Antiretrovirals Promote Metabolic Syndrome through Mitochondrial Stress and Dysfunction: An In Vitro Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. ATP Quantification
2.3. Lipid Peroxidation-TBARS Assay
2.4. Western Blots
2.5. Quantitative PCR
2.5.1. RNA Isolation and Quantification
2.5.2. Quantification of mRNA Expression
2.6. Statistical Analysis
3. Results
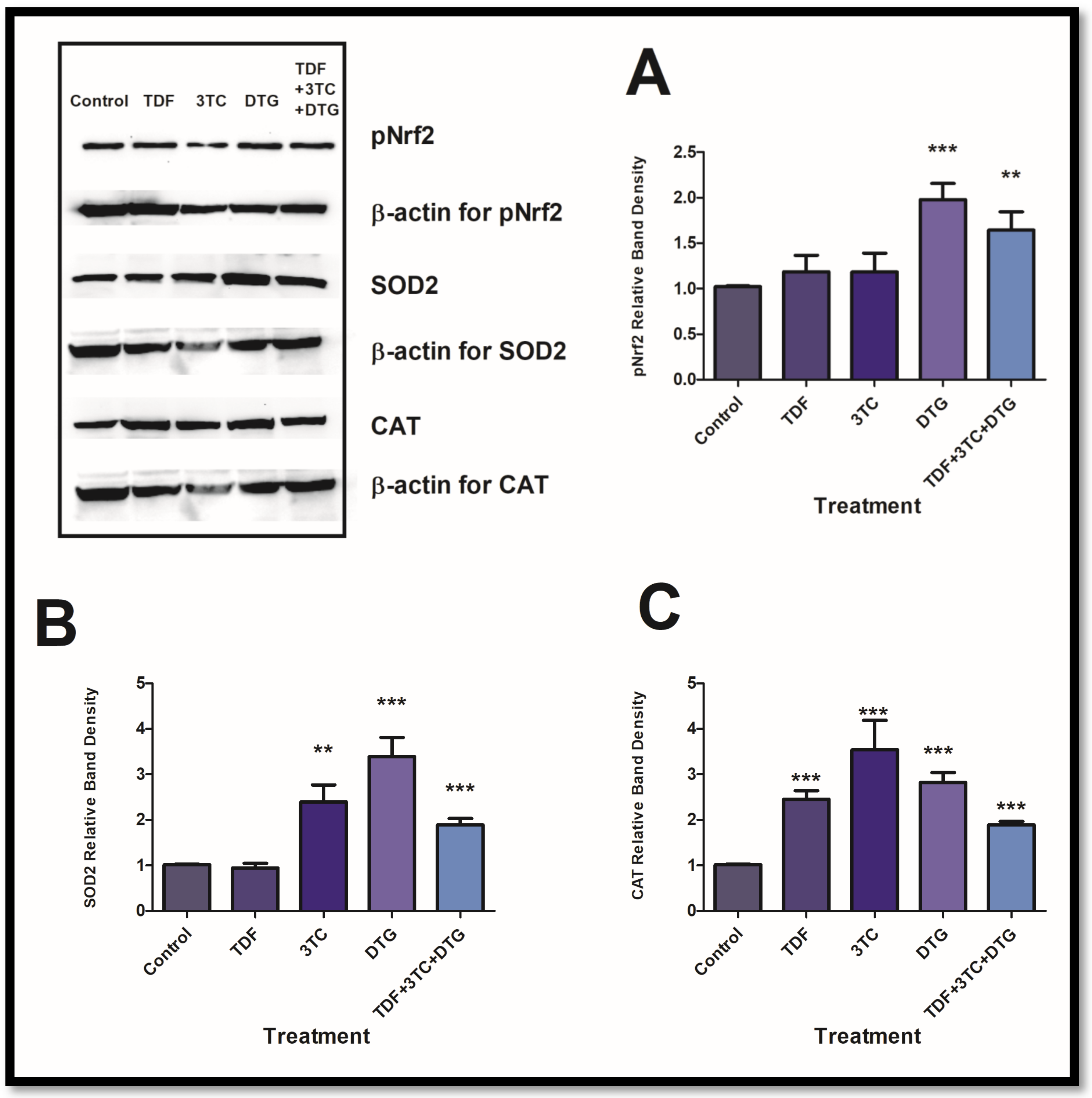
3.1. Antiretrovirals Activated Antioxidant Responses in HepG2 Cells
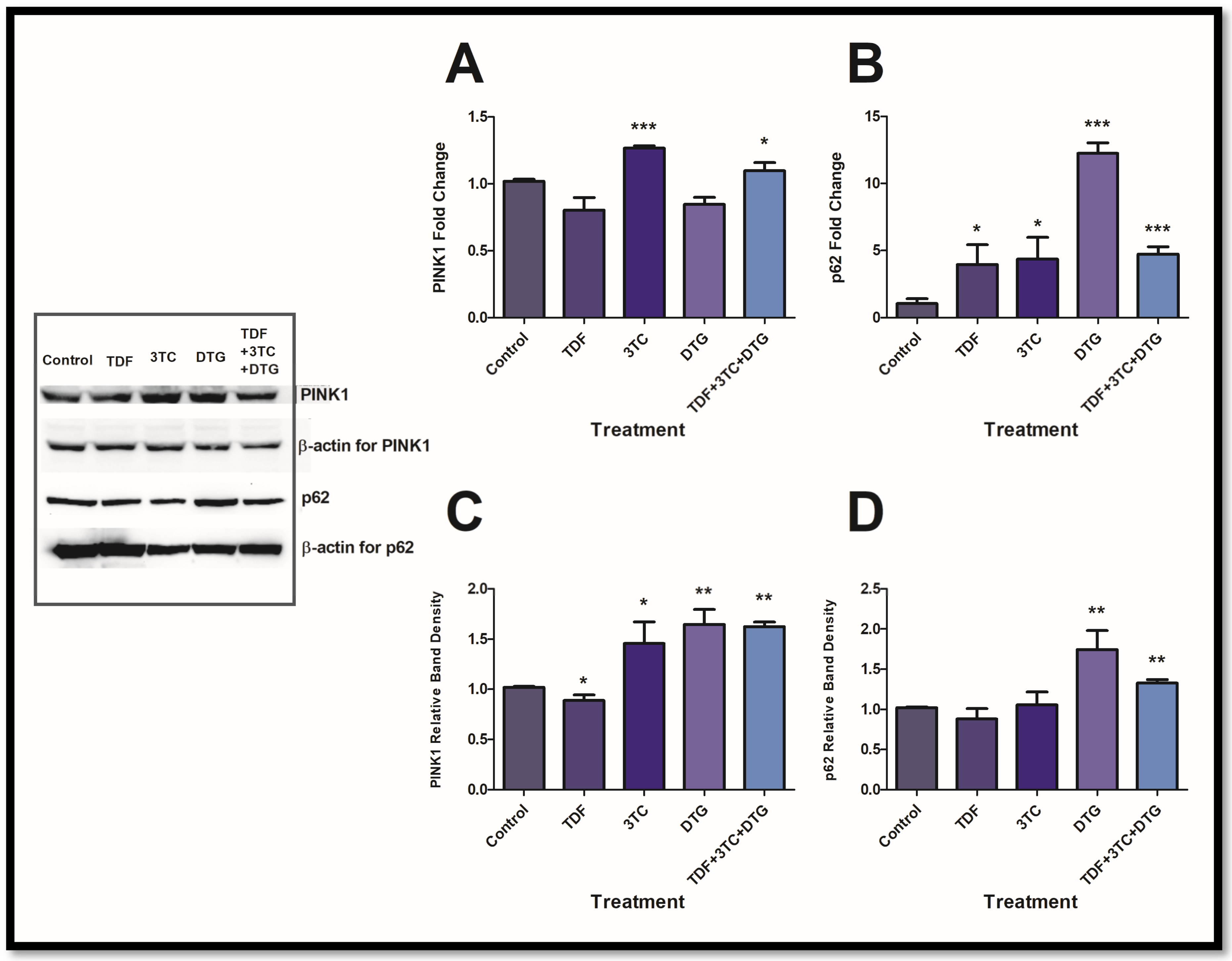
3.2. Antiretrovirals Activated Mitochondrial Maintenance Intermediates
3.3. Antiretrovirals Suppress Essential Mitochondrial Stress Proteins
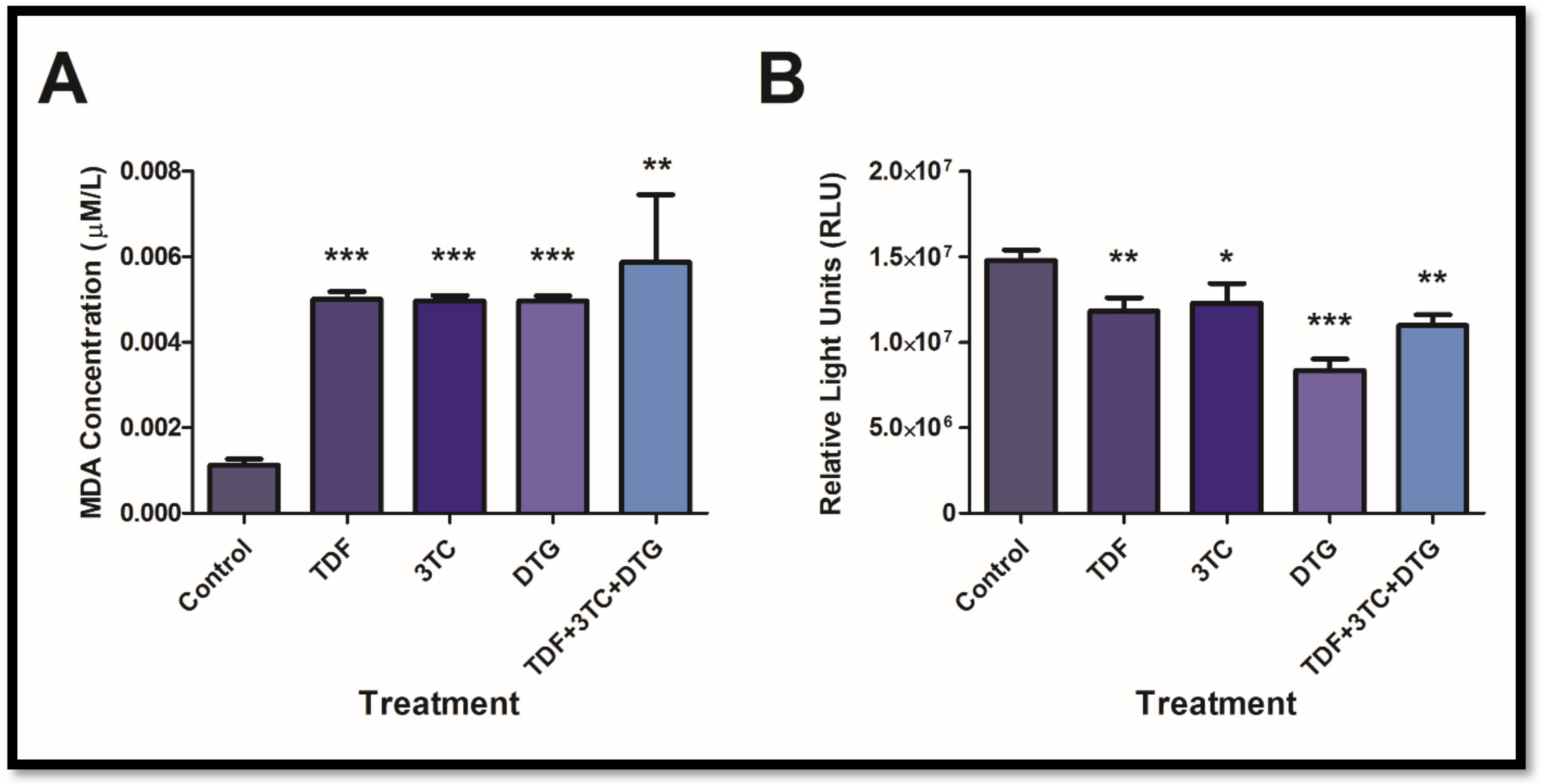
3.4. Antiretrovirals Increased Lipid Peroxidation and Decreased ATP Concentrations
