Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp. ‘Natal Brier’
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Sample Collection
2.2. DNA Extraction, Library Preparation, and Sequencing
2.3. Data Analysis
2.4. Diversity Analysis, Community Composition, Community Function, and Statistical Analysis
3. Results
3.1. Alpha Diversity and Taxonomic Microbial Composition of Bulk Soil and the Rhizosphere of the Rootstock ‘Natal Brier’
3.2. Alpha Diversity, Taxonomic Composition, and Function of Microbial Communities Associated with the Grafted and Non-Grafted ‘Natal Brier’ Rootstock
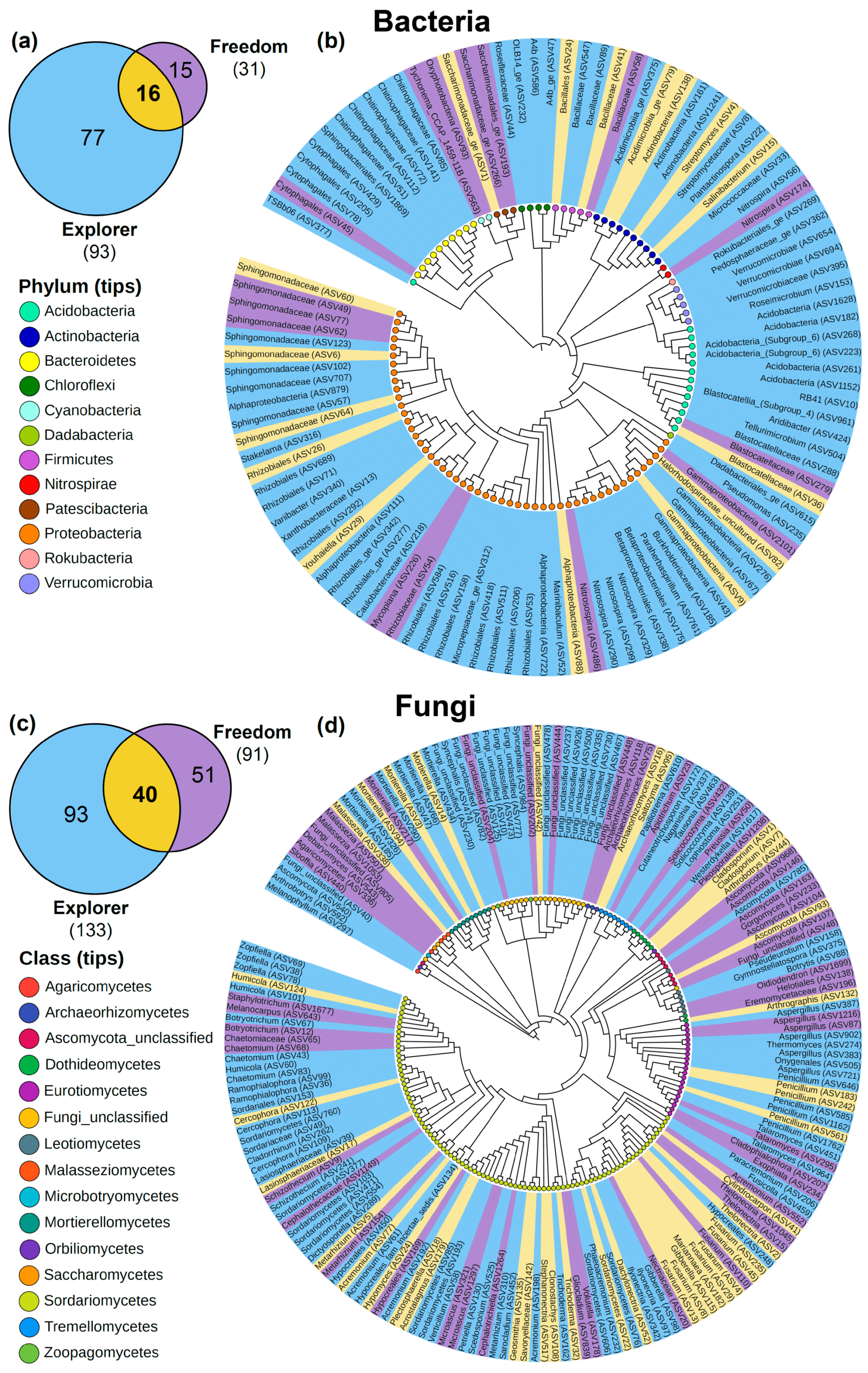
3.3. Microbial Community Composition in the Rhizospheres of ‘Natal Brier’ Rootstock Grafted with Cultivars ‘Freedom’ and ‘Explorer’ and Their Core Microbiome
4. Discussion
4.1. The Rhizosphere Microbiome of Rosa sp. ‘Natal Brier’
4.2. Grafting Changes the Rhizosphere Microbiome of Rose sp. ‘Natal Brier’
4.3. Scion Genotype Determines the Changes in the Rhizosphere Microbiome of Rootstock Rose sp. ‘Natal Brier’
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Expoflores. Reporte Estadístico Anual Expoflores; Expoflores: Quito, Ecuador, 2020. [Google Scholar]
- Leus, L.; Van Laere, K.; De Riek, J.; Van Huylenbroeck, J. Rose. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; pp. 719–767. [Google Scholar]
- The Market Is Shying Away from Older Red Varieties and Is Looking for New Alternatives. Available online: https://www.floraldaily.com/article/9279845/the-market-is-shying-away-from-older-red-varieties-and-is-looking-for-new-alternatives/ (accessed on 9 April 2022).
