Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Innate Immune System
2.1. The Innate Immune System in Physiological Pregnancy
2.1.1. Dendritic Cells
2.1.2. Monocytes/Macrophages
2.1.3. Neutrophils
2.1.4. Natural Killer Cells
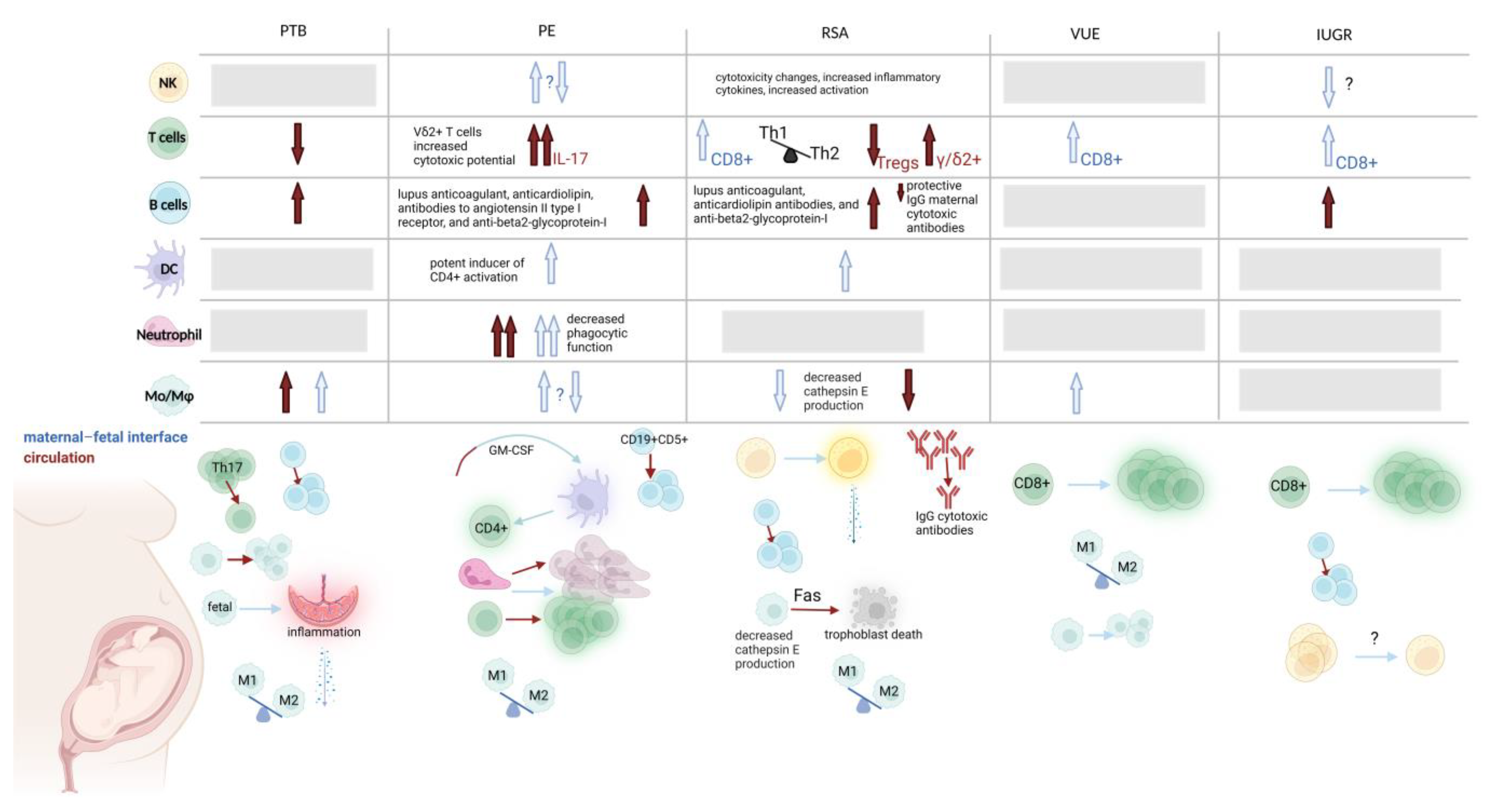
2.2. The Innate Immune System in Pathological Pregnancy
2.2.1. Dendritic Cells
2.2.2. Monocytes/Macrophages
2.2.3. Neutrophils
2.2.4. Natural Killer Cells
3. The Adaptive Immune System in Pregnancy
3.1. The Adaptive Immune System in Physiological Pregnancy
3.1.1. T Cells
3.1.2. B Cells
3.2. The Adaptive Immune System in Pathological Pregnancy
3.2.1. T Cells
3.2.2. B Cells
4. Innate Immune Interaction with Adaptive Immune Responses
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medawar, P.B.; Medawar, P.B. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953, 7, 1953. [Google Scholar]
- Mold, J.E.; Michaëlsson, J.; Burt, T.D.; Muench, M.O.; Beckerman, K.P.; Busch, M.P.; Lee, T.H.; Nixon, D.F.; McCune, J.M. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008, 322, 1562–1565. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Pace, D.; Ritson, A. Immunoregulatory cells in human decidua: Morphology, immunohistochemistry and function. Reprod. Nutr. Dev. 1988, 28, 1599–1613. [Google Scholar] [CrossRef]
- Abelius, M.S.; Janefjord, C.; Ernerudh, J.; Berg, G.; Matthiesen, L.; Duchén, K.; Nilsson, L.J.; Jenmalm, M.C. The placental immune milieu is characterized by a Th2- and anti-inflammatory transcription profile, regardless of maternal allergy, and associates with neonatal immunity. Am. J. Reprod. Immunol. 2015, 73, 445–459. [Google Scholar] [CrossRef]
- Gardner, M.B.; Luciw, P.A. Macaque models of human infectious disease. ILAR J. 2008, 49, 220–255. [Google Scholar] [CrossRef] [PubMed]
- Fettke, F.; Schumacher, A.; Costa, S.D.; Zenclussen, A.C. B cells: The old new players in reproductive immunology. Front. Immunol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Muzzio, D.O.; Soldati, R.; Ehrhardt, J.; Utpatel, K.; Evert, M.; Zenclussen, A.C.; Zygmunt, M.; Jensen, F. B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol. Reprod. 2014, 91, 115. [Google Scholar] [CrossRef]
- Lessin, D.L.; Hunt, J.S.; King, C.R.; Wood, G.W. Antigen expression by cells near the maternal-fetal interface. Am. J. Reprod. Immunol. Microbiol. 1988, 16, 1–7. [Google Scholar] [CrossRef]
- Presicce, P.; Cappelletti, M.; Senthamaraikannan, P.; Ma, F.; Morselli, M.; Jackson, C.M.; Mukherjee, S.; Miller, L.A.; Pellegrini, M.; Jobe, A.H.; et al. TNF-Signaling Modulates Neutrophil-Mediated Immunity at the Feto-Maternal Interface during LPS-Induced Intrauterine Inflammation. Front. Immunol. 2020, 11, 558. [Google Scholar] [CrossRef]
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663. [Google Scholar] [CrossRef]
- Laskarin, G.; Kämmerer, U.; Rukavina, D.; Thomson, A.W.; Fernandez, N.; Blois, S.M. Antigen-presenting cells and materno-fetal tolerance: An emerging role for dendritic cells. Am. J. Reprod. Immunol. 2007, 58, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Brien, M.E.; Duval, C.; Palacios, J.; Boufaied, I.; Hudon-Thibeault, A.A.; Nadeau-Vallée, M.; Vaillancourt, C.; Sibley, C.P.; Abrahams, V.M.; Jones, R.L.; et al. Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction. J. Immunol. 2017, 198, 443–451. [Google Scholar] [CrossRef]
