Leucine Repeat Rich Kinase 1 Controls Osteoclast Activity by Managing Lysosomal Trafficking and Secretion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Proteins, Plasmids, and Antibodies
2.2. Generation of Lentivirus, Transduction and Transfection
2.3. Mice, Bone Slice, and Primary Osteoclast Culture
2.4. Immunofluorescent Staining of Osteoclasts on Bone Slices
2.5. Immunoprecipitation and Western Blot
2.6. Mass Spectrometer Analyses
2.7. Acridine Orange Staining
2.8. RNA Extraction and Real-Time PCR
2.9. Statistical Analyses
3. Results
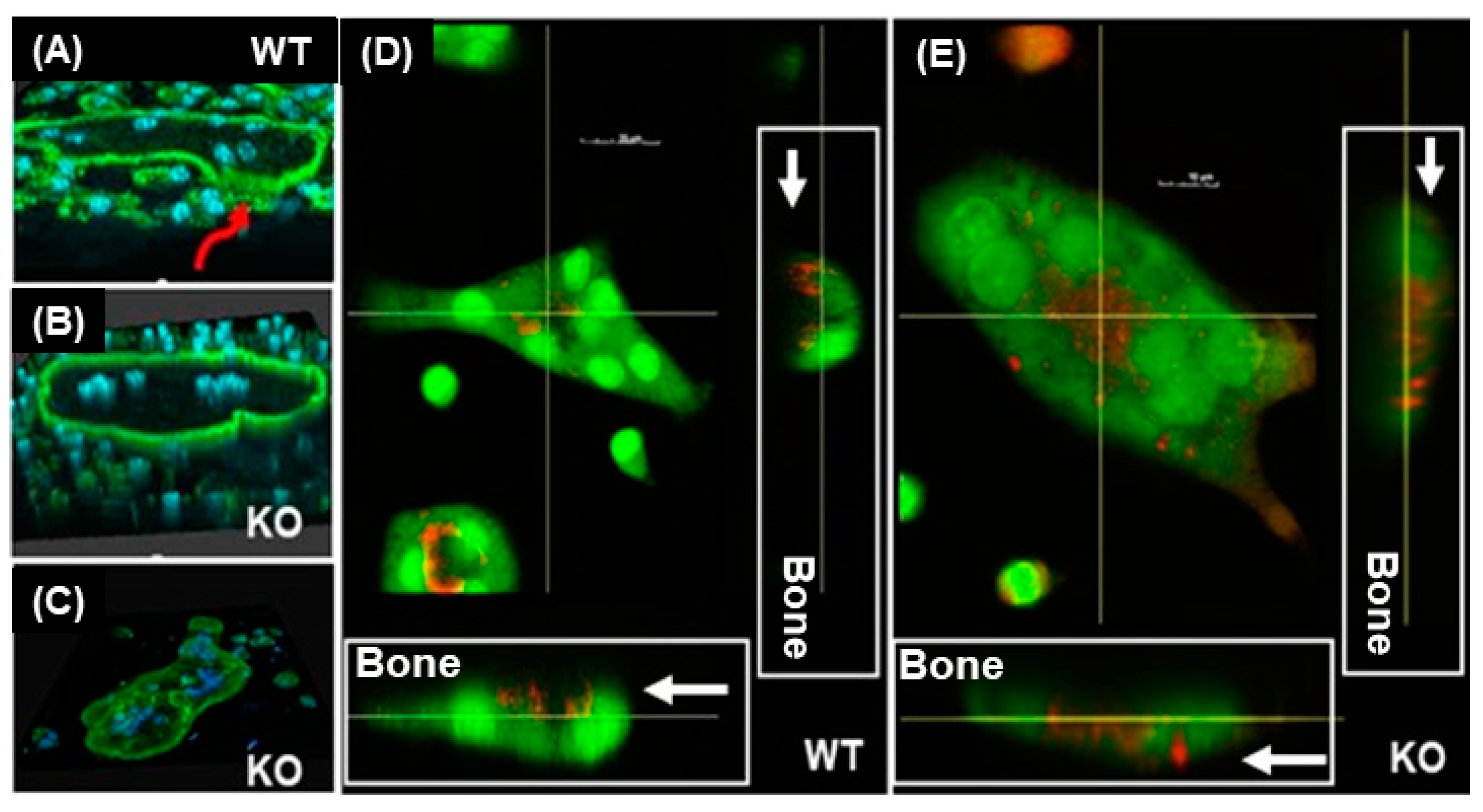
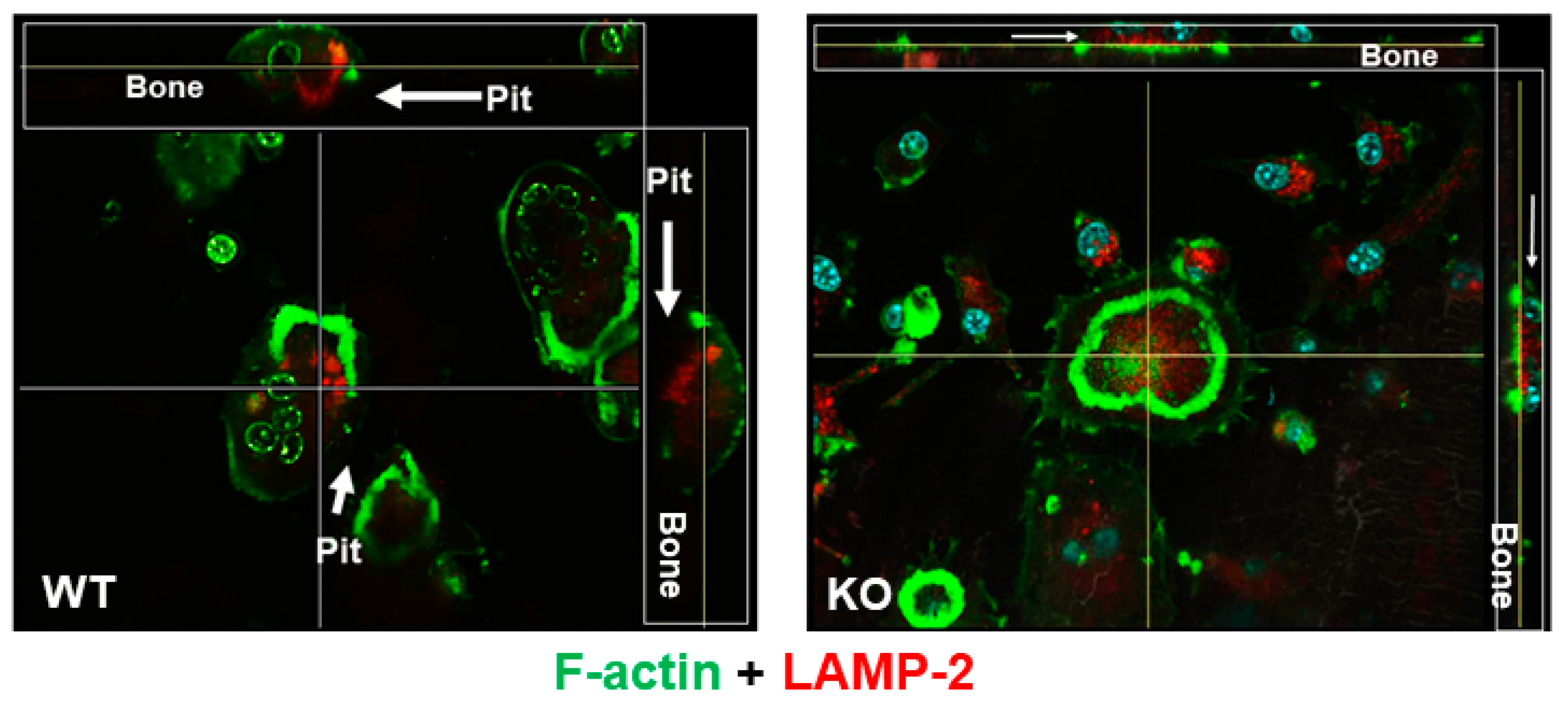
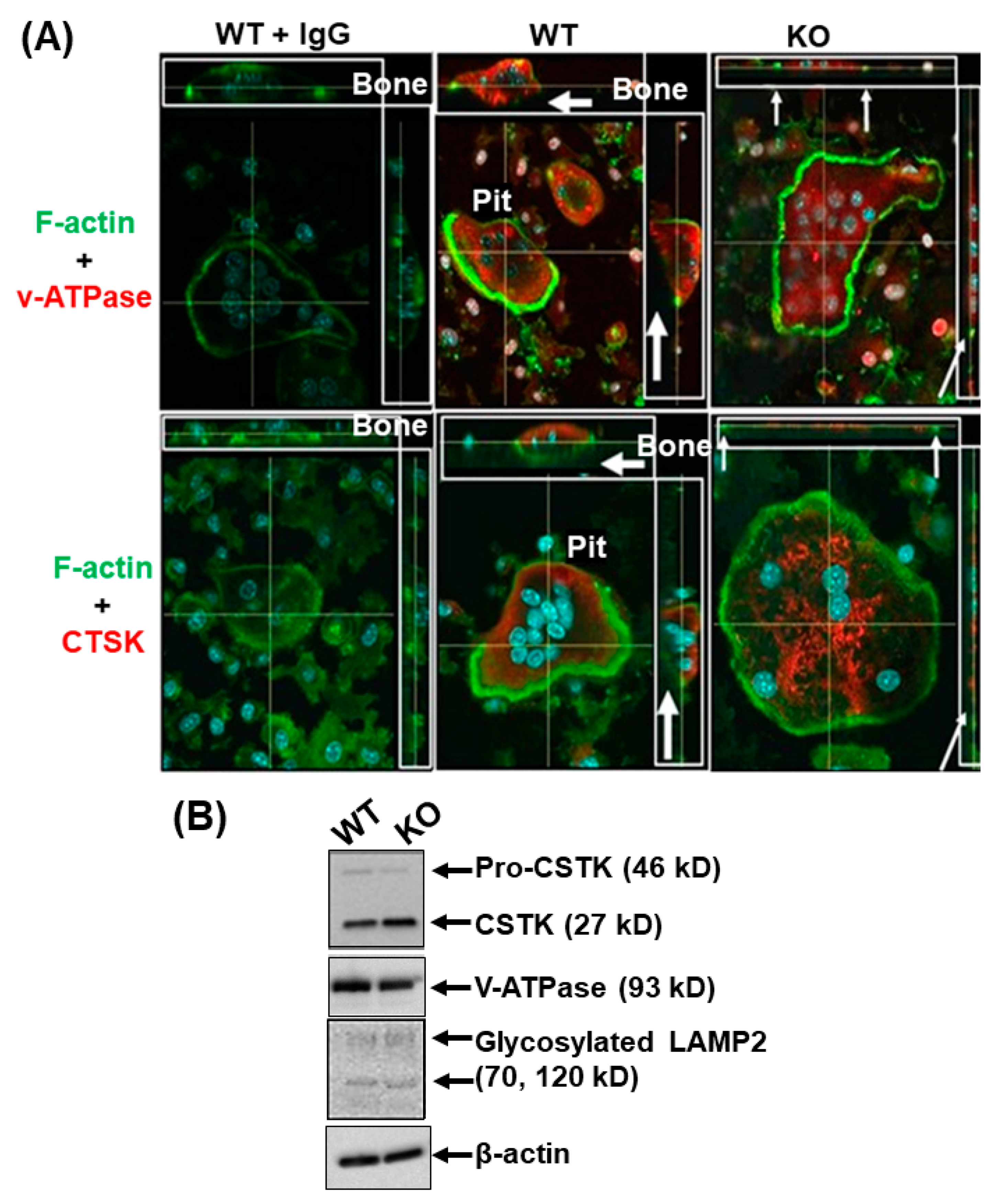
3.1. Lack of LRRK1 in Osteoclasts Distrupts Cytoskeleton Arrangement and Impairs Lysosomal Distribution and Extracellular Acid Secretion
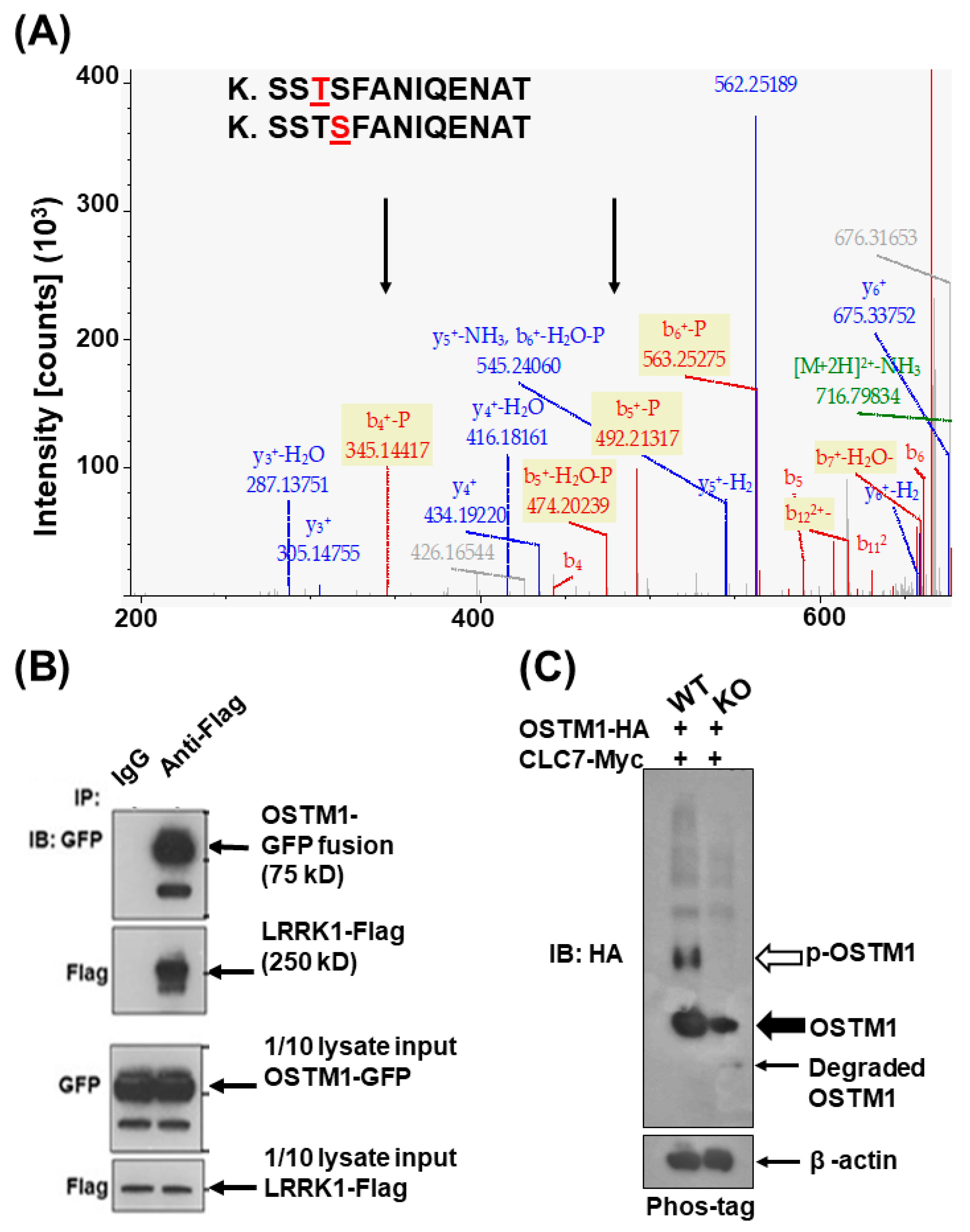
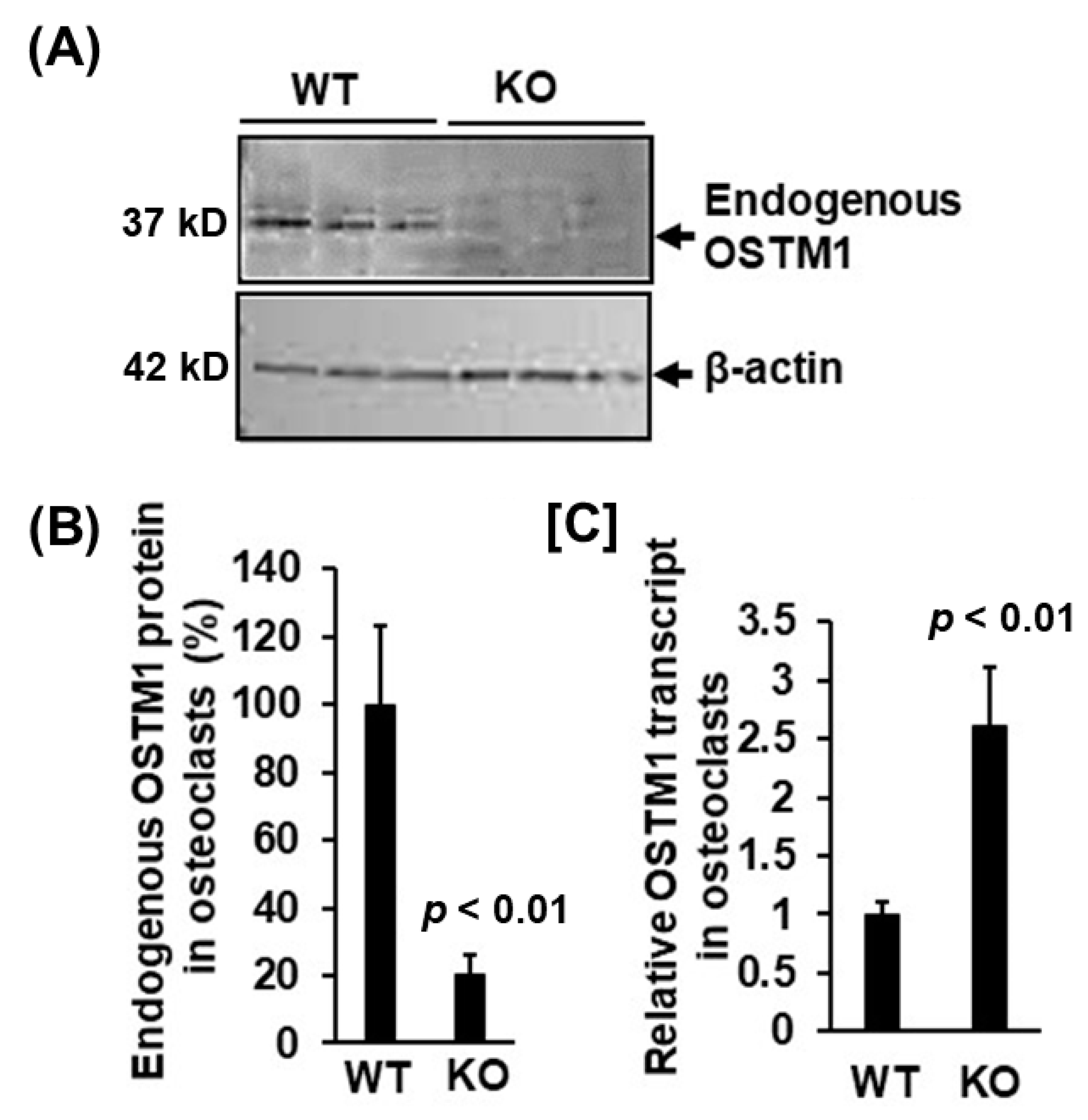
3.2. LRRK1 Interacts and Stabolizes Osteoperosis Specific Transmembrane Protein 1 in Osteoclasts
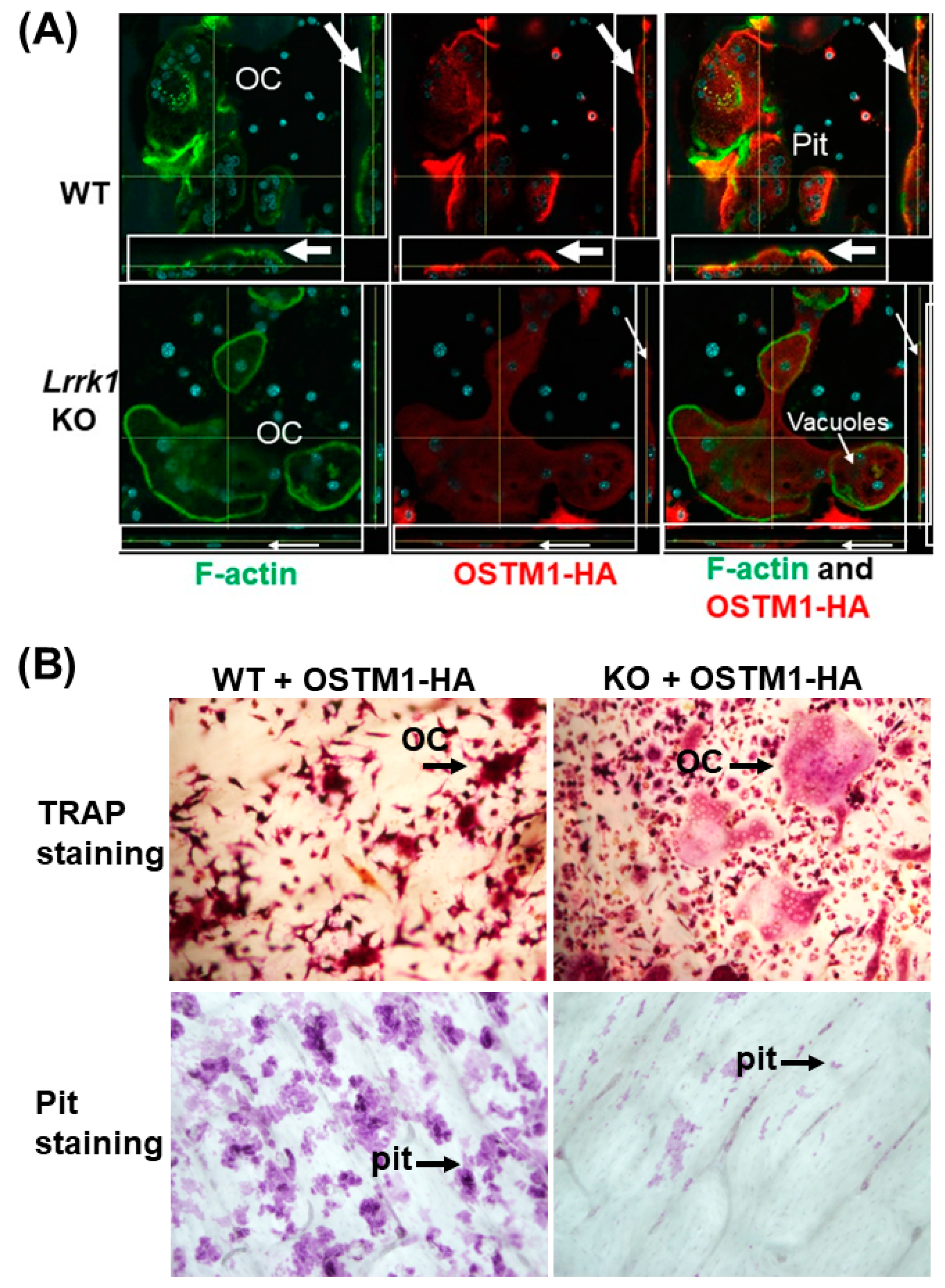
3.3. Exdogenous OSTM1 Overexpressed in LRRK1 Deficient Osteoclasts Is Not Positioned at the Peripheral Ruffled Border
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orwig, D.L.; Chiles, N.; Jones, M.; Hochberg, M.C. Osteoporosis in men: Update 2011. Rheum. Dis. Clin. North Am. 2011, 37, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Ortendahl, J.D.; Vanderpuye-Orgle, J.; Grauer, A.; Arellano, J.; Lemay, J.; Harmon, A.L.; Broder, M.S.; Singer, A.J. Healthcare Policy Changes in Osteoporosis Can Improve Outcomes and Reduce Costs in the United States. JBMR Plus 2019, 3, e10192. [Google Scholar] [CrossRef]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.P.; Delaisse, J.M. Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 2021, 145, 115850. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M. Long-term safety of bisphosphonates. J. Clin. Endocrinol. Metab. 2005, 90, 1897–1899. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Hayes, A.; Hunzelman, J.L.; Wyland, J.J.; Lee, H.; Neer, R.M. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N. Engl. J. Med. 2003, 349, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Denosumab. Limited efficacy in fracture prevention, too many adverse effects. Prescrire Int. 2011, 20, 145–148. [Google Scholar]
- Kidd, L.J.; Cowling, N.R.; Wu, A.C.; Kelly, W.L.; Forwood, M.R. Bisphosphonate treatment delays stress fracture remodeling in the rat ulna. J. Orthop. Res. 2011, 29, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Nase, J.B.; Suzuki, J.B. Osteonecrosis of the jaw and oral bisphosphonate treatment. J. Am. Dent. Assoc. 2006, 137, 1115–1119. [Google Scholar] [CrossRef]
- Taylor, K.H.; Middlefell, L.S.; Mizen, K.D. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br. J. Oral. Maxillofac. Surg. 2010, 48, 221–223. [Google Scholar] [CrossRef]
- Schilcher, J.; Michaelsson, K.; Aspenberg, P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011, 364, 1728–1737. [Google Scholar] [CrossRef]
- Sellmeyer, D.E. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 2010, 304, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Oursler, M.J.; Khosla, S. Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned. Endocr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Dempster, D.W.; Ding, B.; Dent-Acosta, R.; San Martin, J.; Grauer, A.; Wagman, R.B.; Zanchetta, J. Bone remodeling in postmenopausal women who discontinued denosumab treatment: Off-treatment biopsy study. J. Bone Miner. Res. 2011, 26, 2737–2744. [Google Scholar] [CrossRef]
