Biotests in Cyanobacterial Toxicity Assessment—Efficient Enough or Not?
Abstract
Simple Summary
Abstract
1. Introduction
Bioassays in Cyanobacterial Toxicity Testing
2. Bioassays with Vertebrate Animal Models
2.1. Mouse Bioassay
Aquatic Vertebrate Animal Models
2.2. Danio Rerio Bioassay
2.2.1. Developmental and Genotoxic Effects—D. rerio
2.2.2. Oxidative Stress Induction—D. rerio
2.3. Oryzias Latipes Bioassay
| Model Organism | Observed Parameter | Sample Type | Exposure Duration | Effective Concentration of the Agent | Main Observed Effect | Reference |
|---|---|---|---|---|---|---|
| Mouse | Mortality rates | MC-LR containing bloom samples—i.p. injection | 24 h | LC50 = 22–250 mg/L dry weight (dw) | Lethality | [132] |
| DNA damage | Purified MC-LR—i.p. injection and oral exposure | 24 h | 2 and 4 mg/kg dw | DNA lesions induced in the liver, kidney, intestine, and colon | [133] | |
| Lung damage after chronic exposure | Purified MC-LR—oral exposure | 12 months | 5–40 µg/L | Lung impairment—thickening of the alveolar septa | [134] | |
| Clinical changes, hystopathological changes, serum indicators of hepatic toxicity, and general homeostasis | Purified MC-LA—oral exposure | 24 h | 3 mg/kg | Weight loss; elevated liver/body weight; liver score; serum levels of ALT, AST, GLDH; BUN/creatinine ratios and total serum bilirubin; reduced serum glucose | [59] | |
| Purified MC-LR—oral exposure | 5 mg/kg | Weight loss; elevated liver/body weight; serum levels of ALT, AST, GLDH; liver score; reduced serum glucose | ||||
| Purified MC-LY—oral exposure | 5 mg/kg | Weight loss; elevated liver/body weight; serum levels of ALT, AST, GLDH; liver score; reduced serum glucose | ||||
| Purified MC-RR—oral exposure | 22 mg/kg | Weight loss, reduced serum glucose | ||||
| Purified MC-YR—oral exposure | 7 mg/kg | Weight loss, elevated liver score, BUN/creatinine ratios, reduced serum glucose | ||||
| Chronic exposure reproductive effects | Purified MC-LR—oral exposure | 6 months | 30–120 µg/L | Testis structure loss, cell abscission and blood–testis barrier (BTB) damage | [135] | |
| 12 months | 1–120 µg/L | |||||
| Histopathological effects | Purified ANTX-a—i.p. injection | 15 days | 0.5–1 μg/l | Fatty liver degeneration, congestion, inflammation and necrosis, morphological kidney alterations, testis structure loss, and decrease in the number of elongated spermatids | [136] | |
| Survivorship | Bloom samples—i.p. injection | 24 h | LC50 = 445.45 mg dw/kg | Mortality | [137] | |
| Survivorship | Bloom samples—i.p. injection | 24 h | LC50 = 20–908 mg dw/kg | Mortality | [138] | |
| Medaka fish (Oryzias latipes) | Survival and developmental toxicity | Purified MC-LR | 10 days | 1–10 µg/mL | Up to 90% reduction in survival rates and altered hatching rate | [139] |
| Survival and developmental toxicity | Microcystis laboratory culture | 5 days | 13–46 × 106 cells/mL | Decreased heart rate | [140] | |
| Bloom samples | 15 days | 56.3–244 × 106 cells/mL | Up to 100% reduction in survival rates, altered hatching rate, reduced body length, yolk sac edema, decreased heart rate | |||
| Toxicity after oral exposure | Purified ANTX-a | 10 days | LC50 = 11.5 µg/g | Decreased survival at higher doses, no accumulation in tissues, and recovery after 24 h of exposure to lower doses | [141] | |
| Reproductive toxicity after chronic exposure | Purified MC-LR | 28 days | 1–5 µg/L | Liver glycogen storage loss and cellular damages, altered fecundity and hatching rate, induction of circadian-rhythm-related genes | [129] | |
| Crude extracts of M. aeruginosa PCC7806 | ||||||
| Survival and developmental toxicity | P. agardhii extract | 11 days | 10–50 × 10−3 mg/mL | Up to 81% reduction in survival rates, hepatobiliary abnormalities, altered hatching rate | [142] | |
| Chronic exposure effects | Purified MC-LR | 30 days | 5 µg/L | Histopathological modifications of the female and male gonads | [126] | |
| Zebrafish (Danio rerio) | Survival and developmental toxicity | Purified CYN—direct immersion | 5 days | Up to 50 µg/mL | No adverse effects observed | [96] |
| Purified CYN—microinjection | LC50 = 4.5 fmol/embryo | Up to ~15% reduction in survival rates | ||||
| Survival and developmental toxicity | Purified CYN | 96 hpf | 200–2000 nM | Reduction in survival rates, decreased heart rate | [143] | |
| 20–2000 nM | Reduction in body length, reduced eye size, pericardial edema, curved spine, tail deformity, uninflated swim bladder, altered hatching rate | |||||
| Survival and developmental toxicity | Purified MC-LR—microinjection | 48 hpf | 300–900 nM | Up to 82.5% decrease in survival rates, tail deformity, pericardial edema, blastomere coherence inhibition | [100] | |
| Survival and developmental toxicity | Purified CYN | 120 hpf | 500–2000 µg/L | Up to 40% decrease in survival rates, reduction in body length | [144] | |
| 10–2000 µg/L | Pericardial edema, yolk sac edema, swim bladder abnormalities | |||||
| Survival and developmental toxicity | Purified STX | 7 days | 481 ± 40 µg/L | Edema of the eyes, pericardial and yolk sac edema, swim bladder abnormalities, craniofacial deformities, decreased mobility | [116] | |
| Survival and developmental toxicity | Crude extracts | 48 h | 30–71 µg/mL dw | Decreased survival and developmental malformations | [145] | |
| Crude extracts of M. aeruginosa PCC7806 | 12 µg/mL dw | |||||
| Enzyme activity alteration | Purified MC-LR—direct immersion. | 24 h | 100 μg/L | Increased AChE activity (27%), increased ache gene expression (17%) | [115] | |
| Purified MC-LR—microinjection | No effect on AChE acitivity | |||||
| Developmental toxicity | Purified MC-LR | 30 days | 5 and 20 µg/L | Dose-dependent reduction in SOD, CAT, and GPx activities | [101] |
3. Bioassays with Invertebrate Animal Models
3.1. Artemia Salina Bioassay
3.1.1. Whole-Organism Responses—A. salina
3.1.2. Mediation of Cyanobacterial Toxicity—A. salina
3.2. Daphnia sp. Bioassays
3.2.1. Whole-Organism Responses—Daphnia
3.2.2. Mediation of Cyanobacterial Toxicity—Daphnia
3.3. Thamnocephalus Platyurus Bioassay
3.4. Chironomus Bioassays
| Model Organism | Observed Parameter | Sample Type | Possible Causative Agent | Exposure Duration | Effective Concentration of the Agent | Reference |
|---|---|---|---|---|---|---|
| Artemia salina | Survivorship | Crude extracts— Microcystis PCC-7813 | MC-LR | 18 h | ~1 mg/g dw | [156] |
| Bloom samples | / | 24 h | 0.5–5 mg/mL dw | [149] | ||
| Purified toxin | MC-RR | LC50 = 5 µg/mL | ||||
| Bloom samples | MC-LR, MC-RR, NOD | 22–24 h | LC50 = 3–17 mg/L | [157] | ||
| Filtered cultures | Anatoxin-a | LC50 = 2–14 mg/L | ||||
| Atypical movement | Fractionated extracts | Anatoxin-a | EC50 = 1–13 µg/L dw | |||
| Survivorship | Bloom samples | MC-LR | 24 h | LC50 = 0.47–2.44 mg dw/L | [132] | |
| Bloom samples | CYN | 48 h | LC50 = 2.8–3.4 ug Chl a/mL | [167] | ||
| C. raciborskii extracts | CYN | 48 h | LC50 (24 h) = 3.31–5.44 mg/mL dw LC50 (48 h) = 1.68–2.42 mg/mL dw | [164] | ||
| Purified toxin | CYN | LC50 (24 h) = 4.48 µg/mL LC50 (48 h) = 2.86 µg/mL | ||||
| Purified toxin | MC-LR | LC50 (24 h) = 4.58 µg/mL LC50 (48 h) = 2.8 µg/mL | ||||
| Purified toxin | MC-LR | 18 h | LC50 (24 h) = 3.75 µg/mL | [169] | ||
| Crude extract | / | 24 h | LC50 = 0.7–7.9 mg/mL dw | [216] | ||
| Toxin fraction (concentrated peptides) | / | LC50 = 6.8–12.9 mg/mL dw | ||||
| Purified toxin | MC-LR | 18 h | LC50 = 6.8 µg/mL | [170] | ||
| Crude extracts of M. aeruginosa | MC | LC50 = 0.8–33.58 mg/mL dw | ||||
| Crude extracts | / | 48 h | 700–6950 µg/mL dw | [145] | ||
| Crude extracts of M. aeruginosa PCC7806 | MC-LR | 81 ± 3 µg/mL dw | ||||
| Crude extracts | MC-LR | 24 h | EC50 = 6.8 ± 2 mg/mL dw | [207] | ||
| 48 h | EC50 = 6.8 ± 2 mg/mL dw | |||||
| Toxin fraction (concentrated peptides) | MC-LR | 24 h | EC50 = 3.5 ± 1.4 mg/mL dw | |||
| 48 h | EC50 = 2.2 ± 0.7 mg/mL dw | |||||
| Daphnia pulex | Survivorship | Purified toxin | MC-LR | 24 h | LC50 (24 h) > 3.32 mg/L | [192] |
| Purified toxin | ANTX-a | LC50 (24 h) > 1.66 mg/L | ||||
| Daphnia magna | M. aeruginosa 7820 cells—ingestion | MC-LR | 4 days | 3.5 × 107 cells/mL | [184] | |
| Daphnia pulex | Survivorship, growth, and reproduction | M. aeruginosa 7820 cells—ingestion | MC-LR | 21 days | 1 × 104–4 × 104 cells/mL | [183] |
| Daphnia longispina | ||||||
| Daphnia galeata | Survivorship | M. aeruginosa PCC7806 cells—ingestion | MC-LR | 5 days | / | [187] |
| Daphnia pulicaria | Molting disruption | Purified toxin | Microviridin J | 4 days | 6.75–12 mg/L | [217] |
| Ceriodaphnia dubia | Survivorship | C. raciborskii T2 | CYN | 7 days | 197.75 × 103–302.56 × 103 filaments/mL | [118] |
| C. raciborskii T3 | 0.218 × 103–5.101 × 103 filaments/mL | |||||
| Ceriodaphnia cornuta | Population growth | Crude extracts—Dolichospermum planctonicum | MC | 20 days | 0.1180–0.3760 µg/L dw | [203] |
| Daphnia similis | Survivorship | Bloom samples | MC | 24 h | LC50 = 186.61 mg/L | [137] |
| Ceriodaphnia silvestrii | LC50 = 155.11 mg/L | |||||
| Daphnia pulex | Crude extract | / | 24 h | LC50 = 0.5–9.2 mg/mL dw | [218] | |
| Toxin fraction (concentrated peptides) | / | LC50 = 2.01–6.06 mg/mL dw | ||||
| Daphnia magna | Cylindrospermopsis raciborskii cells—ingestion | CYN | 72 h | 1.8– 5 × 105 cells/mL | [196] | |
| Daphnia magna | Crude extract | MC-LR | 48 h | EC50 = 6.4 ± 2.3 mg/mL dw | [207] | |
| Toxin fraction (concentrated peptides) | EC50 = 5.5 ± 0.7 mg/mL dw | |||||
| Daphnia pulex | Crude extract | 24 h | EC50 = 1.1 ± 1.2 mg/mL dw | |||
| Toxin fraction (concentrated peptides) | EC50 = 1.1 ± 0.4 mg/mL dw | |||||
| Ceriodaphnia dubia | Crude extract | 48 h | EC50 = 6.6 ± 2 mg/mL dw | |||
| Toxin fraction (concentrated peptides) | EC50 = 6.1 ± 0.4 mg/mL dw | |||||
| Daphnia magna | Crude extracts | / | 48 h | 26–75 µg/mL dw | [145] | |
| Crude extracts of M. aeruginosa PCC7806 | MC-LR | 8 µg/mL dw | ||||
| Daphnia magna | Survivorship, growth, maturation, time to first reproduction, and fecundity | Purified toxin | MC-LR | 2 months | 5–50 µg/L | [219] |
| MC in crude extracts | MC | 5–50 µg/L dw | ||||
| Chironomus riparius | Survivorship | Trichormus variabilis cells—ingestion | MC-LR | 12 days | / | [213] |
| Oxidative stress induction, DNA damage | 5–10 mg of biomass fed every 48 h | |||||
| Survivorship, larval mass reduction, hemoglobin concentration, DNA damage | Purified toxin | MC-LR | 48 h | 0.01 mg/L | [214] | |
| Survivorship | Crude extracts—Plankthothrix agardhii | MC-LR | 96 h | 0.42–0.91 mg MC-LR/L dw | [215] | |
| Purified toxin | MC-LR | 1.66–3.32 mg/L | ||||
| Crude extracts—Dolichospermum lemmermannii | ANTX-a | 96 h | 0.12–0.35 mg/L dw | |||
| Purified toxin | ANTX-a | 3.32 mg ANTX-a/L | ||||
| Thamnocephalus platyurus | Survivorship | Crude extracts—Microcystis aeruginosa | / | 24 h | 0.5–5 mg/mL dw | [205] |
| Crude extracts—Anabaeanaflos-aquae | / | 0.3–5 mg/mL dw | ||||
| Crude extracts—Cylindrospemopsis raciborskii | / | 1–5 mg/mL dw | ||||
| Crude extracts—Aphanizomenon flos-aquae | / | 3–5 mg/mL dw | ||||
| Purified toxin | MC-LR | 24 h | LC50 = 1.8 mg/L | [138] | ||
| Crude extracts | MC-LR | 24 h | LC50 = 0.11 ± 0.3 mg/mL dw | [207] | ||
| Toxin fraction (concentrated peptides) | MC-LR | LC50 = 0.31 ± 0.05 mg/mL dw |
4. In Vitro Bioassays