- Aye, I.L.; Jansson, T.; Powell, T.L. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol. Cell. Endocrinol. 2013, 381, 46–55. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; Roberts, J.M.; von Versen-Höynck, F.; Koch, J.; Edmunds, L.; Hubel, C.A. Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers. Am. J. Physiol. Cell. Physiol. 2009, 297, C440–C450. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; von Versen-Höynck, F.; Roberts, J.M. Uric acid inhibits placental system A amino acid uptake. Placenta 2009, 30, 195–200. [Google Scholar] [CrossRef]
- Lei, J.; Vermillion, M.S.; Jia, B.; Xie, H.; Xie, L.; McLane, M.W.; Sheffield, J.S.; Pekosz, A.; Brown, A.; Klein, S.L.; et al. IL-1 receptor antagonist therapy mitigates placental dysfunction and perinatal injury following Zika virus infection. JCI Insight 2019, 4, e122678. [Google Scholar] [CrossRef]
- Mulla, M.J.; Myrtolli, K.; Potter, J.; Boeras, C.; Kavathas, P.B.; Sfakianaki, A.K.; Tadesse, S.; Norwitz, E.R.; Guller, S.; Abrahams, V.M. Uric acid induces trophoblast IL-1β production via the inflammasome: Implications for the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 2011, 65, 542–548. [Google Scholar] [CrossRef]
- Deshmukh, H.; Way, S.S. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu. Rev. Pathol. 2019, 14, 185–210. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.K.; McCutcheon, C.R.; Petroff, M.G. Impact of Estrogen and Progesterone on Immune Cells and Host-Pathogen Interactions in the Lower Female Reproductive Tract. J. Immunol. 2022, 209, 1437–1449. [Google Scholar] [CrossRef]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Shah, N.M.; Herasimtschuk, A.A.; Boasso, A.; Benlahrech, A.; Fuchs, D.; Imami, N.; Johnson, M.R. Changes in T Cell and Dendritic Cell Phenotype from Mid to Late Pregnancy Are Indicative of a Shift from Immune Tolerance to Immune Activation. Front. Immunol. 2017, 8, 1138. [Google Scholar] [CrossRef]
- Leno-Durán, E.; Muñoz-Fernández, R.; Olivares, E.G.; Tirado-González, I. Liaison between natural killer cells and dendritic cells in human gestation. Cell. Mol. Immunol. 2014, 11, 449–455. [Google Scholar] [CrossRef]
- Tagliani, E.; Shi, C.; Nancy, P.; Tay, C.S.; Pamer, E.G.; Erlebacher, A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med. 2011, 208, 1901–1916. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, U.; Eggert, A.O.; Kapp, M.; McLellan, A.D.; Geijtenbeek, T.B.; Dietl, J.; van Kooyk, Y.; Kämpgen, E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 2003, 162, 887–896. [Google Scholar] [CrossRef]
- Tirado-González, I.; Muñoz-Fernández, R.; Prados, A.; Leno-Durán, E.; Martin, F.; Abadía-Molina, A.C.; Olivares, E.G. Apoptotic DC-SIGN+ cells in normal human decidua. Placenta 2012, 33, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Laskarin, G.; Redzović, A.; Rubesa, Z.; Mantovani, A.; Allavena, P.; Haller, H.; Vlastelić, I.; Rukavina, D. Decidual natural killer cell tuning by autologous dendritic cells. Am. J. Reprod. Immunol. 2008, 59, 433–445. [Google Scholar] [CrossRef]
- Collins, M.K.; Tay, C.-S.; Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Investig. 2009, 119, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Moffett, A. Dendritic cells in the human decidua. Biol. Reprod. 2003, 69, 1438–1446. [Google Scholar] [CrossRef]
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal-fetal interface. Expert. Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef]
