Antineoplastic Nature of WWOX in Glioblastoma Is Mainly a Consequence of Reduced Cell Viability and Invasion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Stable Transduction
2.3. Protein Extraction and Western Blot
2.4. Assessment of Redox Potential, Proliferation and Apoptosis
2.5. Adhesion Assay
2.6. Integrin-Mediated Cell Adhesion
2.7. Clonogenic Assay
2.8. Suspension Growth Test
2.9. Three-Dimensional Culture Growth Assay
2.10. Invasion Assay
2.11. Cumulative Degradation of Gelatin Layer
2.12. Gelatin Zymography Assay
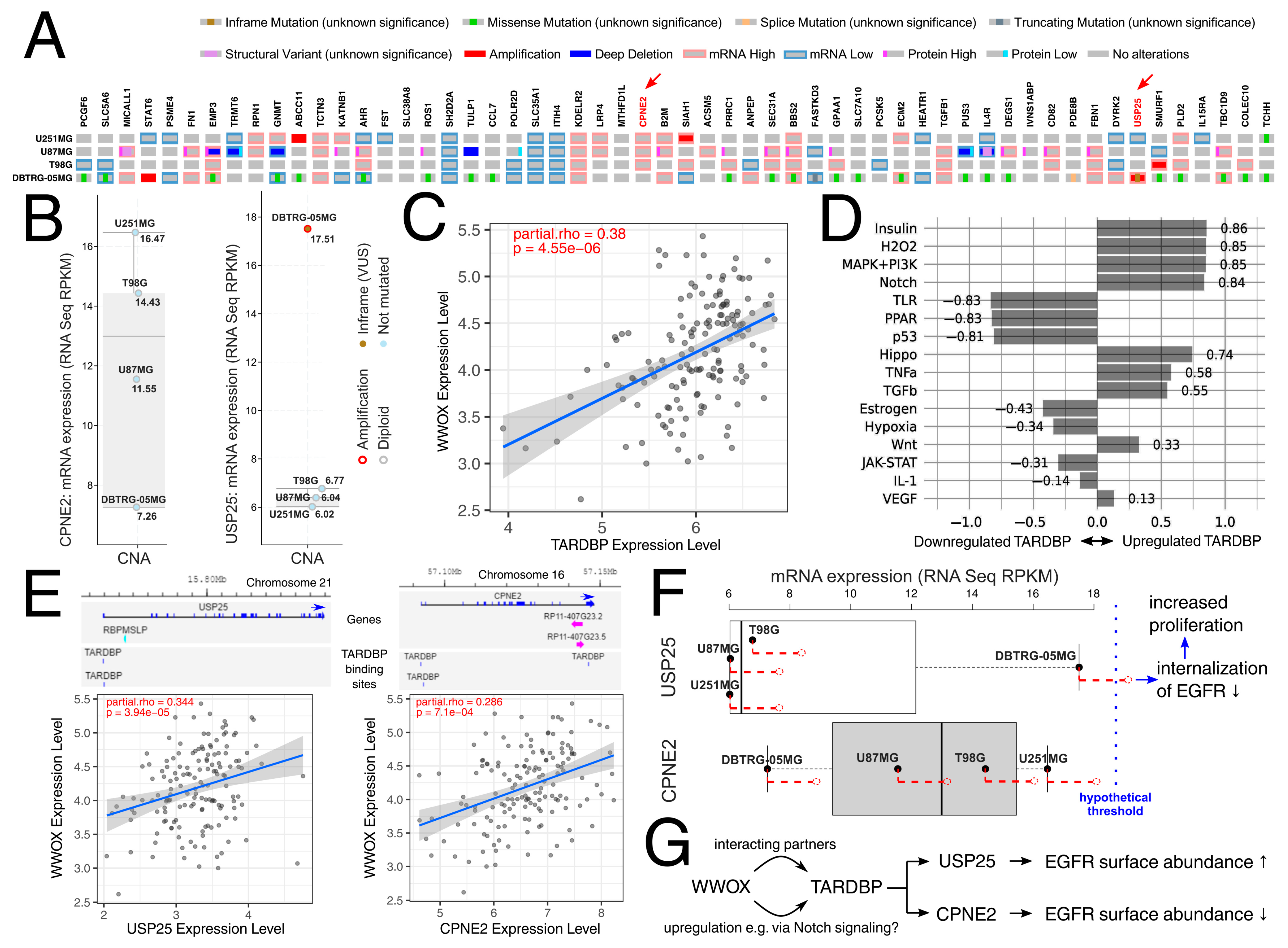
2.13. Bioinformatics Analysis
2.14. Statistical Analysis
3. Results
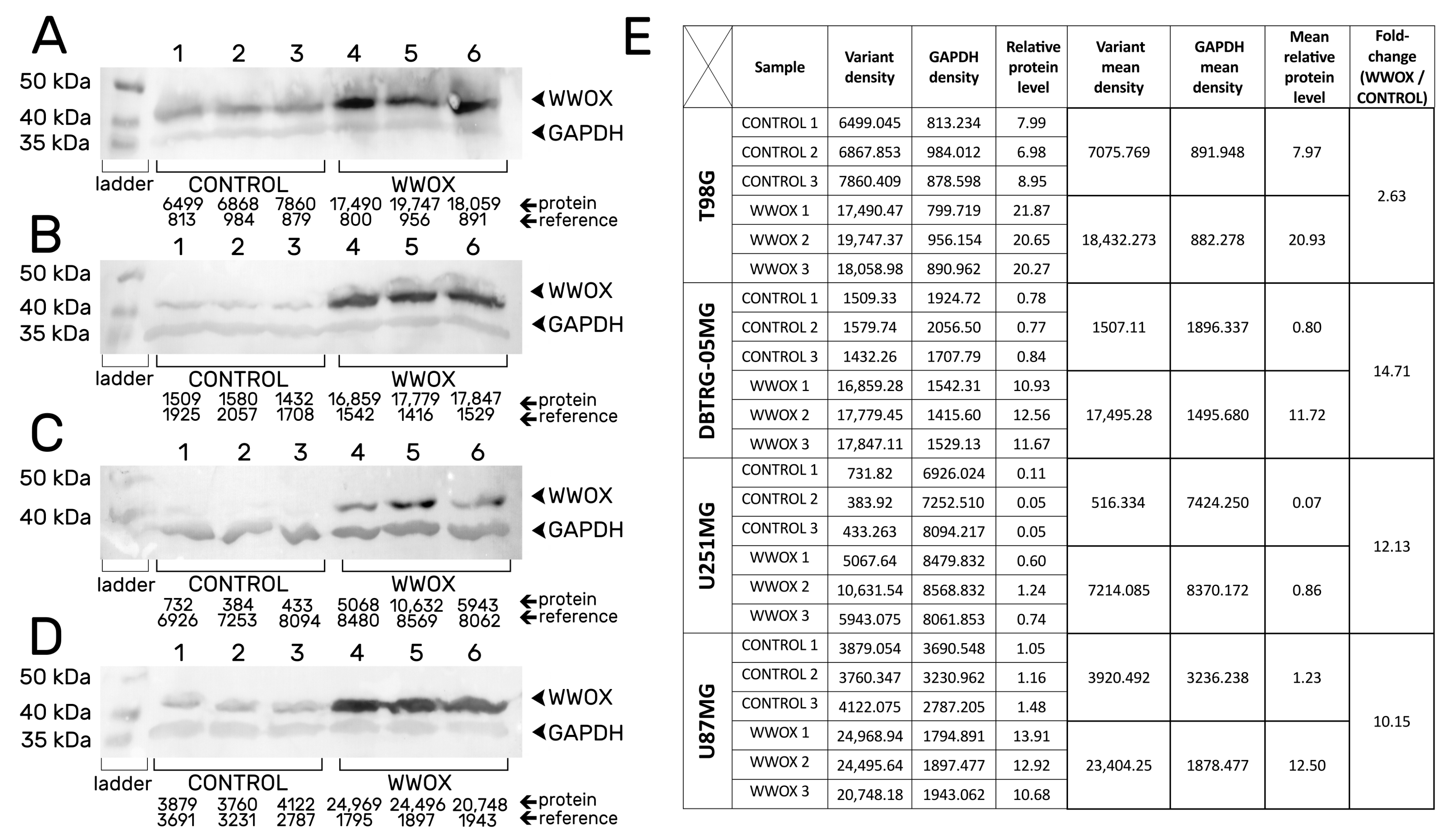
3.1. Confirmation of the Obtaining of Stable Transductants
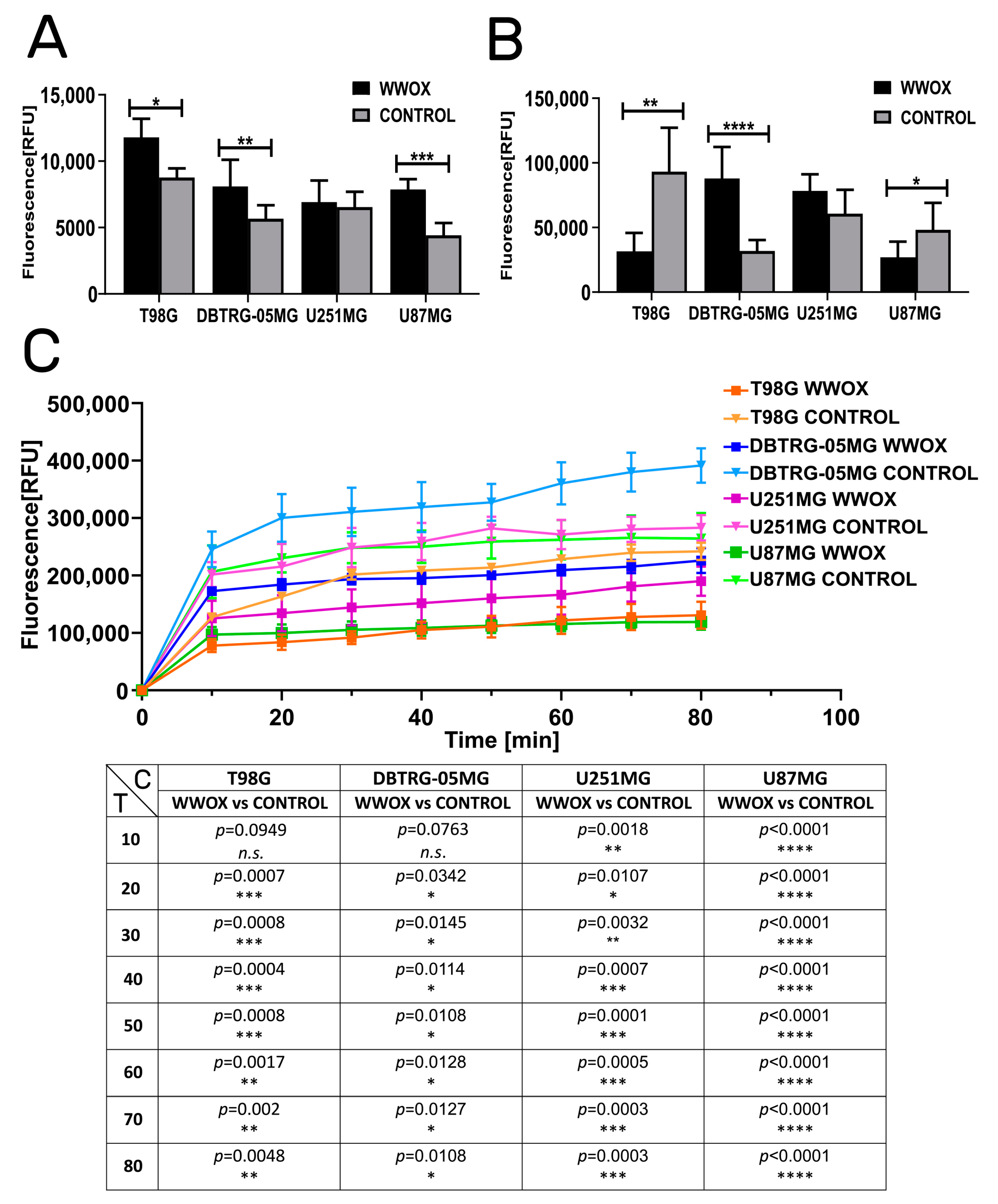
3.2. WWOX Intensified the Apoptosis but Reduced Mitochondrial Redox Potential
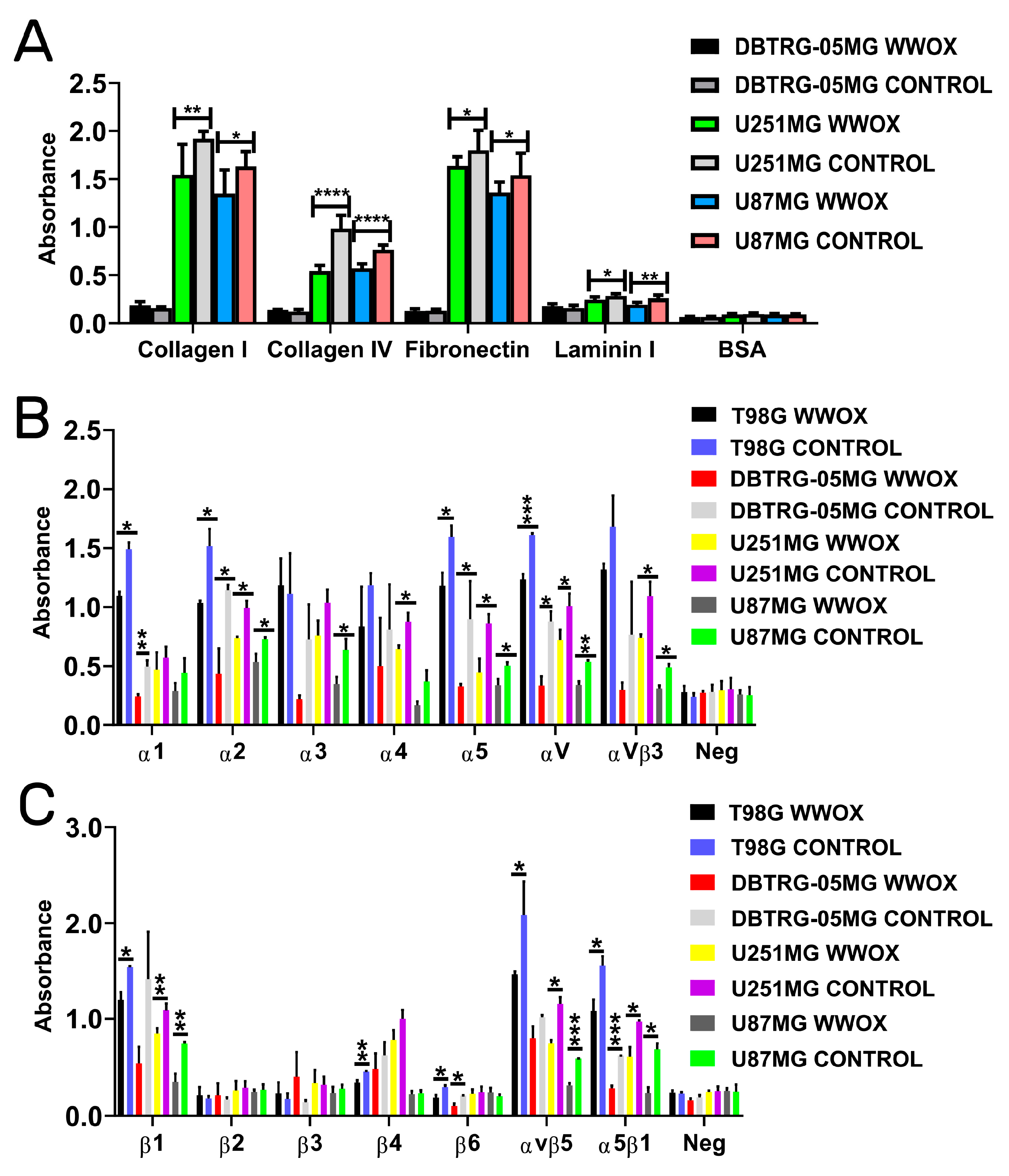
3.3. Overexpression of WWOX Decreased the Adhesion to Collagen I, Collagen IV, Fibronectin, and Laminin I
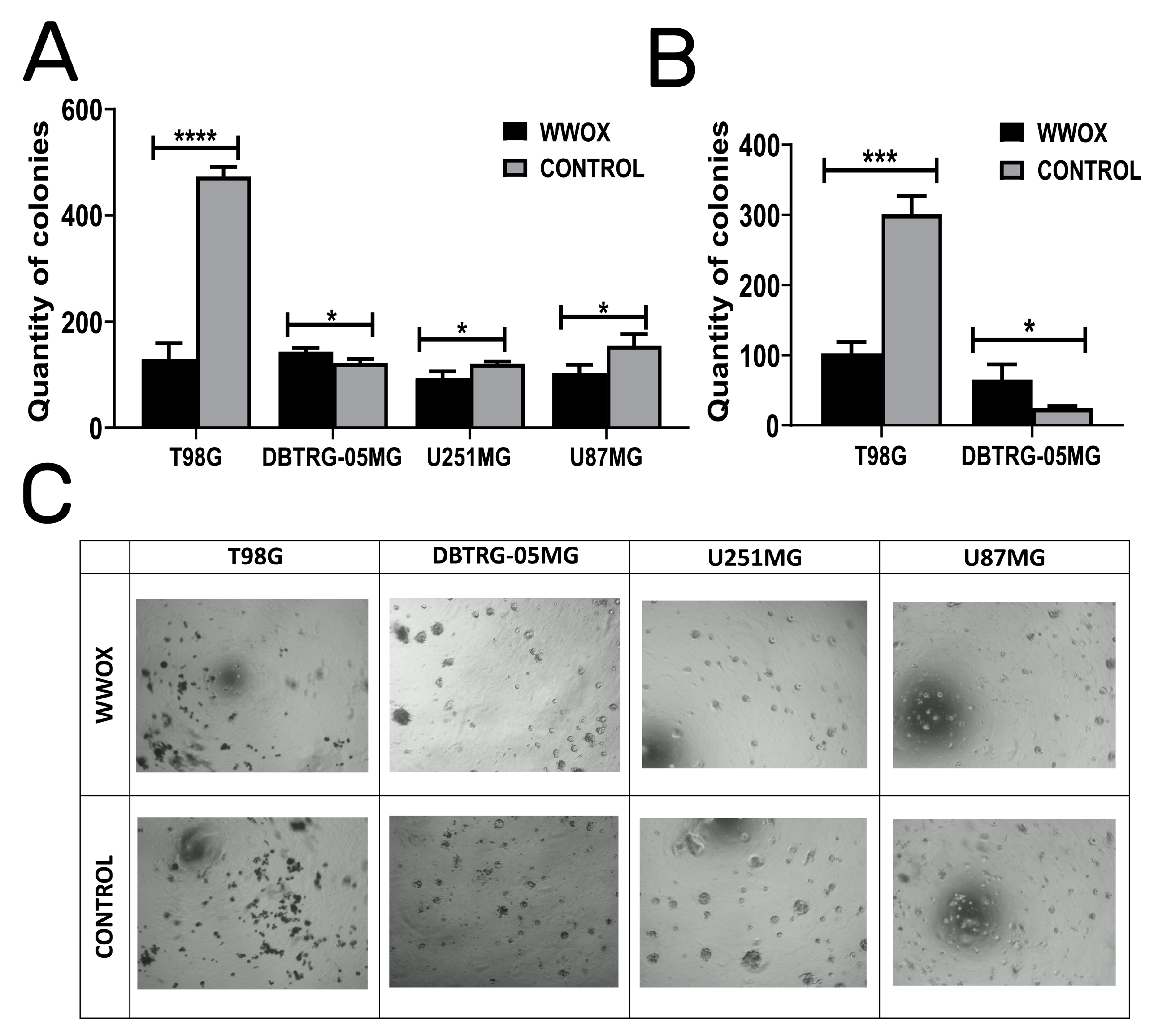
3.4. WWOX Acts in an Opposite Manner in DBTRG-05MG: Enhancing Colony Forming Abilities, Suspension Growth, and Increasing the Size of Dimensional Spheres
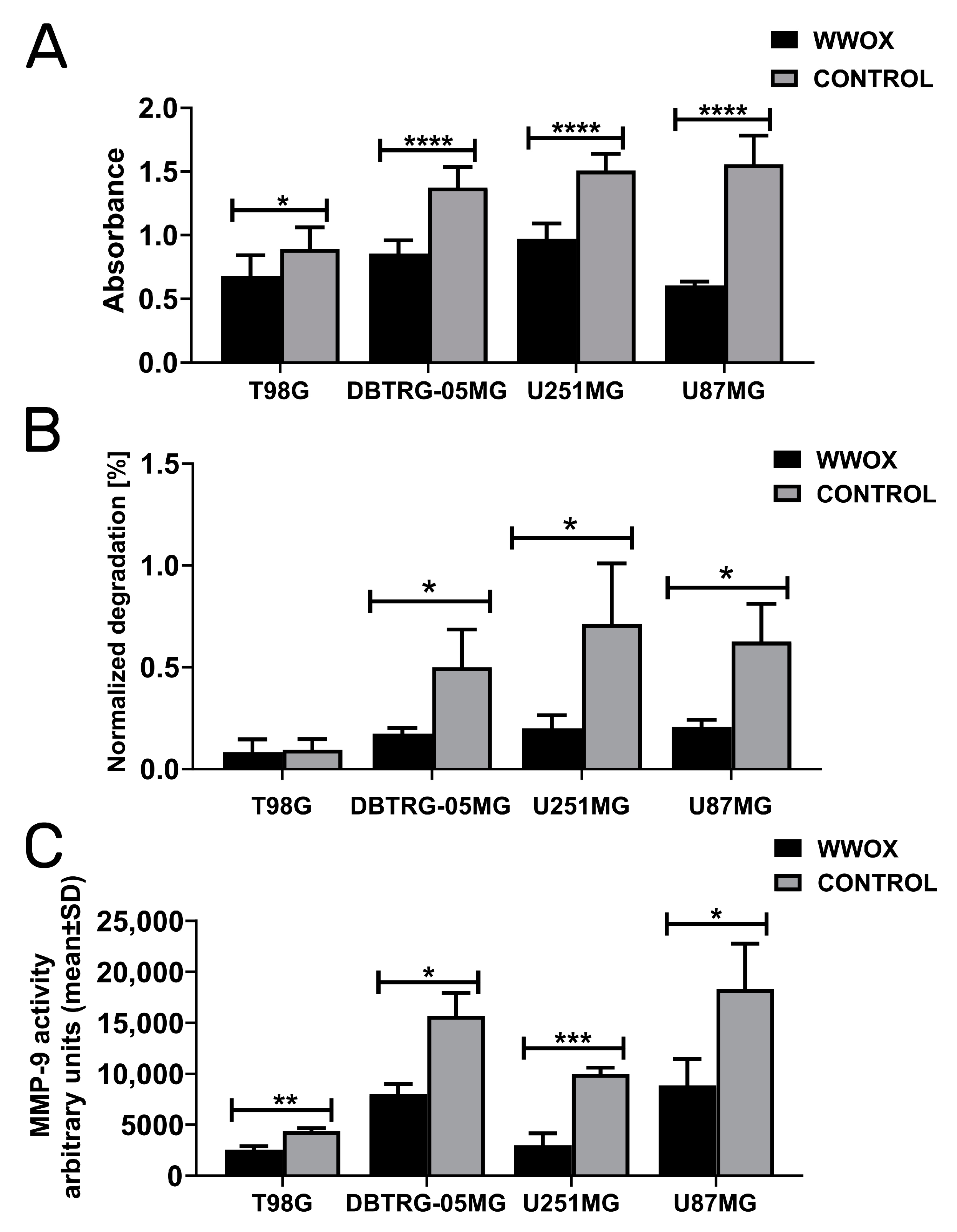
3.5. WWOX Acted Consistently as a Suppressor of Invasion, Gelatin Degradation, and MMP-9
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar] [PubMed]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhu, X.; Liao, H.; Ouyang, L.; Huang, Y.; Syeda, M.Z.; Ying, S. New Era of Mapping and Understanding Common Fragile Sites: An Updated Review on Origin of Chromosome Fragility. Front. Genet. 2022, 13, 906957. [Google Scholar] [CrossRef]
- Smith, D.I.; Zhu, Y.; McAvoy, S.; Kuhn, R. Common fragile sites, extremely large genes, neural development and cancer. Cancer Lett. 2006, 232, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.S.; Salama, S.R.; Lambert, N.; Lambot, M.A.; Coppens, S.; Pedersen, J.S.; Katzman, S.; King, B.; Onodera, C.; Siepel, A.; et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature 2006, 443, 167–172. [Google Scholar] [CrossRef]
- Doan, R.N.; Bae, B.I.; Cubelos, B.; Chang, C.; Hossain, A.A.; Al-Saad, S.; Mukaddes, N.M.; Oner, O.; Al-Saffar, M.; Balkhy, S.; et al. Mutations in Human Accelerated Regions Disrupt Cognition and Social Behavior. Cell 2016, 167, 341–354.e12. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor suppressor WWOX regulates glucose metabolism via HIF1alpha modulation. Cell. Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Joy-Dodson, E.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015, 290, 30728–30735. [Google Scholar] [CrossRef]
- Baryla, I.; Styczen-Binkowska, E.; Pluciennik, E.; Kosla, K.; Bednarek, A.K. The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3326. [Google Scholar] [CrossRef]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Abu-Remaileh, M.; Akkawi, R.; Knani, I.; Udi, S.; Pacold, M.E.; Tam, J.; Aqeilan, R.I. WWOX somatic ablation in skeletal muscles alters glucose metabolism. Mol. Metab. 2019, 22, 132–140. [Google Scholar] [CrossRef]