- Marin, I. The Parkinson disease gene LRRK2: Evolutionary and structural insights. Mol. Biol. Evol. 2006, 23, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Korr, D.; Toschi, L.; Donner, P.; Pohlenz, H.D.; Kreft, B.; Weiss, B. LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal 2006, 18, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Civiero, L.; Vancraenenbroeck, R.; Belluzzi, E.; Beilina, A.; Lobbestael, E.; Reyniers, L.; Gao, F.; Micetic, I.; De Maeyer, M.; Bubacco, L.; et al. Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS ONE 2012, 7, e43472. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, J.; Cheng, S.; Vogel, P.; Mohan, S.; Brommage, R. Targeted disruption of leucine-rich repeat kinase 1 but not leucine-rich repeat kinase 2 in mice causes severe osteopetrosis. J. Bone Miner. Res. 2013, 28, 1962–1974. [Google Scholar] [CrossRef]
- Iida, A.; Xing, W.; Docx, M.K.; Nakashima, T.; Wang, Z.; Kimizuka, M.; Van Hul, W.; Rating, D.; Spranger, J.; Ohashi, H.; et al. Identification of biallelic LRRK1 mutations in osteosclerotic metaphyseal dysplasia and evidence for locus heterogeneity. J. Med. Genet. 2016, 53, 568–574. [Google Scholar] [CrossRef]
- Lange, P.F.; Wartosch, L.; Jentsch, T.J.; Fuhrmann, J.C. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 2006, 440, 220–223. [Google Scholar] [CrossRef]
- Pata, M.; Vacher, J. Ostm1 Bifunctional Roles in Osteoclast Maturation: Insights From a Mouse Model Mimicking a Human OSTM1 Mutation. J. Bone Miner. Res. 2018, 33, 888–898. [Google Scholar] [CrossRef]