4. Discussion
5. Future Recommendations and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
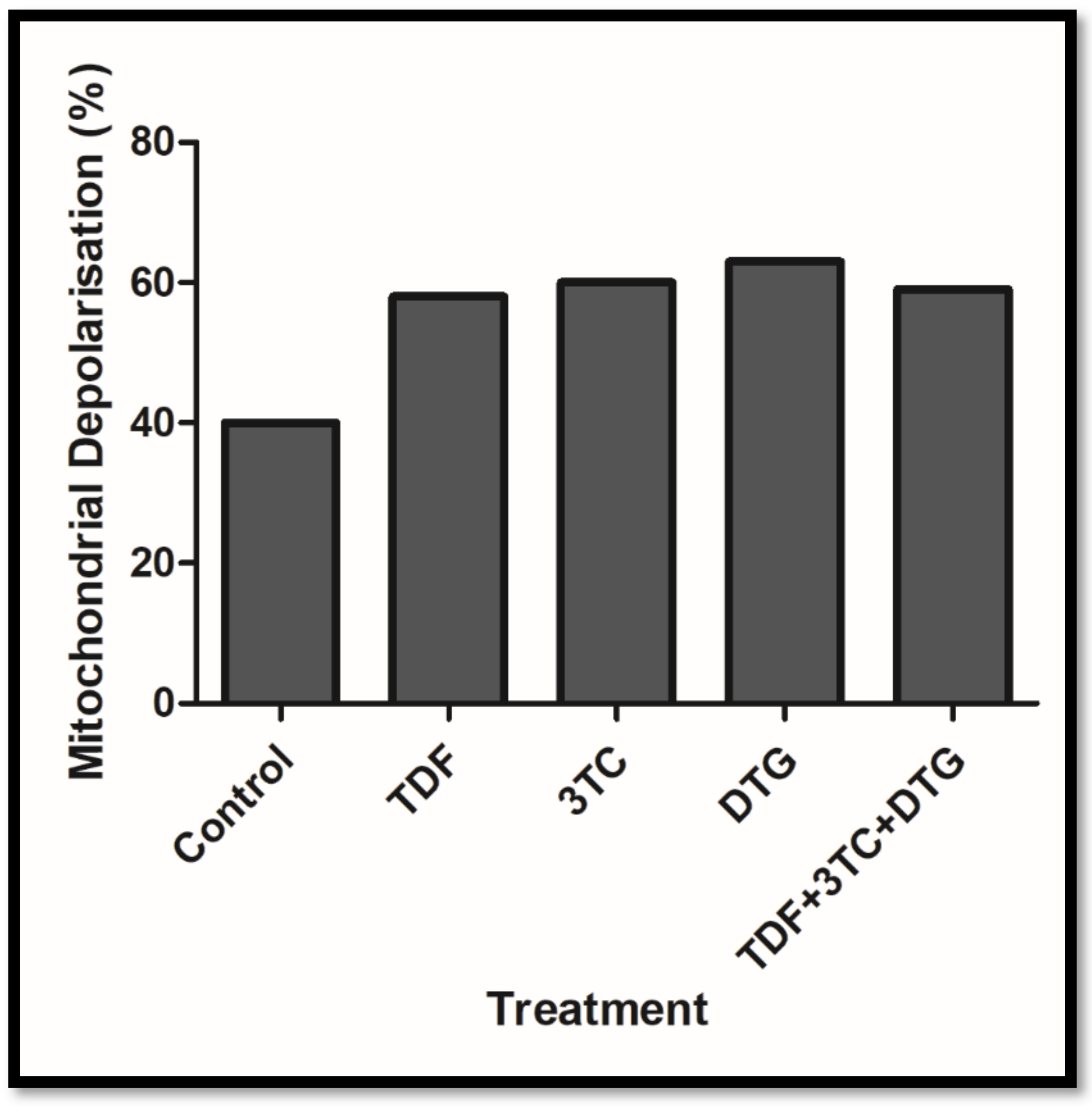
Appendix A. Mitochondrial Depolarisation
References
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2021, 1866, 165838. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Todowede, O.O.; Mianda, S.Z.; Sartorius, B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa—A systematic review and meta-analysis. Syst. Rev. 2019, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 11 February 2022).
- Stats-SA. 2020 Mid-Year Population Estimates. Available online: http://www.statssa.gov.za/?p=13453 (accessed on 11 April 2022).
- WHO. HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 20 January 2022).