| Target Organ/System | Cell Line | Applied Assay | Sample Type | Exposure Duration | Effective Concentration | Observed Effect | Reference |
|---|---|---|---|---|---|---|---|
| Liver | Rainbow trout liver cell line RTL-W1 | Alamar Blue (AB) assay | MC-LR | 48 h | EC50 > 2.5 μM | No effect on cell viability at moderate (0.25 μM) and high (2.5 μM) MC-LR concentrations | [62] |
| CFDA-AM assay | |||||||
| Neutral red (NR) assay | |||||||
| Alamar Blue (AB) assay | Phormidium extracts (five species) showing symptoms of neuro- and hepatotoxicity in mice | 24 h | 0.75, 3.75, and 15 mg/mL dw | Little to no effect on cell viability | [244] | ||
| CFDA-AM assay | |||||||
| MTT colorimetric assay | Crude cyanobacterial extracts | 24 h | 4, 100, 400, and 2000 μg/mL dw | Little to no effect on cell viability | [145] | ||
| MC-producing Microcystis PCC 7806 strain extract | IC50 = 109.16 μg/mL dw | Decrease in cell viability | |||||
| Human hepatocellular carcinoma cell line HepG2 | Alamar Blue (AB) assay | MC-LR | 48 h | EC50 > 2.5 μM | No effect on cell viability at moderate (0.25 μM) and high (2.5 μM) MC-LR concentrations | [62] | |
| CFDA-AM assay | |||||||
| Neutral red (NR) assay | |||||||
| MTT colorimetric assay | Microcystis bloom sample extract | 72 h | IC50 (24 h) = 214.8 μg/mL dw IC50 (72 h) = 211.5 μg/mL dw | Decrease in cell viability | [247] | ||
| Purified microginins | 25–100 µg/mL | Up to 42% decrease in cell viability | |||||
| Tetrazolium salt reduction—MTS assay | Purified CYN | 48 h | EC50 = 3.24 ± 0.73 μg/mL | Cytotoxicity/decrease in cellular viability | [228] | ||
| Purified MC-LR | EC50 = 84.18 ± 4.42 μg/mL | ||||||
| Total protein content—TP assay | Purified CYN | 48 h | EC50 = 3.47 ± 0.41 μg/mL | Cytotoxicity/decrease in cellular viability | |||
| Purified MC-LR | EC50 = 88.02 ± 1.34 μg/mL | ||||||
| MTT colorimetric assay | Purified CYN | 24 h | 1–5 µg/mL | Up to ~50% decrease in cell viability | [230] | ||
| Comet assay | 0.01–5 µg/mL | DNA damage | |||||
| MTT colorimetric assay | Crude cyanobacterial methanolic extracts | - | IC50 = 9–41 µg/mL dw | Strong cytotoxicity | [248] | ||
| MTT colorimetric assay | Crude cyanobacterial extracts | 72 h | EC50 = 49–396 μg/mL dw | Decrease in cell viability | [232] | ||
| MTT colorimetric assay | Crude cyanobacterial methanolic extracts | 24 h | 35–702 μg/mL dw | Decrease in cell viability | [145] | ||
| MTT colorimetric assay | Crude cyanobacterial extracts | 24 h | 0.04–2 mg/mL dw | Decrease in cell viability | [249] | ||
| Neutral red (NR) assay | Purified MC-LR | 24 h | EC50 = 44 µM | Decrease in cell viability | [250] | ||
| M. aeruginosa extract | EC50 = 27 µM dw | ||||||
| Tetrazolium salt reduction—MTS assay | Purified CYN | 72 h | 0.1–5 µg/mL | Concentration-dependent inhibition of cell proliferation | [251] | ||
| Comet assay | Crude extracts of MC-LR containing cyanobacterial blooms | 24 h | 500 µg/mL dw | A low level of DNA damage | [233] | ||
| 48 h | 50, 125, and 500 µg/mL dw | Total damage of DNA, total mortality even at low concentrations | |||||
| MTT colorimetric assay | Crude cyanobacterial aquatic extracts | 24 h | 1:10 (v/v) dilution | >60% of viable cells in most of the cases | [252] | ||
| Methanolic extracts of two Jaaginema strains containing no cyanotoxins | 48 h | 1:10 and 1:50 (v/v) dilutions | <10% of viable cells | ||||
| Human epithelial-like liver adenocarcinoma cells SK-Hep-1 | MTT colorimetric assay | Fischerella major extracts containing microcystins and saxitoxins | 72 h | IC50 = 32.4–>100 µg/mL dw | Strong cytotoxic effects | [234] | |
| Neutral red (NR) assay | 10, 50, and 100 μg/mL dw | Toxic effects significantly decreased after 48 and 72 h | |||||
| Human hepatocellular carcinoma cell line HuH-7 | MTT colorimetric assay | Crude aquatic cyanobacterial extracts | 24 h | 1:10 (v/v) dilutions | >70% of viable cells in almost all cases | [253] | |
| MTT colorimetric assay | Crude cyanobacterial extracts | 48 h | IC50 ≥ 1250 μg/mL dw | Decrease in cell viability | [253] | ||
| Human hepatoma cell line Hep3B | MTT colorimetric assay | Crude cyanobacterial methanolic extracts | 24 h | IC50 = 245.93–296.15 μg/mL dw | Decrease in cell viability | [238] | |
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Cytotoxicity up to 40% | ||||
| Human liver stem cells HL1-hT1 (monolayer cultures) | Alamar Blue (AB) assay | MC-LR | 96 h | EC50 > 10 μM | No cytotoxic effects | [246] | |
| CFDA-AM assay | |||||||
| Neutral red (NR) assay | |||||||
| Alamar Blue (AB) assay | CYN | 96 h | EC50 = 0.61 μM | Inhibition of cell growth and viability | |||
| CFDA-AM assay | EC50 = 2.91 μM | ||||||
| Neutral red (NR) assay | EC50 = 0.75 μM | ||||||
| Primary fish (rainbow trout) hepatocytes | Alamar Blue (AB) assay | MC-LR | 48 h | 250 nM | Decrease in cell viability to ~70% | [62] | |
| CFDA-AM assay | No effect on cell viability | ||||||
| Neutral red (NR) assay | Decrease in cell viability to ~30% | ||||||
| Primary mouse hepatocytes | Alamar Blue (AB) assay | MC-LR | 48 h | 25 nM | Decrease in cell viability to ~20% | ||
| CFDA-AM assay | No effect on cell viability | ||||||
| Neutral red (NR) assay | Decrease in cell viability to ~20% | ||||||
| Kidney | Human kidney cells HEK293 | Tetrazolium salt reduction—MTS assay | Purified CYN | 48 h | 2.5–25 µg/mL | Up to 40% decrease in cell viability | [254] |
| Purified MC-LR | 48 h | 50–200 µg/mL | Up to 20% decrease in cell viability | ||||
| African green monkey kidney cell line—Vero | MTT colorimetric assay | Purified MC-LR | 72 h | 25–200 µM | Cytotoxicity/decrease in cell viability | [255] | |
| Lactate dehydrogenase (LDH) assay | 100–200 µM | ||||||
| Neutral red (NR) assay | 12.5–200 µM | ||||||
| MTT colorimetric assay | M. aeruginosa extract | 11–175 µM | Cytotoxicity/decrease in cell viability | ||||
| Lactate dehydrogenase (LDH) assay | 22–175 µM | ||||||
| Neutral red (NR) assay | Purified MC-LR | 24 h | EC50 = 53 µM | Decrease in cell viability | [250] | ||
| M. aeruginosa extract | EC50 = 34 µM | ||||||
| MTT colorimetric assay | Fractions and subfractions of the cyanobacterial bloom containing MC-LR extract | 72 h | LC50 = 40–>200 µg/mL dw | Cytotoxic effects | [233] | ||
| MTT colorimetric assay | Crude cyanobacterial extracts showing prominent cytotoxicity on other cell lines | 48 h | 1:10 (v/v) dilution | >60% of viable cells | [249] | ||
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 144.97–353.95 μg/mL dw | Decrease in cell viability | [238] | ||
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Cytotoxicity up to ~60% | ||||
| MTT colorimetric assay | Crude cyanobacterial extracts | 48 h | IC50 ≥ 625 μg/mL dw | Decrease in cell viability | [253] | ||
| Colon | Human colon carcinoma cells Caco-2 | MTT colorimetric assay | Crude cyanobacterial extracts | 72 h | EC50 = 58–640 µg/mL dw | Decrease in cell viability | [232] |
| Total protein content—TP assay | Purified CYN | 48 h | EC50 (24 h) = 36.5 ± 2.1 µg/mL EC50 (48 h) = 2.0 ± 0.5 µg/mL | Time/concentration dependent reduction in protein content | [256] | ||
| Neutral red (NR) assay | EC50 (24 h) = 19.0 ± 1.3 µg/mL EC50 (48 h) = 10.0 ± 1.7 µg/mL | Up to 45% decrease in cell viability | |||||
| Tetrazolium salt reduction—MTS assay | EC50 (24 h) = 2.5 ± 0.4 µg/mL EC50 (48 h) = 0.6 ± 0.2 µg/mL | Decrease in cell viability | |||||
| Tetrazolium salt reduction—MTS assay | Purified CYN | 72 h | 0.1–5 µg/mL | Concentration-dependent inhibition of cell proliferation | [251] | ||
| MTT colorimetric assay | Purified MC-LR | 72 h | 50 µM | Up to 90% decrease in cell viability | [76] | ||
| Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay | 120 min | Significant increase in H2O2 levels at 30 min before returning to normal at 120 min | |||||
| MTT colorimetric assay | Purified MC-LR | 48 h | 10 µg/mL | Up to 40% decrease in cell viability | [257] | ||
| Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay | 5 h | 0.2–10 µg/mL | Intracellular ROS formation | ||||
| Comet assay | 16 h | 0.2–10 µg/mL | DNA damage | ||||
| MTT colorimetric assay | Crude aquatic cyanobacterial extracts | 24 h | 1:10 (v/v) dilution | >60% of viable cells | [252] | ||
| Methanolic extracts of two Jaaginema strains containing no cyanotoxins | 48 h | 1:10 and 1:50 (v/v) dilutions | <5% of viable cells | ||||
| Human colorectal carcinoma cell line HCT-116 | MTT colorimetric assay | Crude methanolic cyanobacterial extracts | - | IC50 = 8–27 µg/mL dw | Cytotoxicity | [248] | |
| Human colorectal adenocarcinoma cell line HT-29 | MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 180.82–386.73 μg/mL dw | Decrease in cell viability | [238] | |
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Cytotoxicity up to ~80% | ||||
| Lungs (respiratory system) | Human fetal lung cell line MRC-5 | Colorimetric sulforhodamine B (SRB) assay | Water samples from blooming lakes | 48 h | Raw sample diluted to 10% | Up to ~30% decrease in cell viability | [258] |
| Human lung adenocarcinoma cell line A549 | MTT colorimetric assay | Microcystin- and saxitoxin-producing Nostoc microscopicum (acetic acid extract) | 24 h | IC50 = 173 μg/mL dw | Prominent cytotoxic activity | [235] | |
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 284.20–407.95 μg/mL dw | Decrease in cell viability | [238] | ||
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Low cytotoxicity at all concentrations (~10–20%) | ||||
| Endothelium | Human umbilical vein endothelial cell line HUVEC | Total protein content—TP assay | Purified CYN | 48 h | EC50 (24 h) = 8.5 ± 1.2 µg/mL EC50 (48 h) = 1.5 ± 0.6 µg/mL | Time/concentration-dependent reduction in protein content | [259] |
| Neutral red (NR) assay | EC50 (24 h) = 1.5 ± 0.9 µg/mL EC50 (48 h) = 0.8 ± 0.5 µg/mL | Decrease in cell viability | |||||
| Tetrazolium salt reduction—MTS assay | EC50 (24 h) = 15.5 ± 2.1 µg/mL EC50 (48 h) = 1.5 ± 0.3 µg/mL | ||||||
| Blood | Human peripheral blood mononuclear cells (PBMCs) | MTT colorimetric assay | Crude cyanobacterial extracts | 72 h | EC50 = 28–991 µg/mL dw | Decrease in cell viability | [232] |
| Human peripheral blood lymphocytes (HPBL) | Differential staining (acridine orange and ethidium bromide) | Purified MC-LR | 24 h | 0.1–10 µg/mL | No effect on cell viability | [260] | |
| Comet assay | DNA damage | ||||||
| Comet assay | Purified MC-LR | 24 h | 1–25 µg/mL | DNA damage | [261] | ||
| Human leukemia cell line HL-60 | Lactate dehydrogenase (LDH) assay | Crude methanolic cyanobacterial extracts | 3 h | 20, 100, and 200 μg/mL dw | Cytotoxicity up to 100% | [262] | |
| Human promonocytic cells U-937 | MTT colorimetric assay | Fractions and subfractions of the cyanobacterial bloom containing MC-LR extract | 72 h | LC50 = 17–>200 µg/mL dw | Cytotoxic effects | [233] | |
| Mouse monocytic cells J774 | LC50 = 75–>200 µg/mL dw | ||||||
| Nervous system | Mouse neuroblastoma—Neuro-2a | Colorimetric sulforhodamine B (SRB) assay | Water samples from blooming lakes | 48 h | Raw sample diluted to 10% | Up to ~20% decrease in cell viability | [258] |
| Rat glioma cell line C6 | MTT colorimetric assay | Crude methanolic extracts | 24 h | IC50 = 112.69–164.90 μg/mL dw | Decrease in cell viability | [238] | |
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Cytotoxicity up to ~80% | ||||