- Rajapurohitam, V.; Chalhoub, N.; Benachenhou, N.; Neff, L.; Baron, R.; Vacher, J. The mouse osteopetrotic grey-lethal mutation induces a defect in osteoclast maturation/function. Bone 2001, 28, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, N.; Benachenhou, N.; Rajapurohitam, V.; Pata, M.; Ferron, M.; Frattini, A.; Villa, A.; Vacher, J. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat. Med. 2003, 9, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Goodluck, H.; Qin, X.; Liu, B.; Mohan, S.; Xing, W. Leucine Rich Repeat Kinase 1 Regulates Osteoclast Function by Modulating RAC1/Cdc42 Small GTPase Phosphorylation and Activation. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E772–E780. [Google Scholar] [CrossRef] [PubMed]
- Pandruvada, S.N.; Beauregard, J.; Benjannet, S.; Pata, M.; Lazure, C.; Seidah, N.G.; Vacher, J. Role of Ostm1 Cytosolic Complex with Kinesin 5B in Intracellular Dispersion and Trafficking. Mol. Cell Biol. 2016, 36, 507–521. [Google Scholar] [CrossRef]
- Xing, W.; Singgih, A.; Kapoor, A.; Alarcon, C.M.; Baylink, D.J.; Mohan, S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J. Biol. Chem. 2007, 282, 22052–22061. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Goodluck, H.; Zeng, C.; Pan, S.; Todd, E.M.; Morley, S.C.; Qin, X.; Mohan, S.; Xing, W. LRRK1 regulation of actin assembly in osteoclasts involves serine 5 phosphorylation of L-plastin. J. Cell. Biochem. 2018, 119, 10351–10357. [Google Scholar] [CrossRef]
- Xing, W.; Kim, J.; Wergedal, J.; Chen, S.T.; Mohan, S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol. Cell Biol. 2010, 30, 711–721. [Google Scholar] [CrossRef]
- Baron, R.; Neff, L.; Louvard, D.; Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985, 101, 2210–2222. [Google Scholar] [CrossRef]