- Rosen Tantau’s Freedom Story—The Importance of a Good Variety Name. Available online: https://www.floraldaily.com/article/9029700/the-importance-of-a-good-variety-name/ (accessed on 9 April 2022).
- Casierra-Posada, F.; Paipa Quintero, J.A. Influencia Del Portainjerto Sobre La Calidad de Flor e Incidencia de Plagas y Enfermedades En Rosa (Rosa Sp.). Cienc. y Agric. 2008, 6, 41–48. [Google Scholar]
- Gammon, N.; McFadden, S.E. Effect of Rootstocks on Greenhouse Rose Flower Yield and Leaf Nutrient Levels. Commun. Soil Sci. Plant Anal. 1979, 10, 1171–1184. [Google Scholar] [CrossRef]
- Safi, M.I. Flower Production Related to Re-Blooming Time of Three Rosa Hybrida Cultivars in Response to Rootstock Type. ScienceAsia 2005, 31, 179–181. [Google Scholar] [CrossRef]
- de Vries, D.P. The Vigour of Glasshouse Roses: Scion-Rootstock Relationships: Effects of Phenotypic and Genotypic Variation. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 1993. [Google Scholar]
- de Vries, D.P. Rootstocks|Clonal Rootstocks. In Encyclopedia of Rose Science; Roberts, A.V., Ed.; Elsevier: Oxford, UK, 2003; pp. 651–656. [Google Scholar]
- An Insight in the Selection and Propagation of Roses in Ecuador. Available online: https://www.floraldaily.com/article/9028726/an-insight-in-the-selection-and-propagation-of-roses-in-ecuador/ (accessed on 9 April 2022).
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ling, N.; Ma, J.; Wang, J.; Zhu, C.; Raza, W.; Shen, Y.; Huang, Q.; Shen, Q. Grafting Resulted in a Distinct Proteomic Profile of Watermelon Root Exudates Relative to the Un-Grafted Watermelon and the Rootstock Plant. J. Plant Growth Regul. 2016, 35, 778–791. [Google Scholar] [CrossRef]
- Ling, N.; Zhang, W.; Wang, D.; Mao, J.; Huang, Q.; Guo, S.; Shen, Q. Root Exudates from Grafted-Root Watermelon Showed a Certain Contribution in Inhibiting Fusarium oxysporum f. Sp. Niveum. PLoS ONE 2013, 8, e63383. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-Associated Microbiomes of Wheat under the Combined Effect of Plant Development and Nitrogen Fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of Plant Developmental Stage on Microbial Community Structure and Activity in the Rhizosphere of Three Field Crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jousset, A.; de Boer, W.; Carrión, V.J.; Zhang, T.; Wang, X.; Kuramae, E.E. Legacy of Land Use History Determines Reprogramming of Plant Physiology by Soil Microbiome. ISME J. 2019, 13, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Chaluvadi, S.; Bennetzen, J.L. Species-Associated Differences in the below-Ground Microbiomes of Wild and Domesticated Setaria. Front. Plant Sci. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Berlanas, C.; Berbegal, M.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The Fungal and Bacterial Rhizosphere Microbiome Associated with Grapevine Rootstock Genotypes in Mature and Young Vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef]