| Reproductive system | Human breast cancer cell line MCF-7 | MTT colorimetric assay | Crude cyanobacterial extracts | 72 h | EC50 = 15–361 µg/mL dw | Decrease in cell viability | [232] |
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | - | IC50 = 11–38 µg/mL dw | Decrease in cell viability | [248] | ||
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 133.16–189.45 μg/mL dw | Decrease in cell viability | [238] | ||
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Cytotoxicity up to ~40% | ||||
| Colorimetric sulforhodamine B (SRB) assay | Geitlerinema sp. CNP 1019 strain extract | 48 h | GI50 = 25.7 μg/mL dw | Cytotoxicity | [263] | ||
| Human prostate cancer cell line PC3 | MTT colorimetric assay | Crude cyanobacterial extracts | 72 h | EC50 = 44–339 µg/mL dw | Decrease in cell viability | [232] | |
| Human cervical adenocarcinoma cell line HeLa | MTT colorimetric assay | Microcystins and saxitoxin-producing Nostoc microscopicum (acetic acid extract) | 24 h | IC50 = 270 μg/mL dw | Prominent cytotoxic activity | [235] | |
| MTT colorimetric assay | Fractions and subfractions of the cyanobacterial bloom containing MC-LR extract | 72 h | LC50 = 109.5–>200 µg/mL dw | Cytotoxic effects | [233] | ||
| MTT colorimetric assay | Fischerella major extracts containing microcystins and saxitoxins | 72 h | IC50 = 27–59 µg/mL dw | Strong cytotoxic effects | [234] | ||
| Neutral red (NR) assay | IC50 = 34–95 µg/mL dw | Toxic effects | |||||
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 151.36–209.43 μg/mL dw | Decrease in cell viability | [238] | ||
| Lactate dehydrogenase (LDH) assay | - | 15, 30, 60, and 120 μg/mL dw | Low cytotoxicity at all concentrations (~10–20%) | ||||
| MTT colorimetric assay | Crude methanolic cyanobacterial extracts | 24 h | IC50 = 0.2–>20 mg/mL dw (determined only for selected strains) | 20% of tested extracts exhibited strong cytotoxicity | [236] | ||
| Human normal amniotic cells FL | MTT colorimetric assay | Microcystin- and saxitoxin-producing Nostoc microscopicum (acetic acid extract) | 24 h | IC50 = 253 μg/mL dw | Prominent cytotoxicity | [235] | |
| MTT colorimetric assay | Fischerella major extracts containing microcystins and saxitoxins | 72 h | IC50 = 29–62 μg/mL dw | Strong cytotoxic effects | [234] | ||
| Neutral red (NR) assay | 10, 50, and 100 μg/mL dw | Toxic effects were detected after 24 h, while the cells were able to overcome these effects after 48 and 72 h | |||||
| MTT colorimetric assay | Phormidium extracts (five species) showing symptoms of neuro- and hepatotoxicity in mice | 24 h | 15 mg/mL dw | Decrease in cell viability over 50%, up to ~20% | [244] | ||
| Others | Human dermal fibroblasts (HDF cells) | Tetrazolium salt reduction—MTS assay | Purified CYN | 48 h | IC50 > 5 µg/mL | Concentration-dependent inhibition of cell proliferation | [251] |
| Lactate dehydrogenase (LDH) assay | 72 h | 0.1–5 µg/mL | Cytotoxicity reached only 30% at concentrations above 1 μg/mL | ||||
| Mouse embryonic fibroblast cell line 3T3 | Lactate dehydrogenase (LDH) assay | Crude methanolic cyanobacterial extracts | 3 h | 20, 100, and 200 μg/mL dw | Cytotoxicity up to 100% | [262] | |
| MTT colorimetric assay | Phormidium extracts (five species) showing symptoms of neuro- and hepatotoxicity in mice | 24 h | 15 mg/mL dw | Decrease in cell viability ~50% | [244] | ||
| Human oral cell line KB | Colorimetric sulforhodamine B (SRB) assay | Geitlerinema sp. CNP 1019 strain extract | 48 h | GI50 = 60.1 μg/mL dw | Cytotoxicity | [263] | |
| Human metastatic melanoma cell line A2058 | MTT colorimetric assay | Phormidium extracts (five species) showing symptoms of neuro- and hepatotoxicity in mice | 24 h | 15 mg/mL dw | Decrease in cell viability over 50%, up to ~20% | [244] | |
| Human embryonic rhabdomyosarcoma cell line RD |
5. Limitations and Challenges in Cyanobacterial Toxicity Testing
6. Tracking the Evolution of Bioassays for Cyanotoxin Testing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Simeunović, J.; Svirčev, Z.; Krstić, S.; Lazić, L. Occurrence of Cyanobacterial Blooms in Vojvodina Water Ecosystems. Geogr. Pannonica 2005, 9, 13–19. [Google Scholar] [CrossRef]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First Report of Homoanatoxin-a and Associated Dog Neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human Exposure to Cyanotoxins and Their Effects on Health. Arh. Hig. Rada Toksikol. 2013, 64, 305–316. [Google Scholar] [CrossRef]
- Svirčev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular Aspects of Microcystin-Induced Hepatotoxicity and Hepatocarcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 37–41. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and Potential Carcinogenicity of Cyanobacterial Toxins—A Review. Mutat. Res. Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Szlag, D.C.; Sinclair, J.L.; Southwell, B.; Westrick, J.A. Cyanobacteria and Cyanotoxins Occurrence and Removal from Five High-Risk Conventional Treatment Drinking Water Plants. Toxins 2015, 7, 2198–2220. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of Knowledge and Concerns on Cyanobacterial Blooms and Cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological Effects of Selected Cyanobacterial Secondary Metabolites a Short Review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Meriluoto, J.; Spoof, L.; Codd, G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Chichester, West Sussex, UK, 2017. [Google Scholar]
- Rinehart, K.L.; Harada, K.; Namikoshi, M.; Chen, C.; Harvis, C.A.; Munro, M.H.G.; Blunt, J.W.; Mulligan, P.E.; Beasley, V.R.; Dahlem, A.M. Nodularin, microcystin, and the configuration of Adda. J. Am. Chem. Soc. 1988, 110, 8557–8558. [Google Scholar] [CrossRef]
- Greer, B.; Meneely, J.P.; Elliott, C.T. Uptake and Accumulation of Microcystin-LR Based on Exposure through Drinking Water: An Animal Model Assessing the Human Health Risk. Sci. Rep. 2018, 8, 4913. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins Noureddine. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Merwe, D. Freshwater Cyanotoxins; Academic Press: Cambridge, MA, USA, 2014; pp. 539–548. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G.J. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management; Elsevier: Amsterdam, The Netherlands, 1999; pp. 43–112. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial Microcystin-LR Is a Potent and Specific Inhibitor of Protein Phosphatases 1 and 2A from Both Mammals and Higher Plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R. Cyanobacteria-Toxins in Drinking Water. Encycl. Environ. Microbiol. 2003, 14, 5–12. [Google Scholar] [CrossRef]
- Vasconcelos, V. Cyanobacteria toxins: Diversity and ecological effects. Limnetica 2001, 20, 45–58. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular Mechanisms of Microcystin Toxicity in Animal Cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Moore, R.E. Cylindrospermopsin: A Potent Hepatotoxin from the Blue-Green Alga Cylindrospermopsis raciborskii. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Runnegar, M.T.C.; Jackson, A.R.B.; Falconer, I.R. Severe Hepatotoxicity Caused by the Tropical Cyanobacterium Supply Reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I.R. Cylindrospermopsin Genotoxicity and Cytotoxicity: Role of Cytochrome P-450 and Oxidative Stress. J. Toxicol. Environ. Health Part A 2005, 68, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Ge, J.-L.; Lu, S.C. The Role of Glutathione in the Toxicity of a Novel Cyanobacterial Alkaloid Cylindrospermopsin in Cultured Rat Hepatocytes. Biochem. Biophys. Res. Commun. 1994, 201, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-Induced Protein Synthesis Inhibition and Its Dissociation from Acute Toxicity in Mouse Hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, Re-Naming a Cyanobacterial Organophosphate Toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of Anatoxin-a Poisoning in Dogs from North America. J. Vet. Diagn. Investig. 2008, 20, 89–92. [Google Scholar] [CrossRef]
- Puschner, B.; Pratt, C.; Tor, E.R. Treatment and Diagnosis of a Dog with Fulminant Neurological Deterioration Due to Anatoxin-a Intoxication. J. Vet. Emerg. Crit. Care 2010, 20, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.P.; Edwards, E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a Toxic Alkaloid from Anabaena Flos-Aquae NRC-44h1. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Hyde, E.G.; Carmichael, W.W. Anatoxin-A (S), a Naturally Occurring. J. Biochem. Toxicol. 1991, 6, 195–201. [Google Scholar] [CrossRef]
- Falconer, I.R. Algal Toxins and Human Health; Springer: Berlin/Heidelberg, Germany, 1998; Volume 5, pp. 53–82. [Google Scholar] [CrossRef]
- Sarma, T.A. Handbook of Cyanobacteria; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781466559417. [Google Scholar]
- Solter, P.F.; Beasley, V.R. Phycotoxins. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780124157590. [Google Scholar]
- Adelman, W.J.J.; Fohlmeister, J.F.; Sasner, J.J.; Ikawa, M. Sodium Channels Blocked by Aphantoxin Obtained from the Blue-Green Alga, Aphanizomenon flos-aquae. Toxicon 1982, 20, 513–516. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotox. Res. 2018, 33, 168–177. [Google Scholar] [CrossRef]
- Evans, M.H. Tetrodotoxin, Saxitoxin, and Related Substances: Their Applications in Neurobiology. Int. Rev. Neurobiol. 1972, 15, 83–166. [Google Scholar] [CrossRef]
- Kao, C.Y. Paralytic Shellfish Poisoning. In Algal Toxins in Seafood and Drinking Water; Falconer, I.R., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1993; pp. 75–86. ISBN 0122479904. [Google Scholar]
- Ferrão-filho, A.S.; Kozlowsky-suzuki, B. Cyanotoxins: Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Frigg, R.; Hartmann, S. Models in Science. In The Stanfod Encyclopedia of Philosophy; Zalta, E.N., Ed.; Metaphysics Research Lab; Stanford University: Stanford, CA, USA, 2020. [Google Scholar]
- Hassan, S.; Van Ginkel, S.W.; Hussein, M.; Abskharon, R.; Oh, S.-E. Toxicity Assessment Using Different Bioassays and Microbial Biosensors. Environ. Int. 2016, 92–93, 106–118. [Google Scholar] [CrossRef]
- Gallagher, M.E. Toxicity Testing Requirements, Methods and Proposed Alternatives. Environs 2002, 26, 253–274. [Google Scholar]
- Erzinger, G.S. Bioassays: Advanced Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Freitas, E.C.; Pinheiro, C.; Rocha, O.; Loureiro, S. Can Mixtures of Cyanotoxins Represent a Risk to the Zooplankton? The Case Study of Daphnia magna Straus Exposed to Hepatotoxic and Neurotoxic Cyanobacterial Extracts. Harmful Algae 2014, 31, 143–152. [Google Scholar] [CrossRef]
- Codd, G.A.; Poon, G.K. Cyanobacterial Toxins. Proc. Phytochem. Soc. Eur. 1988, 28, 283–296. [Google Scholar]
- Carmichael, W.W.; Mahmood, N.A.; Hyde, E.G. Natural Toxins from Cyanobacteria (Blue-Green Algae). In Marine Toxins: Origin, Structure, and Molecular Pharmacology; ACS Publications: Washington, DC, USA, 1990; Volume 418, pp. 87–106. [Google Scholar]
- Nicholson, B.; Burch, M. Evaluation of Analytical Methods for the Detection and Quantification of Cyanotoxins in Relation to Australian Drinking Water Guidelines; National Health and Medical Research Council of Australia: Canberra, Australia, 2001.