- Chhoun, P.; Tuot, S.; Harries, A.D.; Kyaw, N.T.T.; Pal, K.; Mun, P.; Brody, C.; Mburu, G.; Yi, S. High prevalence of non-communicable diseases and associated risk factors amongst adults living with HIV in Cambodia. PLoS ONE 2017, 12, e0187591. [Google Scholar] [CrossRef] [PubMed]
- Hyle, E.P.; Naidoo, K.; Su, A.E.; El-Sadr, W.M.; Freedberg, K.A. HIV, tuberculosis, and non-communicable diseases: What is known about the costs, effects, and cost-effectiveness of integrated care? J. Acquir. Immune Defic. Syndr. 2014, 67, S87. [Google Scholar] [CrossRef]
- Araujo, S. Bañó n S, Machuca I, Moreno A, Pé rez-Elıas MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur. J. Endocrinol. 2014, 171, 545–554. [Google Scholar] [CrossRef]
- Aboud, M.; Elgalib, A.; Kulasegaram, R.; Peters, B. Insulin resistance and HIV infection: A review. Int. J. Clin. Pract. 2007, 61, 463–472. [Google Scholar] [CrossRef]
- Su, J.; Liu, J.; Yan, X.-Y.; Zhang, Y.; Zhang, J.-J.; Zhang, L.-C.; Sun, L.-K. Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia–reperfusion injury. Int. J. Mol. Sci. 2017, 18, 1599. [Google Scholar] [CrossRef]
- Salvatori, I.; Valle, C.; Ferri, A.; Carrì, M.T. SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem. Int. 2017, 109, 184–192. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta Vallur, P.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol.-Ren. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Deas, E.; Plun-Favreau, H.; Wood, N.W. PINK1 function in health and disease. EMBO Mol. Med. 2009, 1, 152–165. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62-and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef]
- Murata, H.; Takamatsu, H.; Liu, S.; Kataoka, K.; Huh, N.-h.; Sakaguchi, M. NRF2 regulates PINK1 expression under oxidative stress conditions. PLoS ONE 2015, 10, e0142438. [Google Scholar] [CrossRef]
- Kim, J.-a.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Leavens, K.F.; Birnbaum, M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 200–215. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- WHO. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. Available online: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf (accessed on 12 December 2021).
- Mohan, J.; Ghazi, T.; Chuturgoon, A.A. A Critical Review of the Biochemical Mechanisms and Epigenetic Modifications in HIV- and Antiretroviral-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 12020. [Google Scholar] [CrossRef] [PubMed]
- Ruoß, M.; Damm, G.; Vosough, M.; Ehret, L.; Grom-Baumgarten, C.; Petkov, M.; Naddalin, S.; Ladurner, R.; Seehofer, D.; Nussler, A. Epigenetic modifications of the liver tumor cell line HepG2 increase their drug metabolic capacity. Int. J. Mol. Sci. 2019, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Mitochondrial and oxidative stress response in HepG2 cells following acute and prolonged exposure to antiretroviral drugs. J. Cell. Biochem. 2015, 116, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Inverse association between microRNA-124a and ABCC4 in HepG2 cells treated with antiretroviral drugs. Xenobiotica 2016, 46, 825–830. [Google Scholar] [CrossRef]
- Sibiya, T.; Ghazi, T.; Mohan, J.; Nagiah, S.; Chuturgoon, A.A. Spirulina platensis Ameliorates Oxidative Stress Associated with Antiretroviral Drugs in HepG2 Cells. Plants 2022, 11, 3143. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef]
- Adnan, E.; Rahman, I.A.; Faridin, H. Relationship between insulin resistance, metabolic syndrome components and serum uric acid. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2158–2162. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef]
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 inflammasome activation in obesity-mediated metabolic disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Grdina, D.J.; Murley, J.S.; Miller, R.C.; Mauceri, H.J.; Sutton, H.G.; Thirman, M.J.; Li, J.J.; Woloschak, G.E.; Weichselbaum, R.R. A manganese superoxide dismutase (SOD2)-mediated adaptive response. Radiat. Res. 2013, 179, 115–124. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, H.-W.; Song, S.-E.; Song, D.-K. Catalase and nonalcoholic fatty liver disease. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 1721–1737. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]