- D’Amico, F.; Candela, M.; Turroni, S.; Biagi, E.; Brigidi, P.; Mueller, C.W.; Schmidt, H.; Bega, A.; Vancini, D.; Rampelli, S. The Rootstock Regulates Microbiome Diversity in Root and Rhizosphere Compartments of Vitis vinifera Cultivar Lambrusco. Front. Microbiol. 2018, 9, 2240. [Google Scholar] [CrossRef] [PubMed]
- Vink, S.N.; Dini-Andreote, F.; Höfle, R.; Kicherer, A.; Salles, J.F. Interactive Effects of Scion and Rootstock Genotypes on the Root Microbiome of Grapevines (Vitis spp. L.). Appl. Sci. 2021, 11, 1615. [Google Scholar] [CrossRef]
- Chai, X.; Wang, X.; Li, H.; Xu, X.; Wu, T.; Zhang, X.; Wang, Y.; Han, Z. Apple Scion Cultivars Regulate the Rhizosphere Microbiota of Scion/Rootstock Combinations. Appl. Soil Ecol. 2022, 170, 104305. [Google Scholar] [CrossRef]
- Toju, H.; Okayasu, K.; Notaguchi, M. Leaf-Associated Microbiomes of Grafted Tomato Plants. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Ling, N.; Song, Y.; Raza, W.; Huang, Q.; Guo, S.; Shen, Q. The Response of Root-Associated Bacterial Community to the Grafting of Watermelon. Plant Soil 2015, 391, 253–264. [Google Scholar] [CrossRef]
- Xia, A.N.; Liu, J.; Kang, D.C.; Zhang, H.G.; Zhang, R.H.; Liu, Y.G. Assessment of Endophytic Bacterial Diversity in Rose by High-Throughput Sequencing Analysis. PLoS ONE 2020, 15, e0230924. [Google Scholar] [CrossRef]
- Yim, B.; Baumann, A.; Grunewaldt-Stöcker, G.; Liu, B.; Beerhues, L.; Zühlke, S.; Sapp, M.; Nesme, J.; Sørensen, S.J.; Smalla, K.; et al. Rhizosphere Microbial Communities Associated to Rose Replant Disease: Links to Plant Growth and Root Metabolites. Hortic. Res. 2020, 7, 144. [Google Scholar] [CrossRef]
- Grunewaldt-Stöcker, G.; Popp, C.; Baumann, A.; Fricke, S.; Menssen, M.; Winkelmann, T.; Maiss, E. Observations on Early Fungal Infections with Relevance for Replant Disease in Fine Roots of the Rose Rootstock Rosa Corymbifera “Laxa”. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Yim, B.; Grunewaldt-Stöcker, G.; Liu, B.; Beerhues, L.; Sapp, M.; Nesme, J.; Sørensen, S.J.; Smalla, K.; Winkelmann, T. Rose Replant Disease: Detailed Analyses of Plant Reactions, Root Endophytes and Rhizosphere Microbial Communities. In Proceedings of the XXVI International Eucarpia Symposium Section Ornamentals: Editing Novelty, Erfurt, Germany, 1–4 September 2019; Franken, P., Tränkner, C., Drüge, U., Eds.; International Society for Horticultural Science: Erfurt, Germany, 2020; Volume 1283, pp. 97–104. [Google Scholar]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Spaepen, S.; Law, T.F.; Teixeira, P.J.P.L.; Jones, C.D.; Dangl, J.L. The Effects of Soil Phosphorus Content on Plant Microbiota Are Driven by the Plant Phosphate Starvation Response. PLoS Biol. 2019, 17, e3000534. [Google Scholar] [CrossRef] [PubMed]