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; Volume 22, pp. 41–111. ISBN 0419239308. [Google Scholar]
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; EUR 28624; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Berry, J. Cyanobacterial Toxins in Food-Webs: Implications for Human and Environmental Health. In Current Topics in Public Health; Rodriguez-Morales, A.J., Ed.; Intech Open: Rijeka, Croatia, 2013; pp. 531–589. [Google Scholar]
- Carbis, C.R.; Rawlin, G.T.; Mitchell, G.F.; Anderson, J.W. The Histopathology of Carp, Cyprinus carpio L., Exposed to Microcystins by Gavage, Immersion and Intraperitoneal Administration. J. Fish Dis. 1996, 19, 199–207. [Google Scholar] [CrossRef]
- Tencalla, F.G.; Dietrich, D.R.; Schlatter, C. Toxicity of Microcystis aeruginosa Peptide Toxin to Yearling Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 1994, 30, 215–224. [Google Scholar] [CrossRef]
- Azevedo, S.M.F.O.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. First Report of Microcystins from a Brazilian Isolate of the Cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 1994, 6, 261–265. [Google Scholar] [CrossRef]
- Vasconcelos, V.M.; Sivonen, K.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. Hepatotoxic Microcystin Diversity in Cyanobacterial Blooms Collected in Portuguese Freshwaters. Water Res. 1996, 30, 2377–2384. [Google Scholar] [CrossRef]
- Vieira, J.M.D.S.; Azevedo, M.T.D.P.; De Oliveira Azevedo, S.M.F.; Honda, R.Y.; Corrêa, B. Toxic Cyanobacteria and Microcystin Concentrations in a Public Water Supply Reservoir in the Brazilian Amazonia Region. Toxicon 2005, 45, 901–909. [Google Scholar] [CrossRef]
- Msagati, T.A.M.; Siame, B.A.; Shushu, D.D. Evaluation of Methods for the Isolation, Detection and Quantification of Cyanobacterial Hepatotoxins. Aquat. Toxicol. 2006, 78, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Makita, Y.; Nagata, S.; Tsutsumi, T.; Yoshida, F.; Sekijima, M.; Tamura, S.I.; Ueno, Y. Acute Oral Toxicity of Microcystin-LR, a Cyanobacterial Hepatotoxin, in Mice. Nat. Toxins 1997, 5, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The Toxicity of Cyanobacterial Toxins in the Mouse; II Anatoxin-A. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Miura, G.A.; Matson, C.F.; Dinterman, R.E.; Pace, J.G. Characterization of Chemically Tritiated Microcystin-LR and Its Distribution in Mice. Toxicon 1989, 27, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. [Google Scholar] [CrossRef]
- Lovell, R.A.; Schaeffer, D.J.; Hooser, S.B.; Haschek, W.M.; Dahlem, A.M.; Carmichael, W.W.; Beasley, V.R. Toxicity of Intraperitoneal Doses of Microcystin-LR in Two Strains of Male Mice. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 1989, 9, 221–237. [Google Scholar]
- Kuiper-Goodman, T.; Falconer, I.; Fitzgerald, J. Human Health Aspects. In Toxic Cyanobacteria in Water; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 133–174. ISBN 0419239308. [Google Scholar]
- Boaru, D.A.; Dragoş, N.; Schirmer, K. Microcystin-LR Induced Cellular Effects in Mammalian and Fish Primary Hepatocyte Cultures and Cell Lines: A Comparative Study. Toxicology 2006, 218, 134–148. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Falconer, I.R.; Silver, J. Deformation of Isolated Rat Hepatocytes by a Peptide Hepatotoxin from the Blue-Green Alga Microcystis aeruginosa. Naunyn. Schmiedebergs. Arch. Pharmacol. 1981, 317, 268–272. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V.L. Comparative Toxicity Evaluation of Cyanobacterial Cyclic Peptide Toxin Microcystin Variants (LR, RR, YR) in Mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health Risks Caused by Freshwater Cyanobacteria in Recreational Waters. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 323–347. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Namikoshi, M.; Choi, B.W. Structure and Biosynthesis of Toxins from Blue-Green Algae (Cyanobacteria). J. Appl. Phycol. 1994, 6, 159–176. [Google Scholar] [CrossRef]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron Microscopic Studies on Experimental Poisoning in Mice Induced by Cylindrospermopsin Isolated from Blue-Green Alga Umezakia Natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Xie, C.; Snider, B.B.; Wallace, G.A.; Weinreb, S.M.; Kuhlenkamp, J. In Vitro Hepatotoxicity of the Cyanobacterial Alkaloid Cyclindrospermopsin and Related Synthetic Analogues. Toxicol. Sci. 2002, 67, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Hardy, S.J.; Humpage, A.R.; Froscio, S.M.; Tozer, G.J.; Hawkins, P.R. Hepatic and Renal Toxicity of the Blue-Green Alga (Cyanobacterium) Cylindrospermopsis raciborskii in Male Swiss Albino Mice. Environ. Toxicol. 1999, 14, 143–150. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus Induction and Chromosome Loss in Transformed Human White Cells Indicate Clastogenic and Aneugenic Action of the Cyanobacterial Toxin, Cylindrospermopsin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 472, 155–161. [Google Scholar] [CrossRef]
- Shaw, G.R.; Seawright, A.A.; Lam, P.S. Cylindrospermopsin, a Cyanobacterial Alkaloid: Evaluation of Its Toxicologic Activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute Toxicity of Dihydroanatoxin-a from Microcoleus autumnalis in Comparison to Anatoxin-A. Chemosphere 2021, 263, 127937. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards Clarification of the Biological Role of Microcystins, a Family of Cyanobacterial Toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Lawrence, C.; Adatto, I.; Best, J.; James, A.; Maloney, K. Generation Time of Zebrafish (Danio rerio) and Medakas (Oryzias latipes) Housed in the Same Aquaculture Facility. Lab Anim. 2012, 41, 158–165. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think Small: Zebrafish as a Model System of Human Pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; Van De Venter, M.; Shephard, E.G. The Role of Microcystin-LR in the Induction of Apoptosis and Oxidative Stress in CaCo2 Cells. Toxicon 2004, 43, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.S.; Guha, S. Microcystin Toxicity in a Freshwater Fish, Heteropneustes fossilis (Bloch). Curr. Sci. 2006, 91, 1261–1271. [Google Scholar]
- Lin, W.; Hou, J.; Guo, H.; Li, L.; Wang, L.; Zhang, D. The Synergistic Effects of Waterborne Microcystin-LR and Nitrite on Hepatic Pathological Damage, Lipid Peroxidation and Antioxidant Responses of Male Zebrafish. Environ. Pollut. 2018, 235, 197–206. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Poirier, D.G.; Ortiz Almirall, X.; Bhavsar, S.P.; Sibley, P.K. Assessing the Toxicity of Cell-Bound Microcystins on Freshwater Pelagic and Benthic Invertebrates. Ecotoxicol. Environ. Saf. 2020, 188, 109945. [Google Scholar] [CrossRef]
- Arukwe, A.; Goksøyr, A. Eggshell and Egg Yolk Proteins in Fish: Hepatic Proteins for the next Generation: Oogenetic, Population, and Evolutionary Implications of Endocrine Disruption. Comp. Hepatol. 2003, 2, 4. [Google Scholar] [CrossRef]
- Michael, A.S.; Thomps, C.G.; Abraliov, M. Artemia salina as a Test Organism for Bioassay. Science 1956, 123, 464. [Google Scholar] [CrossRef] [PubMed]
- Fournie, J.W.; Courtney, L.A. Histopathological Evidence of Regeneration Following Hepatotoxic Effects of the Cyanotoxin Microcystin-LR in the Hardhead Catfish and Gulf Killifish. J. Aquat. Anim. Health 2002, 14, 273–280. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Song, L. Cytological Alterations in Isolated Hepatocytes from Common Carp (Cyprinus carpio L.) Exposed to Microcystin-LR. Environ. Toxicol. 2001, 16, 517–522. [Google Scholar] [CrossRef]
- Fischer, W.J.; Dietrich, D.R. Pathological and Biochemical Characterization of Microcystin-Induced Hepatopancreas and Kidney Damage in Carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000, 164, 73–81. [Google Scholar] [CrossRef]
- Arman, T.; Clarke, J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef]
- Oberemm, A.; Fastner, J.; Steinberg, C.E.W. Effects of Microcystin-LR and Cyanobacterial Crude Extracts on Embryo-Larval Development of Zebrafish (Danio rerio). Water Res. 1997, 31, 2918–2921. [Google Scholar] [CrossRef]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of Cyanobacterial Toxins and Aqueous Crude Extracts of Cyanobacteria on the Development of Fish and Amphibians. Environ. Toxicol. 1999, 14, 77–88. [Google Scholar] [CrossRef]
- Black, J.J.; Maccubbin, A.E.; Schiffert, M. A Reliable, Efficient, Microinjection Apparatus and Methodology for the in Vivo Exposure of Rainbow Trout and Salmon Embryos to Chemical Carcinogens. J. Natl. Cancer Inst. 1985, 6, 1123–1128. [Google Scholar]
- Achenbach, J.C.; Leggiadro, C.; Sperker, S.A.; Woodland, C.; Ellis, L.D. Comparison of the zebrafish embryo toxicity assay and the General and Behavioral Embryo Toxicity Chemical Screening. Toxics 2020, 8, 126. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Sutryk, K.; Hebel, A.; Hohlfeld, N.; Pietrasik, A.; Błaszczyk, A. Nodularia spumigena Peptides-Accumulation and Effect on Aquatic Invertebrates. Toxins 2015, 7, 4404–4420. [Google Scholar] [CrossRef] [PubMed]
- Roegner, A.; Truong, L.; Weirich, C.; Pírez-Schirmer, M.; Brena, B.; Miller, T.R.; Tanguay, R. Combined Danio rerio Embryo Morbidity, Mortality and Photomotor Response Assay: A Tool for Developmental Risk Assessment from Chronic CyanoHAB Exposure. Sci. Total Environ. 2019, 697, 134210. [Google Scholar] [CrossRef]
- Sandrini, J.Z.; Bianchini, A.; Trindade, G.S.; Nery, L.E.M.; Marins, L.F.F. Reactive Oxygen Species Generation and Expression of DNA Repair-Related Genes after Copper Exposure in Zebrafish (Danio rerio) ZFL Cells. Aquat. Toxicol. 2009, 95, 285–291. [Google Scholar] [CrossRef]
- Langheinrich, U. Zebrafish: A New Model on the Pharmaceutical Catwalk. BioEssays 2003, 25, 904–912. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; Mclaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 2–8. [Google Scholar] [CrossRef]
- Berry, J.P.; Gibbs, P.D.L.; Schmale, M.C.; Saker, M.L. Toxicity of Cylindrospermopsin, and Other Apparent Metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the Zebrafish (Danio rerio) Embryo. Toxicon 2009, 53, 289–299. [Google Scholar] [CrossRef] [PubMed]
- El Ghazali, I.; Saqrane, S.; Carvalho, A.P.; Ouahid, Y. Compensatory Growth Induced in Zebrafish Larvae after Pre-Exposure to a Microcystis aeruginosa Natural Bloom Extract Containing Microcystins. Int. J. Mol. Sci. 2009, 10, 133–146. [Google Scholar] [CrossRef]