- Steinberg, D.J.; Aqeilan, R.I. WWOX-Related Neurodevelopmental Disorders: Models and Future Perspectives. Cells 2021, 10, 3082. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Hereema, N.A.; Aqeilan, R.I. WWOX modulates the ATR-mediated DNA damage checkpoint response. Oncotarget 2016, 7, 4344–4355. [Google Scholar] [CrossRef]
- Baryla, I.; Styczen-Binkowska, E.; Bednarek, A.K. Alteration of WWOX in human cancer: A clinical view. Exp. Biol. Med. 2015, 240, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Donati, V.; Gaudio, E.; Nicoloso, M.S.; Sundvall, M.; Korhonen, A.; Lundin, J.; Isola, J.; Sudol, M.; Joensuu, H.; et al. Association of Wwox with ErbB4 in breast cancer. Cancer Res. 2007, 67, 9330–9336. [Google Scholar] [CrossRef]
- Bonin, F.; Taouis, K.; Azorin, P.; Petitalot, A.; Tariq, Z.; Nola, S.; Bouteille, N.; Tury, S.; Vacher, S.; Bieche, I.; et al. VOPP1 promotes breast tumorigenesis by interacting with the tumor suppressor WWOX. BMC Biol. 2018, 16, 109. [Google Scholar] [CrossRef]
- Chang, R.; Song, L.; Xu, Y.; Wu, Y.; Dai, C.; Wang, X.; Sun, X.; Hou, Y.; Li, W.; Zhan, X.; et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat. Commun. 2018, 9, 3486. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.; Uner, A.; Guler, N.; Han, S.Y.; Iliopoulos, D.; Hauck, W.W.; McCue, P.; Huebner, K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004, 100, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Khawaled, S.; Suh, S.S.; Abdeen, S.K.; Monin, J.; Distefano, R.; Nigita, G.; Croce, C.M.; Aqeilan, R.I. WWOX Inhibits Metastasis of Triple-Negative Breast Cancer Cells via Modulation of miRNAs. Cancer Res. 2019, 79, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Ren, Y.; Liu, P. Roles of the WWOX in pathogenesis and endocrine therapy of breast cancer. Exp. Biol. Med. 2015, 240, 324–328. [Google Scholar] [CrossRef]
- Pluciennik, E.; Kusinska, R.; Potemski, P.; Kubiak, R.; Kordek, R.; Bednarek, A.K. WWOX--the FRA16D cancer gene: Expression correlation with breast cancer progression and prognosis. Eur. J. Surg. Oncol. 2006, 32, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, K.; Pluciennik, E.; Bednarek, A.K. WWOX Tumor Suppressor Gene in Breast Cancer, a Historical Perspective and Future Directions. Front. Oncol. 2018, 8, 345. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Khalaileh, A.; Pikarsky, E.; Aqeilan, R.I. WWOX controls hepatic HIF1alpha to suppress hepatocyte proliferation and neoplasia. Cell. Death Dis. 2018, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, R.; Huo, J.; Wang, B.; Du, X.; Dai, B.; Zhu, M.; Zhan, Y.; Zhang, D.; Zhang, Y. WWOX activation by toosendanin suppresses hepatocellular carcinoma metastasis through JAK2/Stat3 and Wnt/beta-catenin signaling. Cancer Lett. 2021, 513, 50–62. [Google Scholar] [CrossRef]
- Lin, J.; Wang, B.; Huang, A.M.; Wang, X.J. The relationship between FHIT and WWOX expression and clinicopathological features in hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2010, 18, 357–360. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, S.; Wang, Z. Cloning of WWOX gene and its growth-inhibiting effects on ovarian cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 365–369. [Google Scholar] [CrossRef]