- Blois, S.M.; Klapp, B.F.; Barrientos, G. Decidualization and angiogenesis in early pregnancy: Unravelling the functions of DC and NK cells. J. Reprod. Immunol. 2011, 88, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Tirado-González, I.; Klapp, B.F.; Karimi, K.; Arck, P.C.; Garcia, M.G.; Blois, S.M. The impact of dendritic cells on angiogenic responses at the fetal–maternal interface. J. Reprod. Immunol. 2009, 83, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Chen, X.; Diao, L.; Lian, R.; Zhang, X.; Hu, L.; Zeng, Y. Association of peripheral blood dendritic cells with recurrent pregnancy loss: A case-controlled study. Am. J. Reprod. Immunol. 2016, 76, 326–332. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; Holtz, D.O.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004, 10, 950–958. [Google Scholar] [CrossRef]
- Fainaru, O.; Adini, A.; Benny, O.; Adini, I.; Short, S.; Bazinet, L.; Nakai, K.; Pravda, E.; Hornstein, M.D.; D’Amato, R.J.; et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J. 2008, 22, 522–529. [Google Scholar] [CrossRef]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ Transplant 2016, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.; Haluszczak, C.; Betters, D.; Richard, C.A.; Trucco, M.; DeLoia, J.A. Monocytes are progressively activated in the circulation of pregnant women. J. Leukoc. Biol. 2002, 72, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Hofer, T.P. Toward a refined definition of monocyte subsets. Front. Immunol. 2013, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho, H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H. Macrophage subsets at the maternal-fetal interface. Cell. Mol. Immunol. 2020, 17, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Garcia-Flores, V.; Chin, P.Y.; Groome, H.M.; Bijland, M.T.; Diener, K.R.; Romero, R.; Robertson, S.A. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 2021, 6, e146089. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Sharkey, D.J.; Robertson, S.A.; Zenclussen, A.C. Immune Cells at the Fetomaternal Interface: How the Microenvironment Modulates Immune Cells To Foster Fetal Development. J. Immunol. 2018, 201, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Hauguel-de Mouzon, S.; Guerre-Millo, M. The placenta cytokine network and inflammatory signals. Placenta 2006, 27, 794–798. [Google Scholar] [CrossRef]
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene expression profiling of human decidual macrophages: Evidence for immunosuppressive phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Ando, K.; Hédou, J.J.; Feyaerts, D.; Han, X.; Ganio, E.A.; Tsai, E.S.; Peterson, L.S.; Verdonk, F.; Tsai, A.S.; Marić, I.; et al. A Peripheral Immune Signature of Labor Induction. Front. Immunol. 2021, 12, 725989. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Koumandakis, E.; Koumandaki, I.; Kaklamani, E.; Sparos, L.; Aravantinos, D.; Trichopoulos, D. Enhanced phagocytosis of mononuclear phagocytes in pregnancy. Br. J. Obstet. Gynaecol. 1986, 93, 1150–1154. [Google Scholar] [CrossRef]
- Gezer, C.; Ekin, A.; Ertas, I.E.; Ozeren, M.; Solmaz, U.; Mat, E.; Taner, C.E. High first-trimester neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are indicators for early diagnosis of preeclampsia. Ginekol. Pol. 2016, 87, 431–435. [Google Scholar] [CrossRef]