- Brommage, R.; Liu, J.; Hansen, G.M.; Kirkpatrick, L.L.; Potter, D.G.; Sands, A.T.; Zambrowicz, B.; Powell, D.R.; Vogel, P. High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes. Bone Res. 2014, 2, 14034. [Google Scholar] [CrossRef]
- Hanafusa, H.; Ishikawa, K.; Kedashiro, S.; Saigo, T.; Iemura, S.; Natsume, T.; Komada, M.; Shibuya, H.; Nara, A.; Matsumoto, K. Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat. Commun. 2011, 2, 158. [Google Scholar] [CrossRef]
- Kedashiro, S.; Pastuhov, S.I.; Nishioka, T.; Watanabe, T.; Kaibuchi, K.; Matsumoto, K.; Hanafusa, H. LRRK1-phosphorylated CLIP-170 regulates EGFR trafficking by recruiting p150Glued to microtubule plus ends. J. Cell Sci. 2015, 128, 385–396. [Google Scholar] [CrossRef]
- Yi, T.; Lee, H.L.; Cha, J.H.; Ko, S.I.; Kim, H.J.; Shin, H.I.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H. Epidermal growth factor receptor regulates osteoclast differentiation and survival through cross-talking with RANK signaling. J. Cell. Physiol. 2008, 217, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tamasi, J.; Lu, X.; Zhu, J.; Chen, H.; Tian, X.; Lee, T.C.; Threadgill, D.W.; Kream, B.E.; Kang, Y. Epidermal growth factor receptor plays an anabolic role in bone metabolism in vivo. J. Bone Miner. Res. 2011, 26, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, H.; Kedashiro, S.; Tezuka, M.; Funatsu, M.; Usami, S.; Toyoshima, F.; Matsumoto, K. PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat. Cell Biol. 2015, 17, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.E.; Bernstein, S.E. Hertwig’s anemia: Characterization of the stem cell defect. Blood 1983, 61, 765–769. [Google Scholar] [CrossRef]