- Nunez, M.I.; Rosen, D.G.; Ludes-Meyers, J.H.; Abba, M.C.; Kil, H.; Page, R.; Klein-Szanto, A.J.; Godwin, A.K.; Liu, J.; Mills, G.B.; et al. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 2005, 5, 64. [Google Scholar] [CrossRef]
- Kolat, D.; Kaluzinska, Z.; Pluciennik, E. Fragile Gene WWOX Guides TFAP2A/TFAP2C-Dependent Actions Against Tumor Progression in Grade II Bladder Cancer. Front. Oncol. 2021, 11, 621060. [Google Scholar] [CrossRef]
- Ramos, D.; Abba, M.; Lopez-Guerrero, J.A.; Rubio, J.; Solsona, E.; Almenar, S.; Llombart-Bosch, A.; Aldaz, C.M. Low levels of WWOX protein immunoexpression correlate with tumour grade and a less favourable outcome in patients with urinary bladder tumours. Histopathology 2008, 52, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Tzai, T.S.; Liao, C.Y.; Wang, J.S.; Wu, T.T.; Wang, H.Y.; Wu, C.H.; Yu, C.C.; Lu, P.J. WWOX protein expression varies among RCC histotypes and downregulation of WWOX protein correlates with less-favorable prognosis in clear RCC. Ann. Surg. Oncol. 2013, 20, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kosla, K.; Kaluzinska, Z.; Bednarek, A.K. The WWOX gene in brain development and pathology. Exp. Biol. Med. 2020, 245, 1122–1129. [Google Scholar] [CrossRef]
- Kosla, K.; Nowakowska, M.; Pospiech, K.; Bednarek, A.K. WWOX modulates the gene expression profile in the T98G glioblastoma cell line rendering its phenotype less malignant. Oncol. Rep. 2014, 32, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Yeh, S.T.; Liao, H.F.; Chang, N.S.; Chen, Y.J. Overexpression of WW domain-containing oxidoreductase WOX1 preferentially induces apoptosis in human glioblastoma cells harboring mutant p53. Biomed. Pharmacother. 2012, 66, 433–438. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Yu, P.; Miao, W.; Liu, C.; Pu, Y.; Zhang, C. Methylation of WWOX gene promotes proliferation of osteosarcoma cells. J. BUON 2020, 25, 2708–2713. [Google Scholar]
- Hussain, T.; Lee, J.; Abba, M.C.; Chen, J.; Aldaz, C.M. Delineating WWOX Protein Interactome by Tandem Affinity Purification-Mass Spectrometry: Identification of Top Interactors and Key Metabolic Pathways Involved. Front. Oncol. 2018, 8, 591. [Google Scholar] [CrossRef]
- Taouis, K.; Driouch, K.; Lidereau, R.; Lallemand, F. Molecular Functions of WWOX Potentially Involved in Cancer Development. Cells 2021, 10, 1051. [Google Scholar] [CrossRef]
- Schrock, M.S.; Huebner, K. WWOX: A fragile tumor suppressor. Exp. Biol. Med. 2015, 240, 296–304. [Google Scholar] [CrossRef]
- Guo, W.; Dong, Z.; Dong, Y.; Guo, Y.; Kuang, G.; Yang, Z. Genetic and epigenetic alterations of WWOX in the development of gastric cardia adenocarcinoma. Environ. Mol. Mutagen. 2013, 54, 112–123. [Google Scholar] [CrossRef]