- Cadden, K.A.; Walsh, S.W. Neutrophils, but not lymphocytes or monocytes, infiltrate maternal systemic vasculature in women with preeclampsia. Hypertens Pregnancy 2008, 27, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Nguyen, T.; Lye, S.J. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J. Cell. Mol. Med. 2013, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum. Reprod. 1999, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Belo, L.; Santos-Silva, A.; Rocha, S.; Caslake, M.; Cooney, J.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 2015, 107, 26–30. [Google Scholar] [CrossRef]
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Póka, R. The effect of healthy pregnant plasma and preeclamptic plasma on the phagocytosis index of neutrophil granulocytes and monocytes of nonpregnant women. Hypertens Pregnancy 2017, 36, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lampé, R.; Szucs, S.; Adány, R.; Póka, R. Granulocyte superoxide anion production and regulation by plasma factors in normal and preeclamptic pregnancy. J. Reprod. Immunol. 2011, 89, 199–206. [Google Scholar] [CrossRef]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Amsalem, H.; Kwan, M.; Hazan, A.; Zhang, J.; Jones, R.L.; Whittle, W.; Kingdom, J.C.; Croy, B.A.; Lye, S.J.; Dunk, C.E. Identification of a novel neutrophil population: Proangiogenic granulocytes in second-trimester human decidua. J. Immunol. 2014, 193, 3070–3079. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Pace, D.; Morrison, L.; Bulmer, J.N. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J. Clin. Pathol. 1989, 42, 35–39. [Google Scholar] [CrossRef]
- Xiong, S.; Sharkey, A.M.; Kennedy, P.R.; Gardner, L.; Farrell, L.E.; Chazara, O.; Bauer, J.; Hiby, S.E.; Colucci, F.; Moffett, A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J. Clin. Investig. 2013, 123, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mariee, N.; Jiang, L.; Liu, Y.; Wang, C.C.; Li, T.C.; Laird, S. Measurement of uterine natural killer cell percentage in the periimplantation endometrium from fertile women and women with recurrent reproductive failure: Establishment of a reference range. Am. J. Obstet. Gynecol. 2017, 217, 680.e1–680.e6. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Schiessl, B.; Kirkley, M.; Innes, B.A.; Cooper, A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Hiby, S.E.; Verma, S.; Burrows, T.; Gardner, L.; Loke, Y.W. Uterine NK cells and trophoblast HLA class I molecules. Am. J. Reprod. Immunol. 1997, 37, 459–462. [Google Scholar] [CrossRef]
- Wilkens, J.; Male, V.; Ghazal, P.; Forster, T.; Gibson, D.A.; Williams, A.R.; Brito-Mutunayagam, S.L.; Craigon, M.; Lourenco, P.; Cameron, I.T.; et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J. Immunol. 2013, 191, 2226–2235. [Google Scholar] [CrossRef]
- Hickey, M.; Crewe, J.; Goodridge, J.P.; Witt, C.S.; Fraser, I.S.; Doherty, D.; Christiansen, F.T.; Salamonsen, L.A. Menopausal hormone therapy and irregular endometrial bleeding: A potential role for uterine natural killer cells? J. Clin. Endocrinol. Metab. 2005, 90, 5528–5535. [Google Scholar] [CrossRef]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef]