- Yourstone, S.M.; Lundberg, D.S.; Dangl, J.L.; Jones, C.D. MT-Toolbox: Improved Amplicon Sequencing Using Molecule Tags. BMC Bioinform. 2014, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Salas Gonzales, I. Isaisg/Ohchibi: Iskali (v1.0.0). Zenodo. 2019. Available online: https://zenodo.org/record/2593691#.ZEjhZs5BxPY (accessed on 10 October 2021).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package “Vegan” 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 2 February 2021).
- R-Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 2 February 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Khosh-Khui, M.; Salehi, H. Growth and Flower Quality of Four Rosa Hybrida L. Cultivars in Response to Propagation by Stenting or Cutting in Soilless Culture. Sci. Hortic. 2009, 119, 302–305. [Google Scholar] [CrossRef]
- Pourghorban, M.; Azadi, P.; Khaghani, S.; Mirzakhani, A.; Changizi, M.; Edrisi, B. Propagation of Three Cultivars of Rosa Hybrida L. through Stenting Method. Int. J. Hortic. Sci. Technol. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Khosh-Khui, M.; Zargarian, M. Effects of Four Rootstocks on Growth and Development of Three Rose Scion Cultivars. In Proceedings of the V International Symposium on Rose Research and Cultivation, Gifu, Japan, 24–29 May 2009; Ueda, Y., Ed.; International Society for Horticultural Science: Gifu, Japan, 2010; pp. 207–212. [Google Scholar]
- Van de Pol, P.A.; Pierik, R.L.M. Newest Developments in Rose (Rosa Hybrida) Progapation. Rev. Chapingo 1995, 3, 15–22. [Google Scholar]
- Cabrera, R.I. Rose Yield, Dry Matter Partitioning and Nutrient Status Responses to Rootstock Selection. Sci. Hortic. 2002, 95, 75–83. [Google Scholar] [CrossRef]
- Safi, M.I.; Sawwan, J.S. Rootstock Effects on Yield and Mineral Composition of Rose Cut Flowers. J. Appl. Hortic. 2004, 06, 94–98. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D.; Teixeira, P.J.P.L.; Dangl, J.L. The Plant Microbiome: From Ecology to Reductionism and Beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root Exudates Drive Soil-Microbe-Nutrient Feedbacks in Response to Plant Growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Alturkey, H. The Rootstock-Scion Combination Drives Microorganisms’ Selection and Recruitment in Grapevine Rhizosphere. Master’s Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2020. [Google Scholar]