- Banne, E.; Abudiab, B.; Abu-Swai, S.; Repudi, S.R.; Steinberg, D.J.; Shatleh, D.; Alshammery, S.; Lisowski, L.; Gold, W.; Carlen, P.L.; et al. Neurological Disorders Associated with WWOX Germline Mutations-A Comprehensive Overview. Cells 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, B.; Al Mutairi, F.; Al Hashem, A. The fragile site WWOX gene and the developing brain. Exp. Biol. Med. 2015, 240, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim. Biophys. Acta 2014, 1846, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.C.; Yang, Y.T.; Chen, Y.C.; Kuo, Y.M.; Sze, C.I. Role of WWOX/WOX1 in Alzheimer’s disease pathology and in cell death signaling. Front. Biosci. 2013, 5, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, B.; AlHashem, A.; AlShahwan, S.; Alkuraya, F.S.; Gedela, S.; Zuccoli, G. Severe CNS involvement in WWOX mutations: Description of five new cases. Am. J. Med. Genet. A 2015, 167A, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Liu, C.C.; Chen, S.T.; Yap, Y.V.; Chang, N.S.; Sze, C.I. WW domain-containing oxidoreductase in neuronal injury and neurological diseases. Oncotarget 2014, 5, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ho, P.C.; Lee, I.T.; Chen, Y.A.; Chu, C.H.; Teng, C.C.; Wu, S.N.; Sze, C.I.; Chiang, M.F.; Chang, N.S. WWOX Phosphorylation, Signaling, and Role in Neurodegeneration. Front. Neurosci. 2018, 12, 563. [Google Scholar] [CrossRef]
- Repudi, S.; Kustanovich, I.; Abu-Swai, S.; Stern, S.; Aqeilan, R.I. Neonatal neuronal WWOX gene therapy rescues Wwox null phenotypes. EMBO Mol. Med. 2021, 13, e14599. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature reprinted in Nature 2013, 494, 506. https://doi.org/10.1038/nature11903. 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012, 14 (Suppl. S5), v1–v49. [Google Scholar] [CrossRef]
- Oike, T.; Suzuki, Y.; Sugawara, K.; Shirai, K.; Noda, S.E.; Tamaki, T.; Nagaishi, M.; Yokoo, H.; Nakazato, Y.; Nakano, T. Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma: Japanese mono-institutional results. PLoS ONE 2013, 8, e78943. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Chang, P.D.; Chow, D.S.; Yang, P.H.; Filippi, C.G.; Lignelli, A. Predicting Glioblastoma Recurrence by Early Changes in the Apparent Diffusion Coefficient Value and Signal Intensity on FLAIR Images. AJR Am. J. Roentgenol. 2017, 208, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Peignan, L.; Garrido, W.; Segura, R.; Melo, R.; Rojas, D.; Carcamo, J.G.; San Martin, R.; Quezada, C. Combined use of anticancer drugs and an inhibitor of multiple drug resistance-associated protein-1 increases sensitivity and decreases survival of glioblastoma multiforme cells in vitro. Neurochem. Res. 2011, 36, 1397–1406. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chiang, M.F.; Chen, Y.J. Role of WW domain proteins WWOX in development, prognosis, and treatment response of glioma. Exp. Biol. Med. 2015, 240, 315–323. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Jacob, K. Invasion assays. Curr. Protoc. Cell Biol. 2001, 12, Unit 12. [Google Scholar] [CrossRef]