- Quenby, S.; Nik, H.; Innes, B.; Lash, G.; Turner, M.; Drury, J.; Bulmer, J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. 2009, 24, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.A.; Kopcow, H.D.; Rybalov, B.; Boyson, J.E.; Orange, J.S.; Schatz, F.; Masch, R.; Lockwood, C.J.; Schachter, A.D.; Park, P.J.; et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003, 198, 1201–1212. [Google Scholar] [CrossRef]
- Kopcow, H.D.; Allan, D.S.; Chen, X.; Rybalov, B.; Andzelm, M.M.; Ge, B.; Strominger, J.L. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc. Natl. Acad. Sci. USA 2005, 102, 15563–15568. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Evans, J.H.; Crespo, Â.C.; Strominger, J.L. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2015, 112, 13312–13317. [Google Scholar] [CrossRef]
- Ferreira, L.M.R.; Meissner, T.B.; Tilburgs, T.; Strominger, J.L. HLA-G: At the Interface of Maternal-Fetal Tolerance. Trends Immunol. 2017, 38, 272–286. [Google Scholar] [CrossRef]
- Zhuang, B.; Shang, J.; Yao, Y. HLA-G: An Important Mediator of Maternal-Fetal Immune-Tolerance. Front. Immunol. 2021, 12, 744324. [Google Scholar] [CrossRef]
- Qian, Z.D.; Huang, L.L.; Zhu, X.M. An immunohistochemical study of CD83- and CD1a-positive dendritic cells in the decidua of women with recurrent spontaneous abortion. Eur. J. Med. Res. 2015, 20, 2. [Google Scholar] [CrossRef]
- Liu, S.; Wei, H.; Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4+ dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12998. [Google Scholar] [CrossRef]
- Negishi, Y.; Shima, Y.; Takeshita, T.; Takahashi, H. Distribution of invariant natural killer T cells and dendritic cells in late pre-term birth without acute chorioamnionitis. Am. J. Reprod. Immunol. 2017, 77, e12658. [Google Scholar] [CrossRef]
- Lu, H.Q.; Hu, R. Lasting Effects of Intrauterine Exposure to Preeclampsia on Offspring and the Underlying Mechanism. AJP Rep. 2019, 9, e275–e291. [Google Scholar] [CrossRef] [PubMed]
- Bizargity, P.; Bonney, E.A. Dendritic cells: A family portrait at mid-gestation. Immunology 2009, 126, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Ding, Y. Lnc-DC mediates the over-maturation of decidual dendritic cells and induces the increase in Th1 cells in preeclampsia. Am. J. Reprod. Immunol. 2017, 77, e12647. [Google Scholar] [CrossRef]
- Huang, S.J.; Chen, C.P.; Schatz, F.; Rahman, M.; Abrahams, V.M.; Lockwood, C.J. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J. Pathol. 2008, 214, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Zheng, J.; Zhang, H.; Zhao, M.; Liu, X.; Jiang, Y.; Yang, C.; Ren, L.; Liu, Z.; Hu, X. LILRB4 Decrease on uDCs Exacerbate Abnormal Pregnancy Outcomes Following. Front. Microbiol. 2018, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Witmer, M.D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl. Acad. Sci. USA 1978, 75, 5132–5136. [Google Scholar] [CrossRef]
- Sharma, A.M.; Birkett, R.; Lin, E.T.; Ernst, L.M.; Grobman, W.A.; Swaminathan, S.; Abdala-Valencia, H.; Misharin, A.V.; Bartom, E.T.; Mestan, K.K. Placental dysfunction influences fetal monocyte subpopulation gene expression in preterm birth. JCI Insight 2022, 7, e155482. [Google Scholar] [CrossRef]
- Agrawal, V.; Jaiswal, M.K.; Pamarthy, S.; Katara, G.K.; Kulshrestha, A.; Gilman-Sachs, A.; Hirsch, E.; Beaman, K.D. Role of Notch signaling during lipopolysaccharide-induced preterm labor. J. Leucoc. Biol. 2016, 100, 261–274. [Google Scholar] [CrossRef]