- Poudel, R.; Jumpponen, A.; Kennelly, M.M.; Rivard, C.L.; Gomez-Montano, L.; Garrett, K.A. Rootstocks Shape the Rhizobiome: Rhizosphere and Endosphere Bacterial Communities in the Grafted Tomato System. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Norton, J.M.; Stark, J.M. Regulation and Measurement of Nitrification in Terrestrial Systems. In Methods in Enzymology; Klotz, M.G., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 486, pp. 343–368. [Google Scholar]
- Narayanasamy, M.; Dhanasekaran, D.; Thajuddin, N. Chapter 11—Frankia. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Senthil Kumar, M., Annapurna, K., Krishna Kumar, A.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 185–211. ISBN 9780128234143. [Google Scholar]
- Bennett, R.A.; Lynch, J.M. Bacterial Growth and Development in the Rhizosphere of Gnotobiotic Cereal Plants. Microbiology 1981, 125, 95–102. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.M.; Zhou, X.; Zhang, A.M.; Cai, L. Disease-Induced Changes in Plant Microbiome Assembly and Functional Adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated Root Exudates Stimulate the Abundance of Saccharimonadales to Improve the Alkaline Phosphatase Activity in Maize Rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, J.Y.; Lim, J.M.; Hamada, M.; Ahn, J.H.; Weon, H.Y.; Suzuki, K.I.; Kwon, S.W. Jatrophihabitans Soli Sp. Nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Ratering, S.; Sadeghi, A.; Pokhrel, S.; Honermeier, B.; Schnell, S. The Response of the Soil Microbiota to Long-Term Mineral and Organic Nitrogen Fertilization Is Stronger in the Bulk Soil than in the Rhizosphere. Genes 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Bünger, W.; Jiang, X.; Müller, J.; Hurek, T.; Reinhold-Hurek, B. Novel Cultivated Endophytic Verrucomicrobia Reveal Deep-Rooting Traits of Bacteria to Associate with Plants. Sci. Rep. 2020, 10, 8692. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Ahn, J.-H.; Weon, H.-Y.; Hong, S.-B.; Seok, S.-J.; Kim, J.-S.; Kwon, S.-W. Niastella Gongjuensis Sp. Nov., Isolated from Greenhouse Soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 3115–3118. [Google Scholar] [CrossRef]
- Muñoz, M.; Faust, J.E.; Schnabel, G. Characterization of Botrytis Cinerea from Commercial Cut Flower Roses. Plant Dis. 2019, 103, 1577–1583. [Google Scholar] [CrossRef]
- Herrera-Leon, F.; Sánchez, F.; Bermudez, A.; Barriga-Medina, N.; Ramírez-Villacís, D.; Herrera, K.; Ruales, C.; Leon-Reyes, A. Alternaria Alternata Causes Bud Blight of Rose (Rosa Sp.) in Cotopaxi, Ecuador. Can. J. Plant Pathol. 2022, 44, 673–679. [Google Scholar] [CrossRef]
- Barguil, B.M.; Viana, F.M.P.; Anjos, R.M.; Cardoso, J.E. First Report of Dry Rot Caused by Fusarium Oxysporum on Rose (Rosa Spp.) in Brazil. Plant Dis. 2009, 93, 766. [Google Scholar] [CrossRef]
- Davies, F.T. Effects of Mycorrhizal Fungi on Rosa Multiflora “Brooks 56” Understock. Acta Hortic. 1986, 189, 117–122. [Google Scholar] [CrossRef]