- Kolat, D.; Kaluzinska, Z.; Bednarek, A.K.; Pluciennik, E. WWOX Loses the Ability to Regulate Oncogenic AP-2gamma and Synergizes with Tumor Suppressor AP-2alpha in High-Grade Bladder Cancer. Cancers 2021, 13, 2957. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Forbes, S.A.; Tang, G.; Bindal, N.; Bamford, S.; Dawson, E.; Cole, C.; Kok, C.Y.; Jia, M.; Ewing, R.; Menzies, A.; et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): A resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010, 38, D652–D657. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Bindal, N.; Bamford, S.; Cole, C.; Kok, C.Y.; Beare, D.; Jia, M.; Shepherd, R.; Leung, K.; Menzies, A.; et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011, 39, D945–D950. [Google Scholar] [CrossRef] [PubMed]
- Kaluzinska, Z.; Kolat, D.; Bednarek, A.K.; Pluciennik, E. PLEK2, RRM2, GCSH: A Novel WWOX-Dependent Biomarker Triad of Glioblastoma at the Crossroads of Cytoskeleton Reorganization and Metabolism Alterations. Cancers 2021, 13, 2955. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Kolmykov, S.; Yevshin, I.; Kulyashov, M.; Sharipov, R.; Kondrakhin, Y.; Makeev, V.J.; Kulakovskiy, I.V.; Kel, A.; Kolpakov, F. GTRD: An integrated view of transcription regulation. Nucleic Acids Res. 2021, 49, D104–D111. [Google Scholar] [CrossRef]
- Rydenfelt, M.; Klinger, B.; Klunemann, M.; Bluthgen, N. SPEED2: Inferring upstream pathway activity from differential gene expression. Nucleic Acids Res. 2020, 48, W307–W312. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.; Wildenhain, J.; Rappsilber, J.; Tyers, M. BoxPlotR: A web tool for generation of box plots. Nat. Methods 2014, 11, 121–122. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, K.T.; Sun, T.Y.; Sze, C.I.; Huang, S.S.; Hsu, L.J.; Chang, N.S. WWOX and Its Binding Proteins in Neurodegeneration. Cells 2021, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Husanie, H.; Abu-Remaileh, M.; Maroun, K.; Abu-Tair, L.; Safadi, H.; Atlan, K.; Golan, T.; Aqeilan, R.I. Loss of tumor suppressor WWOX accelerates pancreatic cancer development through promotion of TGFbeta/BMP2 signaling. Cell Death Dis. 2022, 13, 1074. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Abu-Remaileh, M.; Abu-Odeh, M. The common fragile site FRA16D gene product WWOX: Roles in tumor suppression and genomic stability. Cell. Mol. Life Sci. 2014, 71, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.C.; Cao, Y.; Fung, E.L.W.; Kleppe, S.; Gripp, K.W.; Hertecant, J.; El-Hattab, A.W.; Suleiman, J.; Clark, G.; von Allmen, G.; et al. Expansion of the clinical and molecular spectrum of WWOX-related epileptic encephalopathy. Am. J. Med. Genet. A 2022, 191, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, Y.; Natsumeda, M.; Okada, M.; Saito, R.; Kobayashi, D.; Eda, T.; Watanabe, J.; Saito, S.; Tsukamoto, Y.; Oishi, M.; et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: Establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol. Commun. 2019, 7, 119. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Liu, Y.; Wang, S.; Sun, M.; Zhang, Z.; Zheng, X.; Li, J.; Li, Y. The positive feedback loop of NHE1-ERK phosphorylation mediated by BRAF(V600E) mutation contributes to tumorigenesis and development of glioblastoma. Biochem. Biophys. Res. Commun. 2022, 588, 1–7. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Condouris, S.; Xing, M. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J. Clin. Endocrinol. Metab. 2007, 92, 2264–2271. [Google Scholar] [CrossRef]