- Bürk, M.R.; Troeger, C.; Brinkhaus, R.; Holzgreve, W.; Hahn, S. Severely reduced presence of tissue macrophages in the basal plate of pre-eclamptic placentae. Placenta 2001, 22, 309–316. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Matta, P.; Krikun, G.; Koopman, L.A.; Masch, R.; Toti, P.; Arcuri, F.; Huang, S.T.; Funai, E.F.; Schatz, F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: Implications for preeclampsia. Am. J. Pathol. 2006, 168, 445–452. [Google Scholar] [CrossRef]
- Reister, F.; Frank, H.G.; Heyl, W.; Kosanke, G.; Huppertz, B.; Schröder, W.; Kaufmann, P.; Rath, W. The Distribution of Macrophages in Spiral Arteries of the Placental Bed in Pre-eclampsia Differs from that in Healthy Patients. Placenta 1999, 20, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.T.; Peraçoli, J.C.; Bannwart-Castro, C.F.; Romão, M.; Weel, I.C.; Golim, M.A.; de Oliveira, L.G.; Kurokawa, C.S.; Medeiros Borges, V.T.; Peraçoli, M.T. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am. J. Reprod. Immunol. 2014, 72, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Abrahams, V.M. Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol. RB&E 2003, 1, 119. [Google Scholar]
- Goto, S.; Ozaki, Y.; Suzumori, N.; Yasukochi, A.; Kawakubo, T.; Furuno, T.; Nakanishi, M.; Yamamoto, K.; Sugiura-Ogasawara, M. Role of cathepsin E in decidual macrophage of patients with recurrent miscarriage. Mol. Hum. Reprod. 2014, 20, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Aschkenazi, S.; Straszewski, S.; Verwer, K.M.; Foellmer, H.; Rutherford, T.; Mor, G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol. Reprod. 2002, 66, 1853–1861. [Google Scholar] [CrossRef]
- Neale, D.; Demasio, K.; Illuzi, J.; Chaiworapongsa, T.; Romero, R.; Mor, G. Maternal serum of women with pre-eclampsia reduces trophoblast cell viability: Evidence for an increased sensitivity to Fas-mediated apoptosis. J. Matern. Fetal Neonatal Med. 2003, 13, 39–44. [Google Scholar] [CrossRef]
- Malyshkina, A.I.; Sotnikova, N.Y.; Grigushkina, E.V.; Kroshkina, N.V.; Talanova, I.E. Prediction of the outcome of pregnancy in women with reccurent miscarriage. Klin Lab Diagn 2021, 66, 618–622. [Google Scholar] [CrossRef]
- Egal, E.; Rodrigues, N.d.M.; Mariano, F.; Souza, R.; Scarini, J.; Sabino, W.; Aguiar, L.; Montalli, V.; Gondak, R.; Soares, C.; et al. Immunohistochemical Study of M1 and M2 Macrophages Population in Chronic Villitis of the Placenta. Am. J. Clin. Pathol. 2019, 152, S102. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Leng, Y.; Garcia-Flores, V.; Miller, D.; Jacques, S.M.; Hassan, S.S.; Faro, J.; Alsamsam, A.; et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am. J. Obstet. Gynecol. 2017, 217, 693.e1–693.e16. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Moon, J.B.; Shim, S.S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef]
- Ganer Herman, H.; Schreiber, L.; Miremberg, H.; Ben Zvi, M.; Bar, J.; Kovo, M. Histological chorioamnionitis at term according to labor onset: A prospective controlled study. J. Perinatol. 2019, 39, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, Y.; Agrawal, V.; Crawford, S.E.; Fitchev, P.; Qu, X.; Klein, J.; Hirsch, E. Depletion of polymorphonuclear leukocytes has no effect on preterm delivery in a mouse model of Escherichia coli-induced labor. Am. J. Obstet. Gynecol. 2015, 213, 697.e1–697.e10. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.F.; Catalano, R.D.; Wade, J.; Rossi, A.G.; Norman, J.E. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor. J. Immunol. 2014, 192, 2315–2325. [Google Scholar] [CrossRef]