- Wang, W.-J.; Mao, L.-F.; Lai, H.-L.; Wang, Y.-W.; Jiang, Z.-B.; Li, W.; Huang, J.-M.; Xie, Y.-J.; Xu, C.; Liu, P. Dolutegravir derivative inhibits proliferation and induces apoptosis of non-small cell lung cancer cells via calcium signaling pathway. Pharmacol. Res. 2020, 161, 105129. [Google Scholar] [CrossRef]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Pennington, J.D.; Andresson, T.; Rees, D.M.; Bosley, A.D.; Fearnley, I.M.; Ham, A.; Flynn, C.R.; Hill, S.; Rose, K.L. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient-and exercise-induced stress. Antioxid. Redox Signal. 2014, 21, 551–564. [Google Scholar] [CrossRef]
- Tian, X.Y.; Ma, S.; Tse, G.; Wong, W.T.; Huang, Y. Uncoupling protein 2 in cardiovascular health and disease. Front. Physiol. 2018, 9, 1060. [Google Scholar] [CrossRef]
- Adikwu, E.; Nelson, B.; Wolfe Atuboyedia, O. Melatonin and alpha lipoic acid as possible therapies for lopinavir/ritonavir-induced hepatotoxicity in albino rats. Physiol. Pharmacol. 2016, 20, 287–295. [Google Scholar]
- Hamed, M.; Aremu, G.; Akhigbe, R. Concomitant administration of HAART aggravates anti-Koch-induced oxidative hepatorenal damage via dysregulation of glutathione and elevation of uric acid production. Biomed. Pharmacother. 2021, 137, 111309. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- George, J.W.; Mattingly, J.E.; Roland, N.J.; Small, C.M.; Lamberty, B.G.; Fox, H.S.; Stauch, K.L. Physiologically Relevant Concentrations of Dolutegravir, Emtricitabine, and Efavirenz Induce Distinct Metabolic Alterations in HeLa Epithelial and BV2 Microglial Cells. Front. Immunol. 2021, 12, 639378. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, H.; Abraham, P.; Isaac, B. Mitochondrial dysfunction and electron transport chain complex defect in a rat model of tenofovir disoproxil fumarate nephrotoxicity. J. Biochem. Mol. Toxicol. 2014, 28, 246–255. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Company | Catalogue Number |
|---|---|---|
| Anti-PINK1 antibody [N4/15] | Abcam | ab186303 |
| Anti-SIRT3 | Abcam | ab264041 |
| Recombinant Anti-Nrf2 (phospho S40) antibody [EP1809Y] | Abcam | ab76026 |
| Anti-SQSTM1/p62 antibody [2C11]—BSA and Azide free | Abcam | ab56416 |
| SOD2 (D9V9C) Rabbit mAb | Cell Signalling | 13194S |
| Catalase (D4P7B) Rabbit mAb | Cell Signalling | 12980S |
| UCP2 (D1O5V) Rabbit mAb | Cell Signalling | 89326S |
| Gene | Sequence (5′-3′) | Annealing Temperature (°C) | |
|---|---|---|---|
| PINK1 | Forward Reverse | GGAGGAGTATCTGATAGGGCAG AACCCGGTGCTCTTTGTCAC | 57 |
| p62 | Forward Reverse | CAGAGAAGCCCATGGACAG AGCTGCCTTGTACCCACATC | 60 |
| GAPDH | Forward Reverse | TCCACCACCCTGTTGCTGTA ACCACAGTCCATGCCATCAC | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, J.; Ghazi, T.; Sibiya, T.; Chuturgoon, A.A. Antiretrovirals Promote Metabolic Syndrome through Mitochondrial Stress and Dysfunction: An In Vitro Study. Biology 2023, 12, 580. https://doi.org/10.3390/biology12040580
Mohan J, Ghazi T, Sibiya T, Chuturgoon AA. Antiretrovirals Promote Metabolic Syndrome through Mitochondrial Stress and Dysfunction: An In Vitro Study. Biology. 2023; 12(4):580. https://doi.org/10.3390/biology12040580
Chicago/Turabian StyleMohan, Jivanka, Terisha Ghazi, Thabani Sibiya, and Anil A. Chuturgoon. 2023. "Antiretrovirals Promote Metabolic Syndrome through Mitochondrial Stress and Dysfunction: An In Vitro Study" Biology 12, no. 4: 580. https://doi.org/10.3390/biology12040580
APA StyleMohan, J., Ghazi, T., Sibiya, T., & Chuturgoon, A. A. (2023). Antiretrovirals Promote Metabolic Syndrome through Mitochondrial Stress and Dysfunction: An In Vitro Study. Biology, 12(4), 580. https://doi.org/10.3390/biology12040580