- Cartmill, A.D.; Alarcón, A.; Valdez-Aguilar, L.A. Arbuscular Mycorrhizal Fungi Enhance Tolerance of Rosa Multiflora Cv. Burr to Bicarbonate in Irrigation Water. J. Plant Nutr. 2007, 30, 1517–1540. [Google Scholar] [CrossRef]
- Davies, F.T. Effects of VA-Mycorrhizal Fungi on Growth and Nutrient Uptake of Cuttings of Rosa Multiflora in Two Container Media with Three Levels of Fertilizer Application. Plant Soil 1987, 104, 31–35. [Google Scholar] [CrossRef]
- Garmendia, I.; Mangas, V.J. Comparación de Cuatro Modelos de Estado Estacionario de Complejidad Creciente Para El Cálculo Del Requerimiento de Lixiviación En Suelos Agrícolas Con Riesgo de Salinizarse. Span. J. Agric. Res. 2012, 10, 222–237. [Google Scholar] [CrossRef]
- Pinior, A.; Grunewaldt-Stöcker, G.; Von Alten, H.; Strasser, R.J. Mycorrhizal Impact on Drought Stress Tolerance of Rose Plants Probed by Chlorophyll a Fluorescence, Proline Content and Visual Scoring. Mycorrhiza 2005, 15, 596–605. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates Harmful Effects of Drought Stress on Damask Rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Mazur, S.; Nadziakiewicz, M.; Kurzawińska, H.; Nawrocki, J. Effectiveness of Mycorrhizal Fungi in the Protection of Juniper, Rose, Yew and Highbush Blueberry against Alternaria Alternata. Folia Hortic. 2019, 31, 117–127. [Google Scholar] [CrossRef]
- Auge, R.M.; Schekel, K.A.; Wample, R.L. Osmotic Adjustment in Leaves of VA Mycorrhizal and Nonmycorrhizal Rose Plants in Response to Drought Stress. Plant Physiol. 1986, 82, 765–770. [Google Scholar] [CrossRef]
- Zhang, K.; Bonito, G.; Hsu, C.M.; Hameed, K.; Vilgalys, R.; Liao, H.L. Mortierella Elongata Increases Plant Biomass among Non-Leguminous Crop Species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Hung, P.M.; Wattanachai, P.; Kasem, S.; Poeaim, S. Efficacy of Chaetomium Species as Biological Control Agents against Phytophthora Nicotianae Root Rot in Citrus. Mycobiology 2015, 43, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I.J. A Comparative Study of Phosphate Solubilization and the Host Plant Growth Promotion Ability of Fusarium Verticillioides RK01 and Humicola Sp. KNU01 under Salt Stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Leger, R.J., St.; Wang, J.B. Metarhizium: Jack of All Trades, Master of Many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef]