- Lindsay, J.; Metcalf, J.S.; Codd, G.A. Protection against the Toxicity of Microcystin-LR and Cylindrospermopsin in Artemia salina and Daphnia Spp. by Pre-Treatment with Cyanobacterial Lipopolysaccharide (LPS). Toxicon 2006, 48, 995–1001. [Google Scholar] [CrossRef]
- Keil, C.; Forchert, A.; Fastner, J.; Szewzyk, U.; Rotard, W.; Chorus, I.; Krätke, R. Toxicity and Microcystin Content of Extracts from a Planktothrix Bloom and Two Laboratory Strains. Water Res. 2002, 36, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Chien, M.S.; Wu, F.J.; Chou, H.N.; Lee, S.J. Inhibition of Embryonic Development by Microcystin-LR in Zebrafish, Danio rerio. Toxicon 2005, 45, 303–308. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, Q.; Chen, Y.; Wu, K.; Zhang, X. Microcystin-LR Exposure to Adult Zebrafish (Danio rerio) Leads to Growth Inhibition and Immune Dysfunction in F1 Offspring, a Parental Transmission Effect of Toxicity. Aquat. Toxicol. 2014, 155, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, S. Immune-Relevant and Antioxidant Activities of Vitellogenin and Yolk Proteins in Fish. Nutrients 2015, 7, 8818–8829. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Nguyen, T.; Bobe, J. Multiple Vitellogenins in Zebrafish (Danio rerio): Quantitative Inventory of Genes, Transcripts and Proteins, and Relation to Egg Quality. Fish Physiol. Biochem. 2018, 44, 1509–1525. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, L.; Yan, Y. Chemosphere Microcystin-LR Impairs Zebrafish Reproduction by Affecting Oogenesis and Endocrine System. Chemosphere 2015, 120, 115–122. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhou, Y.; Yang, J.; Li, S.; Hu, D.; Wang, J.; Chen, J.; Li, G. Waterborne Exposure to Microcystin-LR Alters Thyroid Hormone Levels and Gene Transcription in the Hypothalamic-Pituitary-Thyroid Axis in Zebrafish Larvae. Chemosphere 2012, 87, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Giesy, J.P.; Chen, F.; Shi, T.; Chen, J.; Xie, P. Microcystin-LR Affects the Hypothalamic-Pituitary-Inter-Renal (HPI) Axis in Early Life Stages (Embryos and Larvae) of Zebrafish. Environ. Pollut. 2018, 241, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, C.; Chen, L.; Wang, L.; Li, J.; Chen, Y.; Jin, J.; Kawan, A.; Zhang, X. Sex-Dependent Effects of Microcystin-LR on Hypothalamic-Pituitary-Gonad Axis and Gametogenesis of Adult Zebrafish. Sci. Rep. 2016, 6, 22819. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, R.; Li, D.; Hu, Q.; Wang, Y. Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio rerio). Toxins 2015, 7, 337–352. [Google Scholar] [CrossRef]
- Guo, S. Using Zebrafish to Assess the Impact of Drugs on Neural Development and Function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef]
- Qian, H.; Liu, G.; Lu, T.; Sun, L. Developmental Neurotoxicity of Microcystis aeruginosa in the Early Life Stages of Zebrafish. Ecotoxicol. Environ. Saf. 2018, 151, 35–41. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, W.; Liu, C.; Li, L.; Yu, L.; Zhao, S.; Li, G. Microcystin-LR Exposure Induces Developmental Neurotoxicity in Zebrafish Embryo. Environ. Pollut. 2016, 213, 793–800. [Google Scholar] [CrossRef]
- Kist, L.W.; Rosemberg, D.B.; Pereira, T.C.B.; De Azevedo, M.B.; Richetti, S.K.; De Castro Leão, J.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Microcystin-LR Acute Exposure Increases AChE Activity via Transcriptional Ache Activation in Zebrafish (Danio rerio) Brain. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 247–252. [Google Scholar] [CrossRef]
- Baganz, D.; Siegmund, R.; Staaks, G.; Pflugmacher, S.; Steinberg, C.E.W. Temporal Pattern in Swimming Activity of Two Fish Species (Danio rerio and Leucaspius delineatus) under Chemical Stress Conditions. Biol. Rhythm Res. 2005, 36, 263–276. [Google Scholar] [CrossRef]
- Kist, L.W.; Piato, A.L.; Da Rosa, J.G.S.; Koakoski, G.; Barcellos, L.J.G.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Acute Exposure to Microcystin-Producing Cyanobacterium Microcystis aeruginosa Alters Adult Zebrafish (Danio rerio) Swimming Performance Parameters. J. Toxicol. 2011, 2011, 280304. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Trainer, V.L.; Scholz, N.L. Morphological Abnormalities and Sensorimotor Deficits in Larval Fish Exposed to Dissolved Saxitoxin. Aquat. Toxicol. 2004, 66, 159–170. [Google Scholar] [CrossRef]
- Zagatto, P.A.; Buratini, S.V.; Marcia, A.A.; Ferrao-Filho, A.S. Neurotoxicity of Two Cylindrospermopsis raciborskii (Cyanobacteria) Strains to Mice, Daphnia and Fish. Environ. Toxicol. Chem. 2012, 31, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Pflugmacher, S.; Oberemm, A.; Meems, N.; Beattie, K.A.; Steinberg, C.E.W.; Codd, G.A. Uptake and Effects of Microcystin-LR on Detoxication Enzymes of Early Life Stages of the Zebra Fish (Danio rerio). Environ. Toxicol. Int. J. 1998, 14, 89–95. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Gong, Z.; Hande, M.P.; Dionysiou, D.D.; De, A.A.; Balasubramanian, R. Biochemical Response of Diverse Organs in Adult Danio rerio (Zebrafish) Exposed to Sub-Lethal Concentrations of Microcystin-LR and Microcystin-RR: A Balneation Study. Aquat. Toxicol. 2012, 109, 1–10. [Google Scholar] [CrossRef]
- Cazenave, J.; Wiegand, C.; Bistoni, M. de los A. Attenuating Effects of Natural Organic Matter on Microcystin Toxicity in Zebra Fish (Danio rerio) Embryos—Benefits and Costs of Microcystin Detoxication Attenuating Effects of Natural Organic Matter on Microcystin Toxicity in Zebra Fish (Danio rerio). Environ. Toxicol. 2006, 21, 22–32. [Google Scholar] [CrossRef] [PubMed]
- McLellan, N.L.; Manderville, R.A. Toxic mechanisms of microcystins in mammals. Toxicol. Res. (Camb.) 2017, 24, 391–405. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Damage and Recovery of the Ovary in Female Zebrafish i.p.-Injected with MC-LR. Aquat. Toxicol. 2014, 155, 110–118. [Google Scholar] [CrossRef]
- Shima, A.; Shimada, A. The Japanese Medaka, Oryzias Latipes, as a New Model Organism for Studying Environmental Germ-Cell Mutagenesis. Environ. Health Perspect. 1994, 102, 33–35. [Google Scholar] [CrossRef]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y.; et al. The Medaka Draft Genome and Insights into Vertebrate Genome Evolution. Nature 2007, 447, 714–719. [Google Scholar] [CrossRef]
- Le Manach, S.; Sotton, B.; Huet, H.; Duval, C.; Paris, A.; Marie, A.; Yépremian, C.; Catherine, A.; Mathéron, L.; Vinh, J.; et al. Physiological Effects Caused by Microcystin-Producing and Non-Microcystin Producing Microcystis aeruginosa on Medaka Fish: A Proteomic and Metabolomic Study on Liver. Environ. Pollut. 2018, 234, 523–537. [Google Scholar] [CrossRef]
- Trinchet, I.; Djediat, C.; Huet, H.; Dao, S.P.; Edery, M. Pathological Modifications Following Sub-Chronic Exposure of Medaka Fish (Oryzias Latipes) to Microcystin-LR. Reprod. Toxicol. 2011, 32, 329–340. [Google Scholar] [CrossRef]
- Djediat, C.; Moyenga, D.; Malécot, M.; Comte, K.; Yéprémian, C.; Bernard, C.; Puiseux-Dao, S.; Edery, M. Oral Toxicity of Extracts of the Microcystin-Containing Cyanobacterium Planktothrix agardhii to the Medaka Fish (Oryzias latipes). Toxicon 2011, 58, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Le Manach, S.; Khenfech, N.; Huet, H.; Qiao, Q.; Duval, C.; Marie, A.; Bolbach, G.; Clodic, G.; Djediat, C.; Bernard, C.; et al. Gender-Specific Toxicological Effects of Chronic Exposure to Pure Microcystin-LR or Complex Microcystis aeruginosa Extracts on Adult Medaka Fish. Environ. Sci. Technol. 2016, 50, 8324–8334. [Google Scholar] [CrossRef]
- Qiao, Q.; Le Manach, S.; Huet, H.; Duvernois-Berthet, E.; Chaouch, S.; Duval, C.; Sotton, B.; Ponger, L.; Marie, A.; Mathéron, L.; et al. An Integrated Omic Analysis of Hepatic Alteration in Medaka Fish Chronically Exposed to Cyanotoxins with Possible Mechanisms of Reproductive Toxicity. Environ. Pollut. 2016, 219, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.F.; Zheng, K.; Teh, F.C.; Lehman, P.W.; Teh, S.J. Toxic Threshold of Dietary Microcystin (-LR) for Quart Medaka. Toxicon 2010, 55, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Sotton, B.; Paris, A.; Le Manach, S.; Blond, A.; Lacroix, G.; Millot, A.; Duval, C.; Huet, H.; Qiao, Q.; Labrut, S.; et al. Metabolic Changes in Medaka Fish Induced by Cyanobacterial Exposures in Mesocosms: An Integrative Approach Combining Proteomic and Metabolomic Analyses. Sci. Rep. 2017, 7, 4051. [Google Scholar] [CrossRef] [PubMed]
- Yunes, J.S.; Salomon, P.S.; Matthiensen, A.; Beattie, K.A.; Raggett, S.L.; Codd, G.A. Toxic Blooms of Cyanobacteria in the Patos Lagoon Estuary, Southern Brazil. J. Aquat. Ecosyst. Health 1996, 5, 223–229. [Google Scholar] [CrossRef]
- Gaudin, J.; Huet, S.; Fran, A. In Vivo DNA Damage Induced by the Cyanotoxin Microcystin-LR: Comparison of Intra-Peritoneal and Oral Administrations by Use of the Comet Assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2008, 652, 65–71. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Zhou, W.; Zhao, Q.; Wang, Y. Chronic Exposure to Microcystin-LR Affected Mitochondrial DNA Maintenance and Caused Pathological Changes of Lung Tissue in Mice. Environ. Pollut. 2016, 210, 48–56. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ma, Y.; Chen, X.; Losiewicz, M.D.; Du, X.; Tian, Z.; Zhang, S.; Shi, L.; Yang, F.; et al. Long-Term Exposure to Low Concentrations of MC-LR Induces Blood-Testis Barrier Damage through the RhoA/ROCK Pathway. Ecotoxicol. Environ. Saf. 2022, 236, 113454. [Google Scholar] [CrossRef]
- Aubaeed, M.A.; Abdulkareem, K.F.; Kathim, A.S.; Al-Sultan, E.Y.A. Toxic Effects of Neurotoxins (Anatoxin-a) Purified from Blue-Green Algae Pseudoanbaena Limnetica on Some Organs in Laboratory Mice (Mus musculus L.). Int. J. Pharm. Res. 2020, 12, 2368–2374. [Google Scholar] [CrossRef]
- Sotero-Santos, R.B.; Silva, C.R.D.S.E.; Verani, N.F.; Nonaka, K.O.; Rocha, O. Toxicity of a Cyanobacteria Bloom in Barra Bonita Reservoir (Middle Tietê River, São Paulo, Brazil). Ecotoxicol. Environ. Saf. 2006, 64, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Tarczynska, M.; Nalecz-Jawecki, G.; Romanowska-Duda, Z.; Sawicki, J.; Beattie, K.; Codd, G.; Zalewski, M. Tests for the Toxicity Assessment of Cyanobacterial Bloom Samples. Environ. Toxicol. 2001, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Thermes, V.; De Luze, A.; Puiseux-Dao, S.; Bernard, C.; Joly, J.S.; Bourrat, F.; Edery, M. Effects of Microcystin-LR on Development of Medaka Fish Embryos (Oryzias latipes). Toxicon 2004, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.R.; Frenkel, A.; Harke, M.J.; Jankowiak, J.G.; Gobler, C.J.; McElroy, A.E. Effects of Microcystis on Development of Early Life Stage Japanese Medaka (Oryzias latipes): Comparative Toxicity of Natural Blooms, Cultured Microcystis and Microcystin-LR. Aquat. Toxicol. 2018, 194, 18–26. [Google Scholar] [CrossRef]
