.; Chang, S.-Y.; Hong, E.-H.; Kwon, B.-E.; Kim, H.M.; Kim, Y.-J.; Lee, J.; Cho, H.-J.; Cheon, J.-H.; Ko, H.-J. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget 2014, 5, 12331–12345. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.S.; Hoffner, B.; Winkelmann, J.L.; Abbott, M.E.; Hamid, O.; Carvajal, R.D. Programmed death 1 immune checkpoint inhibitors. Clin. Adv. Hematol. Oncol. 2015, 13, 858–868. [Google Scholar] [PubMed]
- Wang, X.; Wang, G.; Wang, Z.; Liu, B.; Han, N.; Li, J.; Lu, C.; Liu, X.; Zhang, Q.; Yang, Q.; et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol. Immunol. 2019, 109, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kudlaty, E.; Agnihotri, N.; Khojah, A. Hypogammaglobulinaemia and B cell lymphopaenia in Barth syndrome. BMJ Case Rep. 2022, 15, e249254. [Google Scholar] [CrossRef] [PubMed]
- Zegallai, H.M.; Hatch, G.M. Impaired surface marker expression in stimulated Epstein-Barr virus transformed lymphoblasts from Barth Syndrome patients. Sci. Rep. 2022, 12, 6195. [Google Scholar] [CrossRef] [PubMed]
| Cell Line Identifier | Phenotype | TAFAZZIN Mutation | Age at Harvest |
|---|---|---|---|
| 07535 | Healthy control | None | 15 years |
| 07491 | Healthy control | None | 17 years |
| 16408 | Healthy control | None | 12 years |
| 22192 | BTHS | Exon 3, Substitution of cystine for arginine | 10 years |
| 22193 | BTHS | Exon 6, premature stop codon | 10 years |
| 22194 | BTHS | Complete deletion of TAFAZZIN gene | 9 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zegallai, H.M.; Duan, K.; Hatch, G.M. Reduction in mRNA Expression of the Neutrophil Chemoattract Factor CXCL1 in Pseudomonas aeruginosa Treated Barth Syndrome B Lymphoblasts. Biology 2023, 12, 730. https://doi.org/10.3390/biology12050730
Zegallai HM, Duan K, Hatch GM. Reduction in mRNA Expression of the Neutrophil Chemoattract Factor CXCL1 in Pseudomonas aeruginosa Treated Barth Syndrome B Lymphoblasts. Biology. 2023; 12(5):730. https://doi.org/10.3390/biology12050730
Chicago/Turabian StyleZegallai, Hana M., Kangmin Duan, and Grant M. Hatch. 2023. "Reduction in mRNA Expression of the Neutrophil Chemoattract Factor CXCL1 in Pseudomonas aeruginosa Treated Barth Syndrome B Lymphoblasts" Biology 12, no. 5: 730. https://doi.org/10.3390/biology12050730
APA StyleZegallai, H. M., Duan, K., & Hatch, G. M. (2023). Reduction in mRNA Expression of the Neutrophil Chemoattract Factor CXCL1 in Pseudomonas aeruginosa Treated Barth Syndrome B Lymphoblasts. Biology, 12(5), 730. https://doi.org/10.3390/biology12050730