- Lizarraga, S.B.; Margossian, S.P.; Harris, M.H.; Campagna, D.R.; Han, A.P.; Blevins, S.; Mudbhary, R.; Barker, J.E.; Walsh, C.A.; Fleming, M.D.; et al. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development 2010, 137, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Investig. 2000, 105, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Soriano, P.; Montgomery, C.; Geske, R.; Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991, 64, 693–702. [Google Scholar] [CrossRef]
- Croke, M.; Ross, F.P.; Korhonen, M.; Williams, D.A.; Zou, W.; Teitelbaum, S.L. Rac deletion in osteoclasts causes severe osteopetrosis. J. Cell Sci. 2011, 124, 3811–3821. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, B.H.; Saar, K.; Simpson, C.; Troiano, N.; Dallas, S.L.; Tiede-Lewis, L.M.; Nevius, E.; Pereira, J.P.; Weinstein, R.S.; et al. Deletion of Rac in Mature Osteoclasts Causes Osteopetrosis, an Age-Dependent Change in Osteoclast Number and a Reduced Number of Osteoblasts In Vivo. J. Bone Miner. Res. 2016, 31, 864–873. [Google Scholar] [CrossRef]
- Izawa, T.; Zou, W.; Chappel, J.C.; Ashley, J.W.; Feng, X.; Teitelbaum, S.L. C-Src Links a RANK/alphavbeta3 Integrin Complex to the Osteoclast Cytoskeleton. Mol. Cell Biol. 2012, 32, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Zeng, C.; Goodluck, H.; Shen, S.; Mohan, S.; Xing, W. A small molecular inhibitor of LRRK1 identified by homology modeling and virtual screening suppresses osteoclast function, but not osteoclast differentiation, in vitro. Aging 2019, 11, 3250–3261. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Teitelbaum, S.L.; Zou, W.; Zheng, Y.; Johnson, J.F.; Chappel, J.; Ross, F.P.; Zhao, H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J. Clin. Investig. 2010, 120, 1981–1993. [Google Scholar] [CrossRef]