- Colas, S.; Duval, C.; Marie, B. Toxicity, Transfer and Depuration of Anatoxin-a (Cyanobacterial Neurotoxin) in Medaka Fish Exposed by Single-Dose Gavage. Aquat. Toxicol. 2020, 222, 105422. [Google Scholar] [CrossRef] [PubMed]
- Lecoz, N.; Malécot, M.; Quiblier, C.; Puiseux-Dao, S.; Bernard, C.; Crespeau, F.; Edery, M. Effects of Cyanobacterial Crude Extracts from Planktothrix agardhii on Embryo-Larval Development of Medaka Fish, Oryzias latipes. Toxicon 2008, 51, 262–269. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Xiao, G.; Chen, G.; Han, L.; Hu, T. Adverse Effect of Cylindrospermopsin on Embryonic Development in Zebrafish (Danio rerio). Chemosphere 2020, 241, 125060. [Google Scholar] [CrossRef]
- Moraes, A.C.N.; Shah, S.; Magalhães, V.F.; Habibi, H.R. Cylindrospermopsin Impairs Zebrafish (Danio rerio) Embryo Development. Mar. Environ. Res. 2022, 175, 105567. [Google Scholar] [CrossRef]
- Blagojević, D.; Babić, O.; Kaišarević, S.; Stanić, B.; Mihajlović, V.; Davidović, P.; Marić, P.; Smital, T.; Simeunović, J. Evaluation of Cyanobacterial Toxicity Using Different Biotests and Protein Phosphatase Inhibition Assay. Environ. Sci. Pollut. Res. 2021, 28, 49220–49231. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Ortiz Almirall, X.; Simmons, D.B.D.; Lumsden, J.S.; Bhavsar, S.P.; Watson-Leung, T.; Eyken, A.V.; Hankins, G.; Hubbs, K.; Konopelko, P.; et al. Cyanotoxins within and Outside of Microcystis aeruginosa Cause Adverse Effects in Rainbow Trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2021, 55, 10422–10431. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A Microwell Cytotoxicity Assay Using Artemia salina (Brine Shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, J.; Sivonen, K.; Niemelä, S.I.; Huovinen, K. Detection of Toxicity of Cyanobacteria by Artemia salina Bioassay. Environ. Toxicol. Water Qual. 1991, 6, 423–436. [Google Scholar] [CrossRef]
- Vezie, C.; Benoufella, F.; Sivonen, K.; Bertru, G.; Laplanche, A. Detection of Toxicity of Cyanobacterial Strains Using Artemia salina and Microtox® Assays Compared with Mouse Bioassay Results. Phycologia 1996, 35, 198–202. [Google Scholar] [CrossRef]
- Lee, T.-H.; Chen, Y.-M.; Chou, H.-N. Toxicity Assay of Cyanobacterial Strains Using Artemia salina in Comparison with the Mouse Bioassay. Acta Zool. Taiwanica 1999, 10, 1. [Google Scholar]
- Lopes, V.R.; Fernández, N.; Martins, R.F.; Vasconcelos, V. Primary Screening of the Bioactivity of Brackishwater Cyanobacteria: Toxicity of Crude Extracts to Artemia salina Larvae and Paracentrotus lividus Embryos. Mar. Drugs 2010, 8, 471–482. [Google Scholar] [CrossRef]
- Mayorga, P.; Perez, K.R.; Cruz, S.M.; Caceres, A. Comparison of Bioassays Using the Anostracan Crustaceans Artemia Salin a and Thamnocephalus platyurus for Plant Extract Toxicity Screening. Brazilian J. Pharmacogn. 2010, 20, 897–903. [Google Scholar] [CrossRef]
- Dos Santos, H.D.; De Oliveira, F.F.; De Oliveira, R.A. Influence of Solubility of Ethanol Extracts in Artemia salina Tests. Rev. Virtual Quim. 2017, 9, 1535–1545. [Google Scholar] [CrossRef]
- Malbrouck, C.; Kestemont, P. Effects of Microcystins on Fish. Environ. Toxicol. Chem. 2006, 25, 72–86. [Google Scholar] [CrossRef]
- Campbell, D.L.; Lawton, L.A.; Beattie, K.A.; Codd, G.A. Comparative Assessment of the Specificity of the Brine Shrimp and Microtox Assays to Hepatotoxic (Microcystin-LR-Containing) Cyanobacteria. Environ. Toxicol. Water Qual. 1994, 9, 71–77. [Google Scholar] [CrossRef]
- Lahti, K.; Ahtiainen, J.; Rapala, J.; Sivonen, K.; Niemelä, S.I. Assessment of Rapid Bioassays for Detecting Cyanobacterial Toxicity. Lett. Appl. Microbiol. 1995, 21, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chaumot, A.; Ferrari, B.; Geffard, O.; Garric, J. Ecotoxicology, Aquatic Invertebrates, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, ISBN 9780123864550. [Google Scholar]
- Tokodi, N.; Drobac, D.; Lazić, G.; Petrović, T.; Marinović, Z.; Lujić, J.; Malešević, T.P.; Meriluoto, J.; Svirčev, Z. Screening of Cyanobacterial Cultures Originating from Different Environments for Cyanotoxicity and Cyanotoxins. Toxicon 2018, 154, 1–6. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Bürgi, H.R.; Sticher, O. Biological Screening of Cyanobacteria for Antimicrobial and Molluscicidal Activity, Brine Shrimp Lethality, and Cytotoxicity. Pharm. Biol. 1999, 37, 138–143. [Google Scholar] [CrossRef]
- Faich, B.S.; König, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezznto, J.M.; Bachrnann, H. Biological Activities of Cyanobacteria: Evaluation of Extracts and Pure Compounds. Planta Med. 1995, 61, 321–328. [Google Scholar]
- Mian, P.; Heilmann, J.; Bürgi, H.R.; Sticher, O. Biological Screening of Terrestrial and Freshwater Cyanobacteria for Antimicrobial Activity, Brine Shrimp Lethality, and Cytotoxicity. Pharm. Biol. 2003, 41, 243–247. [Google Scholar] [CrossRef]
- Hisem, D.; Hrouzek, P.; Tomek, P.; Tomšíčková, J.; Zapomělová, E.; Skácelová, K.; Lukešová, A.; Kopecký, J. Cyanobacterial Cytotoxicity versus Toxicity to Brine Shrimp Artemia salina. Toxicon 2011, 57, 76–83. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Lindsay, J.; Beattie, K.A.; Birmingham, S.; Saker, M.L.; Törökné, A.K.; Codd, G.A. Toxicity of Cylindrospermopsin to the Brine Shrimp Artemia salina: Comparisons with Protein Synthesis Inhibitors and Microcystins. Toxicon 2002, 40, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Douma, M.; Manaut, N.; Saqrane, S.; El Khalloufi, F.; Oudra, B.; Loudiki, M. Toxicity Assessment and Detection of Cyanobacterial Toxins (Microcystins) in a Mediterranean Natural Lake (Dayete Aoua, Morocco). J. Mater. Environ. Sci. 2017, 8, 3247–3251. [Google Scholar]
- Rapala, J.; Robertson, A.; Negri, A.P.; Berg, K.A.; Tuomi, P.; Lyra, C.; Erkomaa, K.; Lahti, K.; Hoppu, K.; Lepistö, L. First Report of Saxitoxin in Finnish Lakes and Possible Associated Effects on Human Health. Environ. Toxicol. 2005, 20, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Moreno, E.; Carrasco, D.; Paniagua, T.; Wormer, L.; De Hoyos, C.; Sukenik, A. Toxicity of Aphanizomenon ovalisporum (Cyanobacteria) in a Spanish Water Reservoir. Eur. J. Phycol. 2006, 41, 39–45. [Google Scholar] [CrossRef]
- Svirčev, Z.; Obradović, V.; Codd, G.A.; Marjanović, P.; Spoof, L.; Drobac, D.; Tokodi, N.; Petković, A.; Nenin, T.; Simeunović, J.; et al. Massive Fish Mortality and Cylindrospermopsis raciborskii Bloom in Aleksandrovac Lake. Ecotoxicology 2016, 25, 1353–1363. [Google Scholar] [CrossRef]
- Delaney, J.M.; Wilkins, R.M. Toxicity of Microcystin-LR, Isolated from Microcystis aeruginosa, against Various Insect Species. Toxicon 1995, 33, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Akin-Oriola, G.A.; Lawton, L.A. The Detection and Quantification of Cyanobacterial Toxins in Water Using the Brine Shrimp (Artemia salina) Assay. West Afr. J. Appl. Ecol. 2006, 9, 127–132. [Google Scholar] [CrossRef]
- Ito, E.; Takai, A.; Kondo, F.; Masui, H.; Imanishi, S.; Harada, K. ichi Comparison of Protein Phosphatase Inhibitory Activity and Apparent Toxicity of Microcystins and Related Compounds. Toxicon 2002, 40, 1017–1025. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-Crossreactivity and Toxicity Assessment of Conjugation Products of the Cyanobacterial Toxin, Microcystin-LR. FEMS Microbiol. Lett. 2000, 189, 155–158. [Google Scholar] [CrossRef]
- Beattie, K.A.; Ressler, J.; Wiegand, C.; Krause, E.; Codd, G.A.; Steinberg, C.E.W.; Pflugmacher, S. Comparative Effects and Metabolism of Two Microcystins and Nodularin in the Brine Shrimp Artemia salina. Aquat. Toxicol. 2003, 62, 219–226. [Google Scholar] [CrossRef]
- Galanti, L.N.; Amé, M.V.; Wunderlin, D.A. Accumulation and Detoxification Dynamic of Cyanotoxins in the Freshwater Shrimp Palaemonetes Argentinus. Harmful Algae 2013, 27, 88–97. [Google Scholar] [CrossRef]
- Gonçalves-soares, D.; Zanette, J.; Yunes, J.S.; Yepiz-plascencia, G.M.; Bainy, A.C.D. Expression and Activity of Glutathione S-Transferases and Catalase in the Shrimp Litopenaeus Vannamei Inoculated with a Toxic Microcystis aeruginosa Strain. Mar. Environ. Res. 2012, 75, 54–61. [Google Scholar] [CrossRef]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective Efficacy of the Antioxidants Vitamin e and Trolox against Microcystis aeruginosa and Microcystin-LR in Artemia Franciscana Nauplii. J. Toxicol. Environ. Health Part A Curr. Issues 2009, 72, 1567–1575. [Google Scholar] [CrossRef]
- Tessier, A.J.; Leibold, M.A.; Tsao, J. A Fundamental Trade-off in Resource Exploitation by Daphnia and Consequences to Plankton Communities. Ecology 2000, 81, 826–841. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004; ISBN 9789264069947. [Google Scholar]
- OECD. Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Test No. 211; OECD: Paris, France, 2012. [Google Scholar]
- Hebert, P.D.N.; Ward, R.D. Inheritance during Parthenogenesis in Daphnia magna. Genetics 1972, 71, 639–642. [Google Scholar] [CrossRef]
- Colbourne, J.K.; Singan, V.R.; Gilbert, D.G. WFleaBase: The Daphnia Genome Database. BMC Bioinformatics 2005, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A. Physiological Endpoints in Daphnid Acute Toxicity Tests. Sci. Total Environ. 2020, 700, 134400. [Google Scholar] [CrossRef] [PubMed]
- Hietala, J.; Reinikainen, M.; Walls, M. Variation in Life History Responses of Daphnia to Toxic Microcystis aeruginosa. J. Plankton Res. 1995, 17, 2307–2318. [Google Scholar] [CrossRef]
- Nizan, S.; Dimentman, C.; Shilo, M. Acute Toxic Effects of the Cyanobacterium Microcystis aeruginosa on Daphnia magna. Limnol. Oceanogr. 1986, 31, 497–502. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, R.; Son Dao, T.; Wiegand, C. Transgenerational Effects of Microcystin-LR on Daphnia magna. J. Exp. Biol. 2012, 215, 2795–2805. [Google Scholar] [CrossRef]
- Trubetskova, I.L.; Haney, J.F. Effects of Differing Concentrations of Microcystin-Producing Microcystis aeruginosa on Growth, Reproduction, Survivorship and Offspring of Daphnia magna. Arch. Hydrobiol. 2006, 167, 533–546. [Google Scholar] [CrossRef]