- Tymon, L.S.; Morgan, P.; Gundersen, B.; Inglis, D.A. Potential of Endophytic Fungi Collected from Cucurbita Pepo Roots Grown under Three Different Agricultural Mulches as Antagonistic Endophytes to Verticillium Dahliae in Western Washington. Microbiol. Res. 2020, 240, 126535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Jiang, Z.; Bai, Q.; Wu, S.; Wang, Y.; Li, C.; Zeng, X.; Gan, X.; Xie, X.; et al. A New Strain of Volutella citrinella with Nematode Predation and Nematicidal Activity, Isolated from the Cysts of Potato Cyst Nematodes in China. BMC Microbiol. 2021, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Salazar, C.; Rossman, A.Y.; Chaverri, P. The Genus Thelonectria (Nectriaceae, Hypocreales, Ascomycota) and Closely Related Species with Cylindrocarpon-like Asexual States. Fungal Divers. 2016, 80, 411–455. [Google Scholar] [CrossRef]
- Sigler, L.; Lumley, T.C.; Currah, R.S. New Species and Records of Saprophytic Ascomycetes (Myxotrichaceae) from Decaying Logs in the Boreal Forest. Mycoscience 2000, 41, 495–502. [Google Scholar] [CrossRef]
- Mohd Asmadi, N.E.N.; Yin, W.K.; Saleh, N.H.; Ibrahim, N.F.; Lob, S. Fungal Species Associted with Black Spot Disease in Rose. Univ. Malaysia Teren. J. Undergrad. Res. 2020, 2, 45–50. [Google Scholar] [CrossRef]
- Mirtalebi, M.; Banihashemi, Z.; Sabahi, F.; Mafakheri, H. Dieback of Rose Caused by Acremonium sclerotigenumas a New Causal Agent of Rose Dieback in Iran. Span. J. Agric. Res. 2016, 14, 24. [Google Scholar] [CrossRef]
- Moreira, S.I.; Da Consolação Dutra, D.; Rodrigues, A.C.; Rogério De Oliveira, J.; Dev Dhingra, O.; Pereira, O.L. Fungi and Bacteria Associated with Post-Harvest Rot of Ginger Rhizomes in Espírito Santo, Brazil. Trop. Plant Pathol. 2013, 38, 218–226. [Google Scholar]
- Ou, J.H.; Lin, G.C.; Chen, C.Y. Sarocladium Species Associated with Rice in Taiwan. Mycol. Prog. 2020, 19, 67–80. [Google Scholar] [CrossRef]
- Gautier, A.T.; Cochetel, N.; Merlin, I.; Hevin, C.; Lauvergeat, V.; Vivin, P.; Mollier, A.; Ollat, N.; Cookson, S.J. Scion Genotypes Exert Long Distance Control over Rootstock Transcriptome Responses to Low Phosphate in Grafted Grapevine. BMC Plant Biol. 2020, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Zheng, Y.; Peng, L.; Cao, J. Rootstock-Scion Interaction Affects the Composition and Pathogen Inhibitory Activity of Tobacco (Nicotiana tabacum L.) Root Exudates. Plants 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging Genotypes: Graft Union Formation and Scion–Rootstock Interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A. Microbiome Selection Could Spur Next-Generation Plant Breeding Strategies. Front. Microbiol. 2016, 7, 1971. [Google Scholar] [CrossRef]

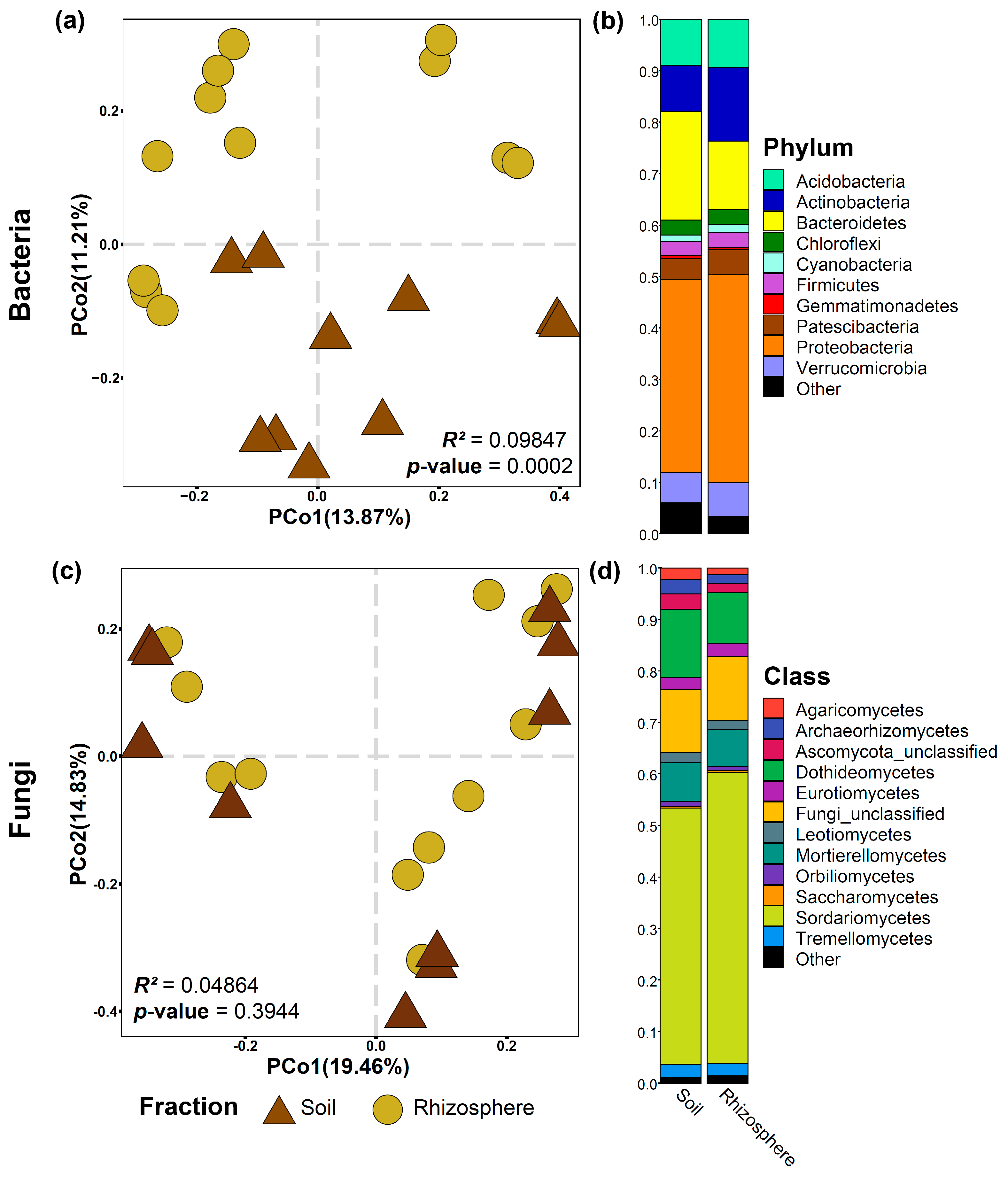
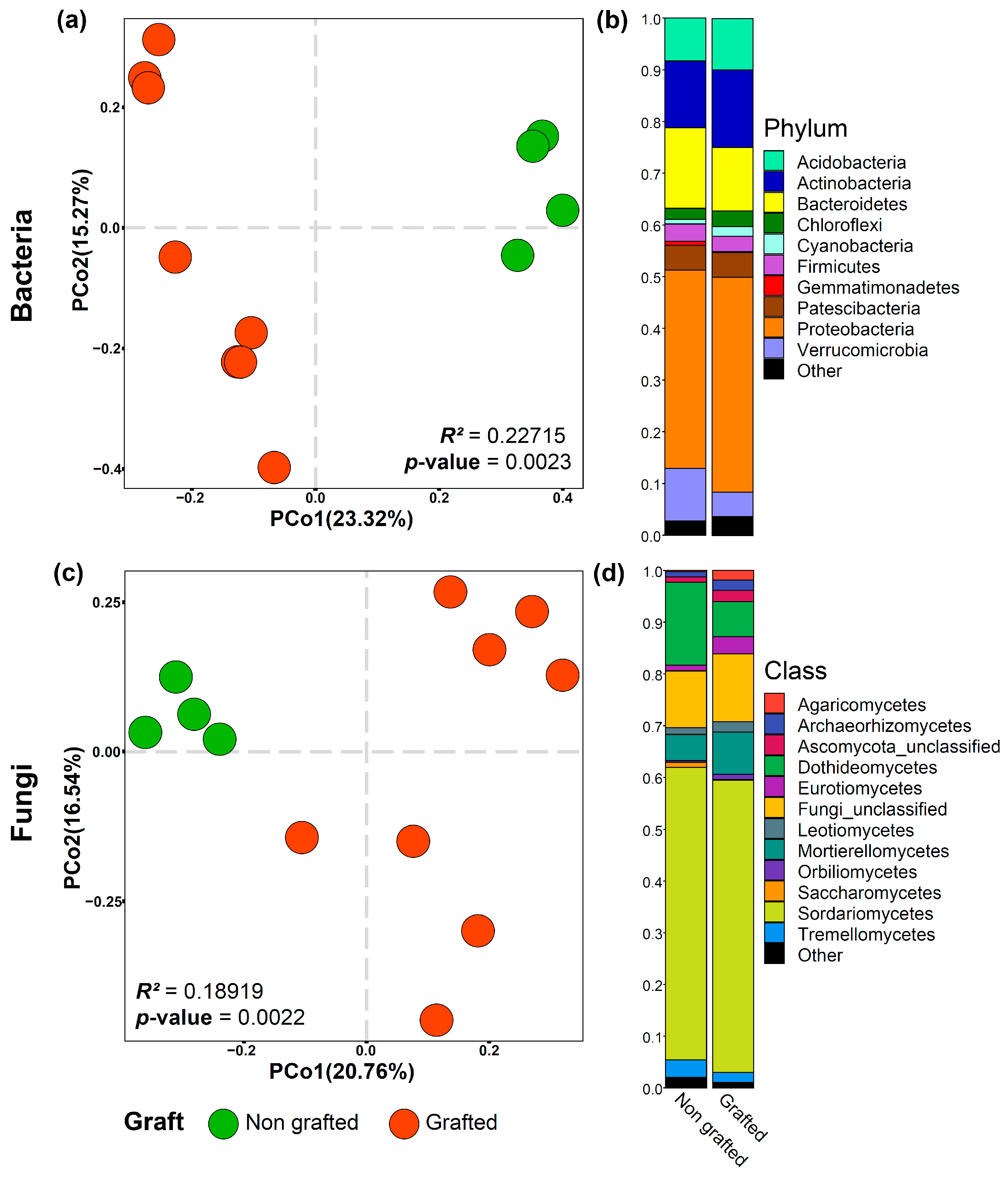
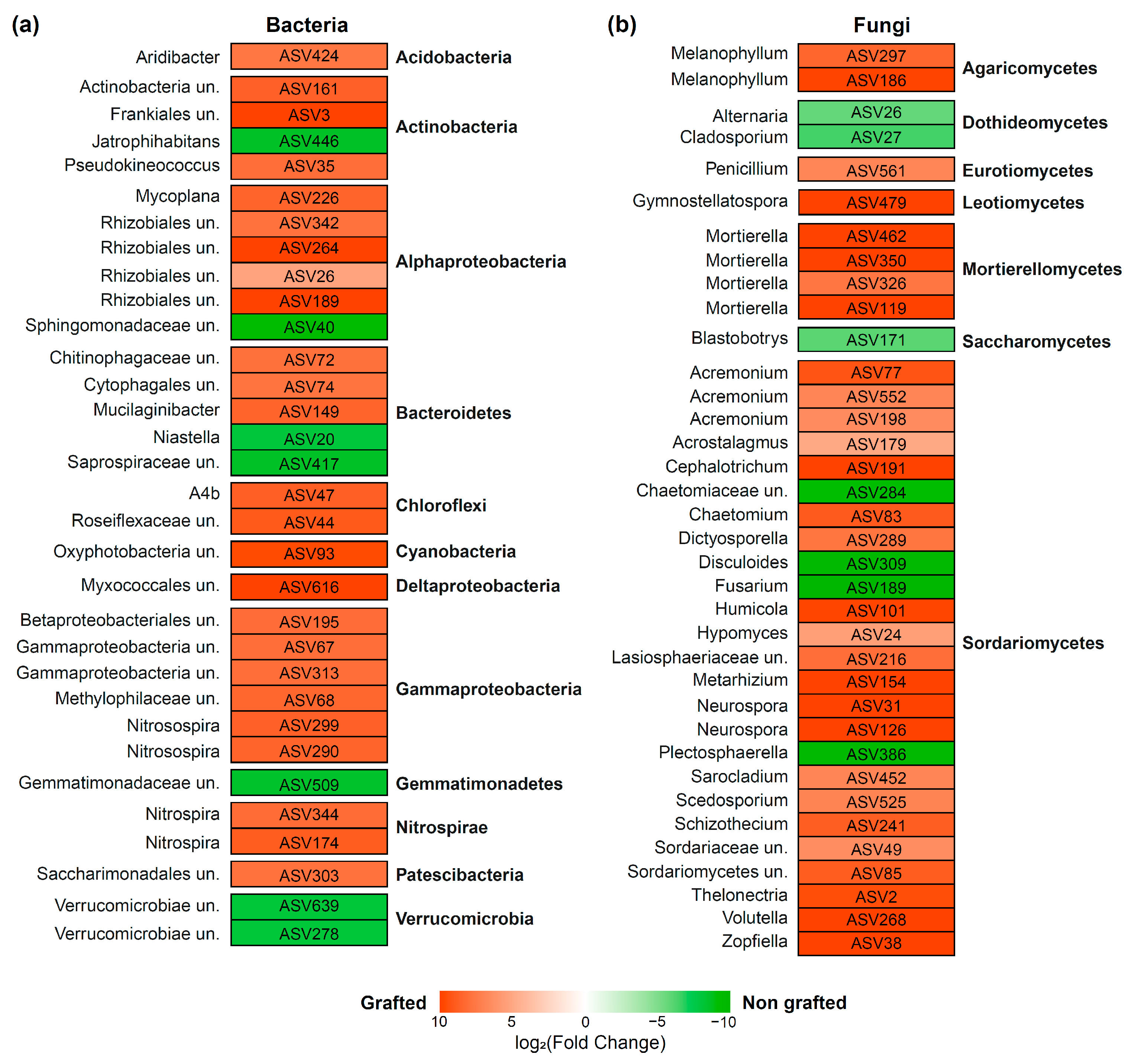
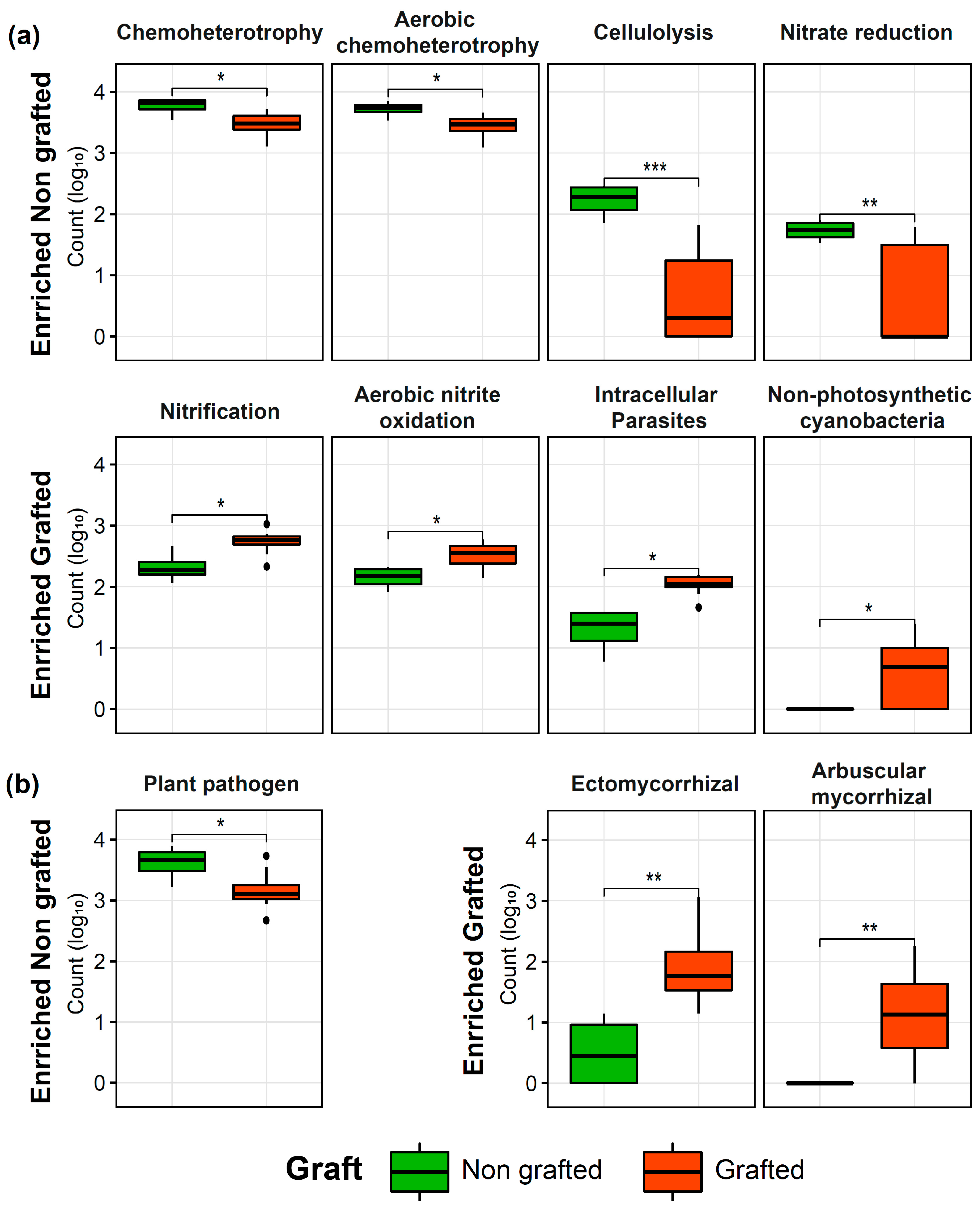

| Bacteria | |||||
| Fraction | Richness | Chao1 | Shannon | Inverse Simpson | Evenness |
| Soil | 800.30 ± 310.99 | 833.29 ± 333.85 | 6.13 ± 0.56 | 308.23 ± 148.14 | 0.93 ± 0.01 |