- Malik, A.U.; Karapetsas, A.; Nirujogi, R.S.; Mathea, S.; Chatterjee, D.; Pal, P.; Lis, P.; Taylor, M.; Purlyte, E.; Gourlay, R.; et al. Deciphering the LRRK code: LRRK1 and LRRK2 phosphorylate distinct Rab proteins and are regulated by diverse mechanisms. Biochem. J. 2021, 478, 553–578. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; Stevens, M.W.; So, L.; Edinger, A.L. Reciprocal effects of rab7 deletion in activated and neglected T cells. Autophagy 2013, 9, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, H.; Yagi, T.; Ikeda, H.; Hisamoto, N.; Nishioka, T.; Kaibuchi, K.; Shirakabe, K.; Matsumoto, K. LRRK1 phosphorylation of Rab7 at S72 links trafficking of EGFR-containing endosomes to its effector RILP. J. Cell Sci. 2019, 132, jcs228809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Laitala-Leinonen, T.; Parikka, V.; Vaananen, H.K. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J. Biol. Chem. 2001, 276, 39295–39302. [Google Scholar] [CrossRef]
- Verhoeven, K.; De Jonghe, P.; Coen, K.; Verpoorten, N.; Auer-Grumbach, M.; Kwon, J.M.; FitzPatrick, D.; Schmedding, E.; De Vriendt, E.; Jacobs, A.; et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 2003, 72, 722–727. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ye, S.; Castro-Gomes, T.; Winchell, C.G.; Andrews, N.W.; Voth, D.E.; Varughese, K.I.; Mackintosh, S.G.; Feng, Y.; Pavlos, N.; et al. PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight 2016, 1, e86330. [Google Scholar] [CrossRef]
- Das, B.K.; Gogoi, J.; Kannan, A.; Gao, L.; Xing, W.; Mohan, S.; Zhao, H. The Cytoplasmic Dynein Associated Protein NDE1 Regulates Osteoclastogenesis by Modulating M-CSF and RANKL Signaling Pathways. Cells 2021, 11, 13. [Google Scholar] [CrossRef]