- Rahman, M.A.; Salajegheh, A.; Smith, R.A.; Lam, A.K. Multiple proliferation-survival signalling pathways are simultaneously active in BRAF V600E mutated thyroid carcinomas. Exp. Mol. Pathol. 2015, 99, 492–497. [Google Scholar] [CrossRef]
- Andrews, L.J.; Thornton, Z.A.; Saincher, S.S.; Yao, I.Y.; Dawson, S.; McGuinness, L.A.; Jones, H.E.; Jefferies, S.; Short, S.C.; Cheng, H.Y.; et al. Prevalence of BRAFV600 in glioma and use of BRAF Inhibitors in patients with BRAFV600 mutation-positive glioma: Systematic review. Neuro Oncol. 2022, 24, 528–540. [Google Scholar] [CrossRef]
- Chen, Y.A.; Sie, Y.D.; Liu, T.Y.; Kuo, H.L.; Chou, P.Y.; Chen, Y.J.; Lee, K.T.; Chen, P.J.; Chen, S.T.; Chang, N.S. Normal cells repel WWOX-negative or -dysfunctional cancer cells via WWOX cell surface epitope 286–299. Commun. Biol. 2021, 4, 753. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.Y.; Melino, G.; Huebner, K.; et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Verma, S.K.; Foster, D.M.; Golden, H.B.; Reneau, J.C.; Watson, L.E.; Singh, H.; Dostal, D.E. Integrins and proximal signaling mechanisms in cardiovascular disease. Front. Biosci. 2009, 14, 2307–2334. [Google Scholar] [CrossRef]
- Revkova, V.A.; Sidoruk, K.V.; Kalsin, V.A.; Melnikov, P.A.; Konoplyannikov, M.A.; Kotova, S.; Frolova, A.A.; Rodionov, S.A.; Smorchkov, M.M.; Kovalev, A.V.; et al. Spidroin Silk Fibers with Bioactive Motifs of Extracellular Proteins for Neural Tissue Engineering. ACS Omega 2021, 6, 15264–15273. [Google Scholar] [CrossRef]
- Ranjan, A.; Bane, S.M.; Kalraiya, R.D. Glycosylation of the laminin receptor (alpha3beta1) regulates its association with tetraspanin CD151: Impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp. Cell. Res. 2014, 322, 249–264. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, L.; Song, H.L.; Adam, P.; Hani, G. Effects of WWOX on ovarian cancer cell attachment in vitro. Zhonghua Zhong Liu Za Zhi 2009, 31, 414–417. [Google Scholar] [PubMed]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef]
- Tabatabai, G.; Weller, M.; Nabors, B.; Picard, M.; Reardon, D.; Mikkelsen, T.; Ruegg, C.; Stupp, R. Targeting integrins in malignant glioma. Target. Oncol. 2010, 5, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kaluzinska, Z.; Kolat, D.; Kosla, K.; Orzechowska, M.; Bednarek, A.K.; Pluciennik, E. In vitro and in silico assessment of the effect of WWOX expression on invasiveness pathways associated with AP-2 transcription factors in bladder cancer. BMC Urol. 2021, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, W.; Li, B.; Lu, C.; Wan, X. WWOX induces apoptosis and inhibits proliferation in cervical cancer and cell lines. Int. J. Mol. Med. 2013, 31, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Liu, F.; Xia, J.H. Effects of miR-670-5p on proliferation, migration and invasion of lung cancer cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2021, 37, 500–505. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Mao, Q.; Lin, J.; Jia, J.; Li, S.; Xiong, W.; Lin, Y.; Liu, Z.; Liu, X.; et al. PDGFR-beta-activated ACK1-AKT signaling promotes glioma tumorigenesis. Int. J. Cancer 2015, 136, 1769–1780. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Zhou, Y.L.; You, Q.J.; Shou, F.; Pang, Q.F.; Chen, J.L. WWOX inhibits the invasion of lung cancer cells by downregulating RUNX2. Cancer Gene Ther. 2016, 23, 433–438. [Google Scholar] [CrossRef]