| Rhizosphere | 672.67 ± 282.47 | 693.54 ± 298.81 | 5.93 ± 0.58 | 244.55 ± 139.39 | 0.93 ± 0.02 |
| No significant differences found | |||||
| Fungi | |||||
| Fraction | Richness * | Chao1 * | Shannon | Inverse Simpson | Evenness |
| Soil | 484.70 ± 95.58 | 532.63 ± 117.35 | 4.42 ± 0.47 | 35.05 ± 20.05 | 0.72 ± 0.06 |
| Rhizosphere | 379.00 ± 97.90 | 405.80 ± 112.84 | 4.24 ± 0.34 | 30.51 ± 12.12 | 0.72 ± 0.05 |
| Bacteria | |||||
| Graft | Richness | Chao1 | Shannon | Inverse Simpson | Evenness |
| Non grafted | 744.25 ± 304.84 | 767.73 ± 326.03 | 6.07 ± 0.57 | 284.25 ± 160.82 | 0.93 ± 0.02 |
| Grafted | 636.88 ± 284.9 | 656.45 ± 300.06 | 5.86 ± 0.61 | 224.71 ± 134.54 | 0.92 ± 0.02 |
| No significant differences found | |||||
| Fungi | |||||
| Graft | Richness * | Chao1 * | Shannon | Inverse Simpson | Evenness |
| Non-grafted | 288 ± 82.16 | 301.99 ± 95.81 | 4.16 ± 0.3 | 33.02 ± 11.88 | 0.74 ± 0.03 |
| Grafted | 424.5 ± 71.2 | 457.7 ± 82.7 | 4.27 ± 0.36 | 29.25 ± 12.85 | 0.71 ± 0.05 |
| Bacteria | |||||
| Variety | Richness | Chao1 | Shannon | Inverse Simpson | Evenness |
| Explorer | 764.75 ± 296.75 | 793.98 ± 314.65 | 6.09 ± 0.46 | 256.68 ± 140.53 | 0.93 ± 0.02 |
| Freedom | 509.00 ± 240.26 | 518.92 ± 246.27 | 5.62 ± 0.71 | 192.73 ± 140.56 | 0.92 ± 0.03 |
| No significant differences found | |||||
| Fungi | |||||
| Variety | Richness | Chao1 | Shannon | Inverse Simpson | Evenness |
| Explorer | 430.5 ± 44.67 | 463.99 ± 53.81 | 4.37 ± 0.29 | 32.53 ± 12.83 | 0.72 ± 0.04 |
| Freedom | 418.5 ± 98.68 | 451.41 ± 113.83 | 4.17 ± 0.45 | 25.97 ± 13.85 | 0.69 ± 0.07 |
| No significant differences found | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Villacis, D.X.; Erazo-Garcia, P.; Quijia-Pillajo, J.; Llerena-Llerena, S.; Barriga-Medina, N.; Jones, C.D.; Leon-Reyes, A. Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp. ‘Natal Brier’. Biology 2023, 12, 663. https://doi.org/10.3390/biology12050663
Ramirez-Villacis DX, Erazo-Garcia P, Quijia-Pillajo J, Llerena-Llerena S, Barriga-Medina N, Jones CD, Leon-Reyes A. Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp. ‘Natal Brier’. Biology. 2023; 12(5):663. https://doi.org/10.3390/biology12050663
Chicago/Turabian StyleRamirez-Villacis, Dario X., Pablo Erazo-Garcia, Juan Quijia-Pillajo, Sol Llerena-Llerena, Noelia Barriga-Medina, Corbin D. Jones, and Antonio Leon-Reyes. 2023. "Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp. ‘Natal Brier’" Biology 12, no. 5: 663. https://doi.org/10.3390/biology12050663
APA StyleRamirez-Villacis, D. X., Erazo-Garcia, P., Quijia-Pillajo, J., Llerena-Llerena, S., Barriga-Medina, N., Jones, C. D., & Leon-Reyes, A. (2023). Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp. ‘Natal Brier’. Biology, 12(5), 663. https://doi.org/10.3390/biology12050663