- Timmons, B.C.; Mahendroo, M.S. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol. Reprod. 2006, 74, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Oylumlu, M.; Ozler, A.; Yildiz, A.; Acet, H.; Polat, N.; Soydinc, H.E.; Yuksel, M.; Ertas, F. New inflammatory markers in pre-eclampsia: Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Clin. Exp. Hypertens. 2014, 36, 503–507. [Google Scholar] [CrossRef]
- Örgül, G.; Aydın Haklı, D.; Özten, G.; Fadiloğlu, E.; Tanacan, A.; Beksaç, M.S. First trimester complete blood cell indices in early and late onset preeclampsia. Turk. J. Obstet. Gynecol. 2019, 16, 112–117. [Google Scholar] [CrossRef]
- Regal, J.F.; Lillegard, K.E.; Bauer, A.J.; Elmquist, B.J.; Loeks-Johnson, A.C.; Gilbert, J.S. Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat. PLoS ONE 2015, 10, e0132063. [Google Scholar] [CrossRef]
- Konečná, B.; Lauková, L.; Vlková, B. Immune activation by nucleic acids: A role in pregnancy complications. Scand. J. Immunol. 2018, 87, e12651. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- Hahn, S.; Giaglis, S.; Hoesli, I.; Hasler, P. Neutrophil NETs in reproduction: From infertility to preeclampsia and the possibility of fetal loss. Front. Immunol. 2012, 3, 362. [Google Scholar] [CrossRef]
- Rabaglino, M.B.; Post Uiterweer, E.D.; Jeyabalan, A.; Hogge, W.A.; Conrad, K.P. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertension 2015, 65, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Milosevic-Stevanovic, J.; Krstic, M.; Radovic-Janosevic, D.; Popovic, J.; Tasic, M.; Stojnev, S. Number of decidual natural killer cells & macrophages in pre-eclampsia. Indian J. Med. Res. 2016, 144, 823–830. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bachmayer, N.; Rafik Hamad, R.; Liszka, L.; Bremme, K.; Sverremark-Ekström, E. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am. J. Reprod. Immunol. 2006, 56, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, W.; Huang, L.; Guan, X.; Lin, W.; Yao, J.; Li, L. Natural killer cells in the pathogenesis of preeclampsia: A double-edged sword. J. Matern. Fetal Neonatal Med. 2022, 35, 1028–1035. [Google Scholar] [CrossRef]
- Wallace, A.E.; Whitley, G.S.; Thilaganathan, B.; Cartwright, J.E. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J. Leukoc. Biol. 2015, 97, 79–86. [Google Scholar] [CrossRef]
- James-Allan, L.B.; Whitley, G.S.; Leslie, K.; Wallace, A.E.; Cartwright, J.E. Decidual cell regulation of trophoblast is altered in pregnancies at risk of pre-eclampsia. J. Mol. Endocrinol. 2018, 60, 239–246. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Arck, P.C.; Hecher, K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 2013, 19, 548–556. [Google Scholar] [CrossRef]
- Chen, X.; Yin, B.; Lian, R.C.; Zhang, T.; Zhang, H.Z.; Diao, L.H.; Li, Y.Y.; Huang, C.Y.; Liang, D.S.; Zeng, Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016, 76, 432–438. [Google Scholar] [CrossRef]