- Wang, G.; Nola, S.; Bovio, S.; Bun, P.; Coppey-Moisan, M.; Lafont, F.; Galli, T. Biomechanical Control of Lysosomal Secretion Via the VAMP7 Hub: A Tug-of-War between VARP and LRRK1. iScience 2018, 4, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Hashimoto, K.; Panchenko, A.R. Phosphorylation in protein-protein binding: Effect on stability and function. Structure 2011, 19, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Faupel, J.; Goebel, I.; Stiller, A.; Beyer, S.; Stockle, C.; Hasan, C.; Bode, U.; Kornak, U.; Kubisch, C. Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum. Mutat. 2004, 23, 471–476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Si, M.; Zeng, C.; Liu, E.K.; Chen, Y.; Vacher, J.; Zhao, H.; Mohan, S.; Xing, W. Leucine Repeat Rich Kinase 1 Controls Osteoclast Activity by Managing Lysosomal Trafficking and Secretion. Biology 2023, 12, 511. https://doi.org/10.3390/biology12040511
Shen S, Si M, Zeng C, Liu EK, Chen Y, Vacher J, Zhao H, Mohan S, Xing W. Leucine Repeat Rich Kinase 1 Controls Osteoclast Activity by Managing Lysosomal Trafficking and Secretion. Biology. 2023; 12(4):511. https://doi.org/10.3390/biology12040511
Chicago/Turabian StyleShen, Sandi, Mingjue Si, Canjun Zeng, Elaine K. Liu, Yian Chen, Jean Vacher, Haibo Zhao, Subburaman Mohan, and Weirong Xing. 2023. "Leucine Repeat Rich Kinase 1 Controls Osteoclast Activity by Managing Lysosomal Trafficking and Secretion" Biology 12, no. 4: 511. https://doi.org/10.3390/biology12040511
APA StyleShen, S., Si, M., Zeng, C., Liu, E. K., Chen, Y., Vacher, J., Zhao, H., Mohan, S., & Xing, W. (2023). Leucine Repeat Rich Kinase 1 Controls Osteoclast Activity by Managing Lysosomal Trafficking and Secretion. Biology, 12(4), 511. https://doi.org/10.3390/biology12040511