- Ferguson, B.W.; Gao, X.; Zelazowski, M.J.; Lee, J.; Jeter, C.R.; Abba, M.C.; Aldaz, C.M. The cancer gene WWOX behaves as an inhibitor of SMAD3 transcriptional activity via direct binding. BMC Cancer 2013, 13, 593. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.H.; Gaur, T.; Stein, G.S.; et al. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, Y.C.; Hu, Y.K.; Fang, L.S.; Li, Q.; Xu, J.; Yan, H.C. WWOX regulates the Elf5/Snail1 pathway to affect epithelial-mesenchymal transition of ovarian carcinoma cells in vitro. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Ma, Z.; Tong, W.; Yang, J. LncRNA WWOX-AS1 inhibits the proliferation, migration and invasion of osteosarcoma cells. Mol. Med. Rep. 2018, 18, 779–788. [Google Scholar] [CrossRef]
- Wagner, E.G.; Altuvia, S.; Romby, P. Antisense RNAs in bacteria and their genetic elements. Adv. Genet. 2002, 46, 361–398. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell. Biol. 2009, 10, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Woo, H.Y.; Kim, H.S. Targeted Genomic Sequencing Reveals Novel TP53 In-frame Deletion Mutations Leading to p53 Overexpression in High-grade Serous Tubo-ovarian Carcinoma. Anticancer. Res. 2019, 39, 2883–2889. [Google Scholar] [CrossRef]
- Bessiere, L.; Todeschini, A.L.; Auguste, A.; Sarnacki, S.; Flatters, D.; Legois, B.; Sultan, C.; Kalfa, N.; Galmiche, L.; Veitia, R.A. A Hot-spot of In-frame Duplications Activates the Oncoprotein AKT1 in Juvenile Granulosa Cell Tumors. EBioMedicine 2015, 2, 421–431. [Google Scholar] [CrossRef]
- Chakraborty, U.; Dinh, T.A.; Alani, E. Genomic Instability Promoted by Overexpression of Mismatch Repair Factors in Yeast: A Model for Understanding Cancer Progression. Genetics 2018, 209, 439–456. [Google Scholar] [CrossRef]
- Nino, C.A.; Wollscheid, N.; Giangreco, G.; Maspero, E.; Polo, S. USP25 Regulates EGFR Fate by Modulating EGF-Induced Ubiquitylation Dynamics. Biomolecules 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Laketa, V.; Zarbakhsh, S.; Traynor-Kaplan, A.; Macnamara, A.; Subramanian, D.; Putyrski, M.; Mueller, R.; Nadler, A.; Mentel, M.; Saez-Rodriguez, J.; et al. PIP(3) induces the recycling of receptor tyrosine kinases. Sci. Signal. 2014, 7, ra5. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Dai, Q.; Wei, J.; Shao, G.; Sun, A.; Yang, W.; Lin, Q. Stress-induced endocytosis and degradation of epidermal growth factor receptor are two independent processes. Cancer Cell. Int. 2016, 16, 25. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Vogt, M.A.; Ehsaei, Z.; Knuckles, P.; Higginbottom, A.; Helmbrecht, M.S.; Kunath, T.; Eggan, K.; Williams, L.A.; Shaw, P.J.; Wurst, W.; et al. TDP-43 induces p53-mediated cell death of cortical progenitors and immature neurons. Sci. Rep. 2018, 8, 8097. [Google Scholar] [CrossRef]