- Rohrlack, T.; Dittmann, E.; Henning, M.; Börner, T.; Kohl, J.G. Role of Microcystins in Poisoning and Food Ingestion Inhibition of Daphnia Galeata Caused by the Cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 1999, 65, 737–739. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Courts, C.; von Elert, E. Target Gene Approaches: Gene Expression in Daphnia magna Exposed to Predator-Borne Kairomones or to Microcystin-Producing and Microcystin-Free Microcystis aeruginosa. BMC Genom. 2009, 10, 527. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Dittmann, E.; Nogueira, I.; Vasconcelos, V.; Börner, T. Ingestion of Microcystins by Daphnia: Intestinal Uptake and Toxic Effects. Limnol. Oceanogr. 2005, 50, 440–448. [Google Scholar] [CrossRef]
- Haney, J.F.; Sasner, J.J.; Ikawa, M. Effects of Products Released by Aphanizomenon Jlos-Aquae and Purified Saxitoxin on the Movements of Daphnia Carinata Feeding Appendages. Limnol. Oceanogr. 1995, 40, 263–272. [Google Scholar] [CrossRef]
- Claska, M.E.; Gilbert, J.J. The Effect of Temperature on the Response of Daphnia to Toxic Cyanobacteria. Freshw. Biol. 1998, 39, 221–232. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Toporowska, M.; Mazur-Marzec, H. Effects of Secondary Metabolites Produced by Different Cyanobacterial Populations on the Freshwater Zooplankters Brachionus calyciflorus and Daphnia Pulex. Environ. Sci. Pollut. Res. 2019, 26, 11793–11804. [Google Scholar] [CrossRef]
- Nandini, S. Responses of Rotifers and Cladocerans to Microcystis aeruginosa (Cyanophyceae): A Demographic Study. Aquat. Ecol. 2000, 34, 227–242. [Google Scholar] [CrossRef]
- Gustafsson, S.; Hansson, L.A. Development of Tolerance against Toxic Cyanobacteria in Daphnia. Aquat. Ecol. 2004, 38, 37–44. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an Enzymatically Formed Glutathione Conjugate of the Cyanobacterial Hepatotoxin Microcystin-LR: The First Step of Detoxication. Biochim. Biophys. Acta-Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, I.C.G.; Saker, M.L.; Pflugmacher, S.; Wiegand, C.; Vasconcelos, V.M. Toxicity of the Cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environ. Toxicol. 2004, 19, 453–459. [Google Scholar] [CrossRef]
- Sadler, T.; Elert, E. Von Dietary Exposure of Daphnia to Microcystins: No in Vivo Relevance of Biotransformation. Aquat. Toxicol. 2014, 150, 73–82. [Google Scholar] [CrossRef]
- Asselman, J.; De Coninck, D.I.M.; Glaholt, S.; Colbourne, J.K.; Janssen, C.R.; Shaw, J.R.; De Schamphelaere, K.A.C. Identification of Pathways, Gene Networks, and Paralogous Gene Families in Daphnia Pulex Responding to Exposure to the Toxic Cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2012, 46, 8448–8457. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Huo, D.; Gao, J.; Xu, Y.; Yang, R.; Yang, Y.; Yu, G. Combined-Methods Elucidate the Multi-Organ Toxicity of Cylindrospermopsin (CYN) on Daphnia magna. Environ. Pollut. 2023, 324, 121250. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Meng, Q.; Zhu, X.; Dai, D.; Zhang, L.; Huang, Y.; Yang, Z. Changes in ITRAQ-Based Proteomic Profiling of the Cladoceran Daphnia magna Exposed to Microcystin-Producing and Microcystin-Free Microcystis aeruginosa. Environ. Sci. Technol. 2016, 50, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Shahmohamadloo, R.S.; Simmons, D.B.D.; Sibley, P.K. Shotgun Proteomics Analysis Reveals Sub-Lethal Effects in Daphnia magna Exposed to Cell-Bound Microcystins Produced by Microcystis aeruginosa. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100656. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Sadler, T.; Motameny, S.; Ben-Khalifa, K.; Frommolt, P.; Altmüller, J.; Konrad, K.; von Elert, E. Deciphering the Genetic Basis of Microcystin Tolerance. BMC Genom. 2014, 15, 776. [Google Scholar] [CrossRef]
- Kozma Törökné, A.; László, E.; Chorus, I.; Fastner, J.; Heinze, R.; Padisák, J.; Barbosa, F.A. Water Quality Monitoring by Thamnotoxkit F(TM) Including Cyanobacterial Blooms. Water Sci. Technol. 2000, 42, 381–385. [Google Scholar] [CrossRef]
- ISO (The International Organization for Standardization). Water Quality—Determination of the Acute Toxicity to Thamnocephalus platyurus (Crustacea, Anostraca) Qualité; ISO (The International Organization for Standardization): Geneva, Switzerland, 2011; Volume 2011. [Google Scholar]
- Torokne, A. The Potential of the Thamnotoxkit Microbiotest for Routine Detection of Cyanobacterial Toxins. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C.R., De Coen, W.M., Eds.; Springer Science: New York, NY, USA, 2000; pp. 533–540. [Google Scholar]
- Tarczynska, M.; Nalecz-Jawecki, G.; Brzychcy, M.; Zalewski, M.; Sawicki, J. The Toxicity of Cyanobacterial Blooms as Detennined by Microbiotests and Mouse Assays. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C.R., De Coen, W.M., Eds.; Springer Science: New York, NY, USA, 2000; Volume 4, pp. 527–532. ISBN 9781461369240. [Google Scholar]
- Maršalek, B.B.L. Comparison of 17 Biotests for Detection of Cyanobacterial Toxicity. Environ. Toxicol. 2004, 19, 310–317. [Google Scholar] [CrossRef]
- OECD. Test No. 235: Chironomus sp., Acute Immobilisation Test; OECD Publishing: Paris, France, 2011; pp. 1–4. [Google Scholar]
- OECD. Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; OECD Publishing: Paris, France, 2010; p. 29. [Google Scholar]
- OECD. Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Water, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004; pp. 1–21. [Google Scholar]
- OECD. Test No. 219: Sediment- Water Chironomid Toxicity Test Using Siked Water; OECD Publishing: Paris, France, 2004; pp. 1–21. [Google Scholar]
- Stanković, N.; Kostić, I.; Jovanović, B.; Savić-Zdravković, D.; Matić, S.; Bašić, J.; Cvetković, T.; Simeunović, J.; Milošević, D. Can Phytoplankton Blooming Be Harmful to Benthic Organisms? The Toxic Influence of Anabaena Sp. and Chlorella Sp. on Chironomus Riparius Larvae. Sci. Total Environ. 2020, 729, 138666. [Google Scholar] [CrossRef]
- Stanković, N.; Jovanović, B.; Kokić, I.K.; Piperac, M.S.; Simeunović, J.; Jakimov, D.; Dimkić, I.; Milošević, D. Toxic Effects of a Cyanobacterial Strain on Chironomus riparius Larvae in a Multistress Environment. Aquat. Toxicol. 2022, 253, 106321. [Google Scholar] [CrossRef]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Accumulation and Effects of Cyanobacterial Microcystins and Anatoxin-a on Benthic Larvae of Chironomus Spp. (Diptera: Chironomidae). Eur. J. Entomol. 2014, 111, 83–90. [Google Scholar] [CrossRef]
- Armitage, P.D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae. Biology and Ecology of Non-Biting Midges, 1st ed.; Chapman & Hall: London, UK, 1995; ISBN 9789401043083. [Google Scholar]
- Kyselková, I.; Maršálek, B.; Kyselková, I.; Maršálek, B. Using of Daphnia magna, Artemia salina and Tubifex tubifex for Cyanobacterial Microcystins Detection. Biologia 2000, 55, 637–643. [Google Scholar]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial Protease Inhibitor Microviridin J Causes a Lethal Molting Disruption in Daphnia Pulicaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A.Z.; Nandini, S.; Sarma, S.S.S. Effect of Crude Extracts of Dolichospermum Planctonicum on the Demography of Plationus Patulus (Rotifera) and Ceriodaphnia Cornuta (Cladocera). Ecotoxicology 2015, 24, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.S.; Do-Hong, L.C.; Wiegand, C. Chronic Effects of Cyanobacterial Toxins on Daphnia magna and Their Offspring. Toxicon 2010, 55, 1244–1254. [Google Scholar] [CrossRef]
- Schirmer, K. Proposal to Improve Vertebrate Cell Cultures to Establish Them as Substitutes for the Regulatory Testing of Chemicals and Effluents Using Fish. Toxicology 2006, 224, 163–183. [Google Scholar] [CrossRef]
- Verma, A.; Verma, M.; Singh, A. Animal Tissue Culture Principles and Applications. In Animal Biotechnology; Verma, A.S., Singh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 1–12. ISBN 9788578110796. [Google Scholar]
- Li, Z. In Vitro Micro-Tissue and -Organ Models for Toxicity Testing. Compr. Biotechnol. Second Ed. 2011, 5, 551–563. [Google Scholar] [CrossRef]
- Moysidou, C.M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Yu, H.; Cook, T.J.; Sinko, P.J. Evidence for Diminished Functional Expression of Intestinal Transporters in Caco-2 Cell Monolayers at High Passages. Pharm. Res. 1997, 14, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell Lines. Spermatogenesis 2012, 2, 44–52. [Google Scholar] [CrossRef]
- Dietrich, D.; Hoeger, S. Guidance Values for Microcystins in Water and Cyanobacterial Supplement Products (Blue-Green Algal Supplements): A Reasonable or Misguided Approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of Cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Guzmán-Guillén, R.; Pichardo, S.; Moreno, F.J.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Cytotoxic and Morphological Effects of Microcystin-LR, Cylindrospermopsin, and Their Combinations on the Human Hepatic Cell Line HepG2. Environ. Toxicol. 2019, 34, 240–251. [Google Scholar] [CrossRef]
- Neumann, C.; Bain, P.; Shaw, G. Studies of the Comparative in Vitro Toxicology of the Cyanobacterial Metabolite DeoxyCylindrospermopsin. J. Toxicol. Environ. Health Part A 2007, 70, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic Effects of the Cyanobacterial Hepatotoxin Cylindrospermopsin in the HepG2 Cell Line. Arch. Toxicol. 2011, 85, 1617–1626. [Google Scholar] [CrossRef]
- Chong, M.W.K.; Gu, K.D.; Lam, P.K.S.; Yang, M.; Fong, W.F. Study on the Cytotoxicity of Microcystin-LR on Cultured Cells. Chemosphere 2000, 41, 143–147. [Google Scholar] [CrossRef]
- Senousy, H.H.; Abd Ellatif, S.; Ali, S. Assessment of the Antioxidant and Anticancer Potential of Different Isolated Strains of Cyanobacteria and Microalgae from Soil and Agriculture Drain Water. Environ. Sci. Pollut. Res. 2020, 27, 18463–18474. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Herrera, C.; Ortíz, I.; Orozco, L.; Robledo, S.; Agudelo, D.; Echeverri, F. Genotoxicity and Cytotoxicity of Three Microcystin-LR Containing Cyanobacterial Samples from Antioquia, Colombia. Toxicon 2018, 154, 50–59. [Google Scholar] [CrossRef]
- Batsalova, T.; Basheva, D.; Bardarov, K.; Bardarov, V.; Dzhambazov, B.; Teneva, I. Assessment of the Cytotoxicity, Antioxidant Activity and Chemical Composition of Extracts from the Cyanobacterium Fischerella Major Gomont. Chemosphere 2019, 218, 93–103. [Google Scholar] [CrossRef]
- Batsalova, T.; Moten, D.; Basheva, D.; Teneva, I.; Dzhambazov, B. In Vitro Cytotoxicity and Antioxidative Potential of Nostoc Microscopicum (Nostocales, Cyanobacteria). Toxicol. Forensic Med. Open J. 2016, 1, 9–17. [Google Scholar] [CrossRef]
- Hrouzek, P.; Kapuścik, A.; Vacek, J.; Voráčová, K.; Paichlová, J.; Kosina, P.; Voloshko, L.; Ventura, S.; Kopecký, J. Cytotoxicity Evaluation of Large Cyanobacterial Strain Set Using Selected Human and Murine in Vitro Cell Models. Ecotoxicol. Environ. Saf. 2016, 124, 177–185. [Google Scholar] [CrossRef]
- Costa, M.; Garcia, M.; Costa-Rodrigues, J.; Costa, M.S.; Ribeiro, M.J.; Fernandes, M.H.; Barros, P.; Barreiro, A.; Vasconcelos, V.; Martins, R. Exploring Bioactive Properties of Marine Cyanobacteria Isolated from the Portuguese Coast: High Potential as a Source of Anticancer Compounds. Mar. Drugs 2014, 12, 98–114. [Google Scholar] [CrossRef]
- Karan, T.; Aydin, A. Anticancer Potential and Cytotoxic Effect of Some Freshwater Cyanobacteria. Trop. J. Pharm. Res. 2018, 17, 2183–2188. [Google Scholar] [CrossRef]
- Sazdova, I.; Keremidarska-Markova, M.; Chichova, M.; Uzunov, B.; Nikolaev, G.; Mladenov, M.; Schubert, R.; Stoyneva-Gärtner, M.; Gagov, H.S. Review of Cyanotoxicity Studies Based on Cell Cultures. J. Toxicol. 2022, 2022, 5647178. [Google Scholar] [CrossRef]
- Humpage, A.R.; Auré, L.; Fanok, S.; Cile Bernard, C.É.; Briand, J.-F.O.; Eaglesham, G.; Papageorgiou, J.; Nicholson, B.; Steffensen, D. Application of the Neuroblastoma Assay for Paralytic Shellfish Poisons To Neurotoxic Freshwater Cyanobacteria: Interlaboratory Calibration and Comparison with Other Methods of Analysis. Environ. Toxicol. Chem. 2007, 26, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at Low Doses Induce Apoptosis and Inflammatory Effects in Murine Brain Cells: Potential Implications for Neurodegenerative Diseases. Toxicol. Rep. 2016, 3, 180–189. [Google Scholar] [CrossRef]
- Pannetier, P.; Fuster, L.; Clérandeau, C.; Lacroix, C.; Gourves, P.Y.; Cachot, J.; Morin, B. Usefulness of RTL-W1 and OLCAB-E3 Fish Cell Lines and Multiple Endpoint Measurements for Toxicity Evaluation of Unknown or Complex Mixture of Chemicals. Ecotoxicol. Environ. Saf. 2018, 150, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Teneva, I.; Asparuhova, D.; Dzhambazov, B.; Mladenov, R.; Schirmer, K. The Freshwater Cyanobacterium Lyngbya Aerugineo-Coerulea Produces Compounds Toxic to Mice and to Mammalian and Fish Cells. Environ. Toxicol. 2003, 18, 9–20. [Google Scholar] [CrossRef]
- Teneva, I.; Dzhambazov, B.; Koleva, L.; Mladenov, R.; Schirmer, K. Toxic Potential of Five Freshwater Phormidium Species (Cyanoprokaryota). Toxicon 2005, 45, 711–725. [Google Scholar] [CrossRef]
- Marić, P.; Ahel, M.; Babić, O.; Simeunović, J.; Smital, T. Ecotoxicological Profiling of Selected Cyanobacterial Strains Using Multi-Endpoint Effect-Directed Analysis. Ecotoxicology 2020, 29, 535–550. [Google Scholar] [CrossRef]
- Basu, A.; Dydowiczová, A.; Čtveráčková, L.; Jaša, L.; Trosko, J.E.; Bláha, L.; Babica, P. Assessment of Hepatotoxic Potential of Cyanobacterial Toxins Using 3D in Vitro Model of Adult Human Liver Stem Cells. Environ. Sci. Technol. 2018, 52, 10078–10088. [Google Scholar] [CrossRef]
- Ujvárosi, A.Z.; Hercog, K.; Riba, M.; Gonda, S.; Filipič, M.; Vasas, G.; Žegura, B. The Cyanobacterial Oligopeptides Microginins Induce DNA Damage in the Human Hepatocellular Carcinoma (HepG2) Cell Line. Chemosphere 2020, 240, 124880. [Google Scholar] [CrossRef]
- Gheda, S.F.; Ismail, G.A. Natural Products from Some Soil Cyanobacterial Extracts with Potent Antimicrobial, Antioxidant and Cytotoxic Activities. An. Acad. Bras. Cienc. 2020, 92, e20190934. [Google Scholar] [CrossRef]
- Bittner, M.; Štern, A.; Smutn, M.; Žegura, B. Cytotoxic and Genotoxic Effects of Cyanobacterial and Algal Extracts—Microcystin and Retinoic Acid Content. Toxins 2021, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.; Alverca, E.; Dias, E.; Sam-Bento, F.; Pereira, P. Involvement of Endoplasmic Reticulum and Autophagy in Microcystin-LR Toxicity in Vero-E6 and HepG2 Cell Lines. Toxicol. Vitr. 2013, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Bain, P.; Shaw, G.; Patel, B. Induction of P53-Regulated Gene Expression in Human Cell Lines Exposed to the Cyanobacterial Toxin Cylindrospermopsin. J. Toxicol. Environ. Health Part A Curr. Issues 2007, 70, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Panou, M.; Konstantinou, D.; Apostolidis, P.; Kasampali, A.; Papadimitriou, S.; Kati, D.; Di Lorenzo, G.M.; Ioakeim, S.; Zervou, S.K.; et al. Diversity, Cyanotoxin Production, and Bioactivities of Cyanobacteria Isolated from Freshwaters of Greece. Toxins 2019, 11, 436. [Google Scholar] [CrossRef]
- Murad, W.; Amin, A.; Khan, M.H.; Mahmood, N.; Ahmed, M. Assessment of Antimicrobial, Antialgal and Cytotoxic Activities of Crude Extracts from Rhizospheric and Freshwater Cyanobacterial Strains. Adv. Life Sci. 2022, 9, 169–176. [Google Scholar]
- Diez-Quijada, L.; Puerto, M.; Gutiérrez-Praena, D.; Turkina, M.V.; Campos, A.; Vasconcelos, V.; Cameán, A.M.; Jos, Á. In Vitro Toxicity Evaluation of Cyanotoxins Cylindrospermopsin and Microcystin-LR on Human Kidney HEK293 Cells. Toxins 2022, 14, 429. [Google Scholar] [CrossRef]
- Dias, E.; Andrade, M.; Alverca, E.; Pereira, P.; Batoréu, M.C.C.; Jordan, P.; Silva, M.J. Comparative Study of the Cytotoxic Effect of Microcistin-LR and Purified Extracts from Microcystis aeruginosa on a Kidney Cell Line. Toxicon 2009, 53, 487–495. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Biochemical and Pathological Toxic Effects Induced by the Cyanotoxin Cylindrospermopsin on the Human Cell Line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef]
- Žegura, B.; Volčič, M.; Lah, T.T.; Filipič, M. Different Sensitivities of Human Colon Adenocarcinoma (CaCo-2), Astrocytoma (IPDDC-A2) and Lymphoblastoid (NCNC) Cell Lines to Microcystin-LR Induced Reactive Oxygen Species and DNA Damage. Toxicon 2008, 52, 518–525. [Google Scholar] [CrossRef]
- Cetojevic-Simin, D.; Svircev, Z.; Baltic, V.V. In Vitro Cytotoxicity of Cyanobacteria from Water Ecosystems of Serbia. J. BUON 2009, 14, 289–294. [Google Scholar] [PubMed]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Alterations Observed in the Endothelial HUVEC Cell Line Exposed to Pure Cylindrospermopsin. Chemosphere 2012, 89, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V.; Filipič, M. Microcystin-LR Induced DNA Damage in Human Peripheral Blood Lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 726, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Krzowski, Ł.; Głąb, J.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Wójcik, A. DNA Damage and Repair in Human Peripheral Blood Lymphocytes Following Treatment with Microcystin-LR. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 559, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Surakka, A.; Sihvonen, L.M.; Lehtimäki, J.M.; Wahlsten, M.; Vuorela, P.; Sivonen, K. Benthic Cyanobacteria from the Baltic Sea Contain Cytotoxic Anabaena, Nodularia, and Nostoc Strains and an Apoptosis-Inducing Phormidium Strain. Environ. Toxicol. 2005, 20, 285–292. [Google Scholar] [CrossRef]
- Maruthanayagam, V.; Nagarajan, M.; Sundararaman, M. Cytotoxicity Assessment of Cultivable Marine Cyanobacterial Extracts in Artemia salina (Brine Shrimp) Larvae and Cancer Cell Lines. Toxin Rev. 2013, 32, 1–9. [Google Scholar] [CrossRef]
- Davidović, P.G.; Blagojević, D.J.; Lazić, G.G.; Simeunović, J.B. Gene Expression Changes in Daphnia magna Following Waterborne Exposure to Cyanobacterial Strains from the Genus Nostoc. Harmful Algae 2022, 115, 102232. [Google Scholar] [CrossRef]
- Porazinski, S.R.; Wang, H.; Furutani-Seiki, M. Essential Techniques for Introducing Medaka to a Zebrafish Laboratory—Towards the Combined Use of Medaka and Zebrafish for Further Genetic Dissection of the Function of the Vertebrate Genome. In Practical Approaches for Implementing Forward Genetic Strategies in Zebrafish; Methods in Molecular Biology; Nair, S., Pelegri, F.J., Eds.; Springer: New York, NY, USA; Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK, 2011; pp. 211–241. [Google Scholar]
- Lyu, K.; Gu, L.; Wang, H.; Zhu, X.; Zhang, L.; Sun, Y.; Huang, Y.; Yang, Z. Transcriptomic Analysis Dissects the Mechanistic Insight into the Daphnia Clonal Variation in Tolerance to Toxic Microcystis. Limnol. Oceanogr. 2019, 64, 272–283. [Google Scholar] [CrossRef]
- Andersen, M.L.; Winter, L.M.F. Animal Models in Biological and Biomedical Research—Experimental and Ethical Concerns. An. Acad. Bras. Cienc. 2019, 91, e20170238. [Google Scholar] [CrossRef]
- Greek, R.; Menache, A. Systematic Reviews of Animal Models: Methodology versus Epistemology. Int. J. Med. Sci. 2013, 10, 206–221. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.; Leusch, F. Bioanalytical Tools in Water Quality Assessment; IWA Publishing: London, UK, 2011; Volume 10, ISBN 9781789061970. [Google Scholar]
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; et al. European Demonstration Program on the Effect-Based and Chemical Identification and Monitoring of Organic Pollutants in European Surface Waters. Sci. Total Environ. 2017, 601–602, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Jefferies, T.M.; Keevil, C.W.; Potter, E. Detection Methods for Cyanobacterial Toxins; Woodhead Publishing: Sawston, UK, 1994. [Google Scholar]
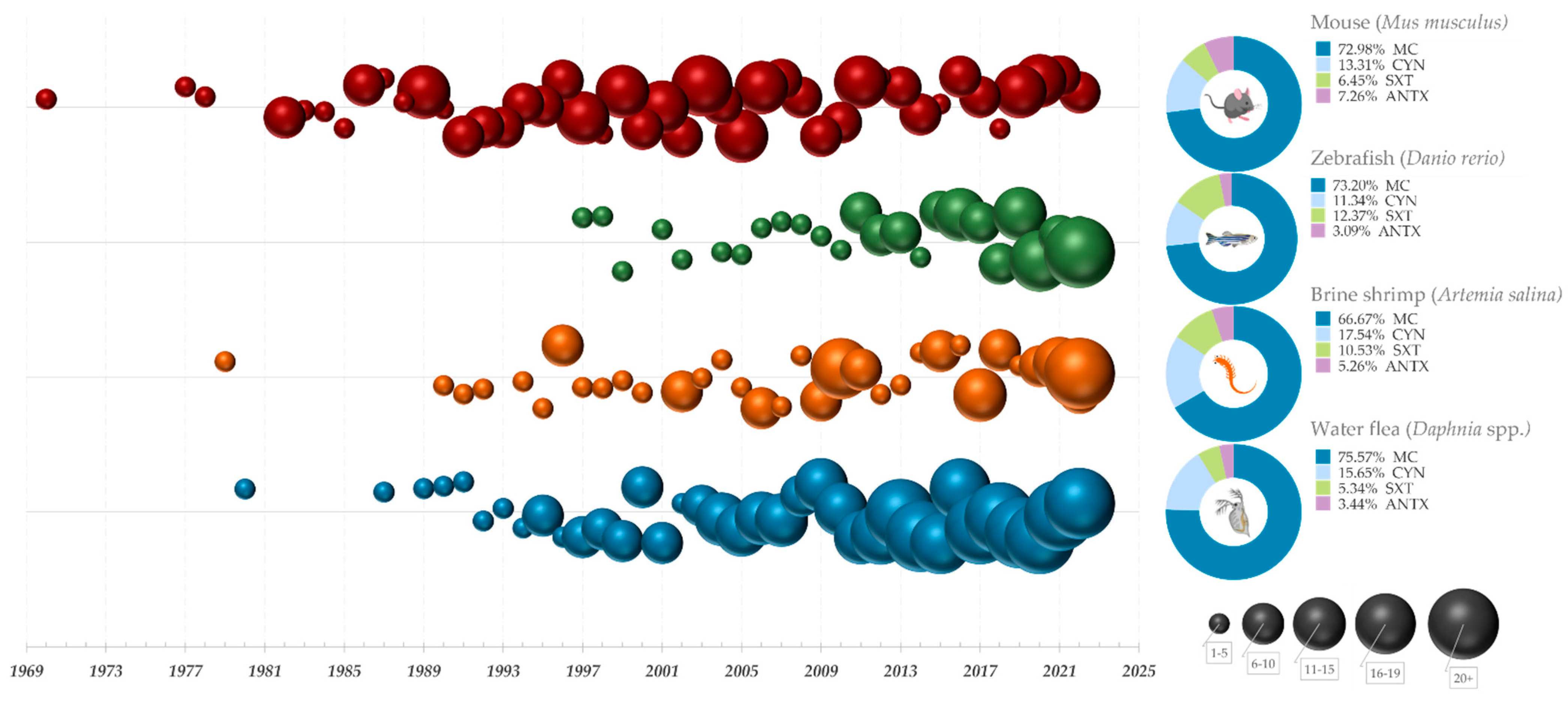
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidović, P.; Blagojević, D.; Meriluoto, J.; Simeunović, J.; Svirčev, Z. Biotests in Cyanobacterial Toxicity Assessment—Efficient Enough or Not? Biology 2023, 12, 711. https://doi.org/10.3390/biology12050711
Davidović P, Blagojević D, Meriluoto J, Simeunović J, Svirčev Z. Biotests in Cyanobacterial Toxicity Assessment—Efficient Enough or Not? Biology. 2023; 12(5):711. https://doi.org/10.3390/biology12050711
Chicago/Turabian StyleDavidović, Petar, Dajana Blagojević, Jussi Meriluoto, Jelica Simeunović, and Zorica Svirčev. 2023. "Biotests in Cyanobacterial Toxicity Assessment—Efficient Enough or Not?" Biology 12, no. 5: 711. https://doi.org/10.3390/biology12050711
APA StyleDavidović, P., Blagojević, D., Meriluoto, J., Simeunović, J., & Svirčev, Z. (2023). Biotests in Cyanobacterial Toxicity Assessment—Efficient Enough or Not? Biology, 12(5), 711. https://doi.org/10.3390/biology12050711