- Li, S.; Xiang, W.; Tian, J.; Wang, H.; Hu, S.; Wang, K.; Chen, L.; Huang, C.; Zhou, J. Bone Marrow-Derived Mesenchymal Stem Cells Differentially Affect Glioblastoma Cell Proliferation, Migration, and Invasion: A 2D-DIGE Proteomic Analysis. Biomed. Res. Int. 2021, 2021, 4952876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.T.; Wang, S.; Huang, S.L.; Zheng, Y.; Wang, C.Q.; Hu, B.Y.; Qin, W.; Zou, T.T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Xu, J.F.; Wang, Y.P.; Zhang, S.J.; Chen, Y.; Gu, H.F.; Dou, X.F.; Xia, B.; Bi, Q.; Fan, S.W. Exosomes containing differential expression of microRNA and mRNA in osteosarcoma that can predict response to chemotherapy. Oncotarget 2017, 8, 75968–75978. [Google Scholar] [CrossRef]
- Batagov, A.O.; Kurochkin, I.V. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol. Direct 2013, 8, 12. [Google Scholar] [CrossRef]
- Slomka, A.; Kornek, M.; Cho, W.C. Small Extracellular Vesicles and Their Involvement in Cancer Resistance: An Up-to-Date Review. Cells 2022, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Han, J.B.; Huang, M.L.; Li, F.; Yang, R.; Chen, S.M.; Tao, Z.Z. MiR-214 Mediates Cell Proliferation and Apoptosis of Nasopharyngeal Carcinoma Through Targeting Both WWOX and PTEN. Cancer Biother. Radiopharm. 2020, 35, 615–625. [Google Scholar] [CrossRef]
- Liu, C.J.; Shen, W.G.; Peng, S.Y.; Cheng, H.W.; Kao, S.Y.; Lin, S.C.; Chang, K.W. miR-134 induces oncogenicity and metastasis in head and neck carcinoma through targeting WWOX gene. Int. J. Cancer 2014, 134, 811–821. [Google Scholar] [CrossRef]
- Chen, T.J.; Gao, F.; Yang, T.; Li, H.; Li, Y.; Ren, H.; Chen, M.W. LncRNA HOTAIRM1 Inhibits the Proliferation and Invasion of Lung Adenocarcinoma Cells via the miR-498/WWOX Axis. Cancer Manag. Res. 2020, 12, 4379–4390. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, B.; Liu, M.; Liu, T.; Zhang, R. A long non-coding RNA TSLD8 inhibits hepatocellular carcinoma by stabilizing WWOX. Biochem. Biophys. Res. Commun. 2019, 516, 526–532. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałuzińska-Kołat, Ż.; Kośla, K.; Kołat, D.; Płuciennik, E.; Bednarek, A.K. Antineoplastic Nature of WWOX in Glioblastoma Is Mainly a Consequence of Reduced Cell Viability and Invasion. Biology 2023, 12, 465. https://doi.org/10.3390/biology12030465
Kałuzińska-Kołat Ż, Kośla K, Kołat D, Płuciennik E, Bednarek AK. Antineoplastic Nature of WWOX in Glioblastoma Is Mainly a Consequence of Reduced Cell Viability and Invasion. Biology. 2023; 12(3):465. https://doi.org/10.3390/biology12030465
Chicago/Turabian StyleKałuzińska-Kołat, Żaneta, Katarzyna Kośla, Damian Kołat, Elżbieta Płuciennik, and Andrzej K. Bednarek. 2023. "Antineoplastic Nature of WWOX in Glioblastoma Is Mainly a Consequence of Reduced Cell Viability and Invasion" Biology 12, no. 3: 465. https://doi.org/10.3390/biology12030465
APA StyleKałuzińska-Kołat, Ż., Kośla, K., Kołat, D., Płuciennik, E., & Bednarek, A. K. (2023). Antineoplastic Nature of WWOX in Glioblastoma Is Mainly a Consequence of Reduced Cell Viability and Invasion. Biology, 12(3), 465. https://doi.org/10.3390/biology12030465