- St Louis, D.; Romero, R.; Plazyo, O.; Arenas-Hernandez, M.; Panaitescu, B.; Xu, Y.; Milovic, T.; Xu, Z.; Bhatti, G.; Mi, Q.S.; et al. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J. Immunol. 2016, 196, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Ntrivalas, E.; Fukuhara, R.; Fujii, S.; Mizunuma, H.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. Correlation between natural cytotoxicity receptors and intracellular cytokine expression of peripheral blood NK cells in women with recurrent pregnancy losses and implantation failures. Am. J. Reprod. Immunol. 2009, 62, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Borzychowski, A.M.; Croy, B.A.; Chan, W.L.; Redman, C.W.; Sargent, I.L. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur. J. Immunol. 2005, 35, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, A.; Alferink, J.; Möller, P.; Hämmerling, G.J.; Arnold, B. T Cell Awareness of Paternal Alloantigens during Pregnancy. Science 1995, 270, 630–633. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Mellor, A.L.; Sivakumar, J.; Chandler, P.; Smith, K.; Molina, H.; Mao, D.; Munn, D.H. Prevention of T cell–driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2001, 2, 64–68. [Google Scholar] [CrossRef]
- Koch, C.A.; Platt, J.L. T cell recognition and immunity in the fetus and mother. Cell. Immunol. 2007, 248, 12–17. [Google Scholar] [CrossRef]
- Jiang, S.-P.; Vacchio, M.S. Cutting Edge: Multiple Mechanisms of Peripheral T Cell Tolerance to the Fetal “Allograft”. J. Immunol. 1998, 160, 3086–3090. [Google Scholar] [CrossRef]
- Glassman, A.B.; Bennett, C.E.; Christopher, J.B.; Self, S. Immunity during pregnancy: Lymphocyte subpopulations and mitogen responsiveness. Ann. Clin. Lab. Sci. 1985, 15, 357–362. [Google Scholar]
- Coulam, C.B.; Silverfield, J.C.; Kazmar, R.E.; Fathman, C.G. T-lymphocyte subsets during pregnancy and the menstrual cycle. Am. J. Reprod. Immunol. 1983, 4, 88–90. [Google Scholar] [CrossRef]
- Watanabe, M.; Iwatani, Y.; Kaneda, T.; Hidaka, Y.; Mitsuda, N.; Morimoto, Y.; Amino, N. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am. J. Reprod. Immunol. 1997, 37, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C. CD4(+)CD25+ T regulatory cells in murine pregnancy. J. Reprod. Immunol. 2005, 65, 101–110. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Robertson, S.A.; Guerin, L.R.; Bromfield, J.J.; Branson, K.M.; Ahlström, A.C.; Care, A.S. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol. Reprod. 2009, 80, 1036–1045. [Google Scholar] [CrossRef]
- Saito, S.; Sasaki, Y.; Sakai, M. CD4(+)CD25high regulatory T cells in human pregnancy. J. Reprod. Immunol. 2005, 65, 111–120. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sharkey, D.J. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001, 13, 243–254. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: Adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Kusanovic, J.P.; Yoo, W.; Dong, Z.; Topping, V.; Gotsch, F.; Yoon, B.H.; Chi, J.G.; Kim, J.S. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: A lesion associated with spontaneous preterm birth. Mod. Pathol. 2010, 23, 1000–1011. [Google Scholar] [CrossRef]
- Kim, I.K.; Bedi, D.S.; Denecke, C.; Ge, X.; Tullius, S.G. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation 2008, 86, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Romero, R.; Xu, Y.; Kim, J.S.; Topping, V.; Yoo, W.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S.; Yoon, B.H.; et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: Chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS ONE 2011, 6, e16806. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Schonkeren, D.; Eikmans, M.; Nagtzaam, N.M.; Datema, G.; Swings, G.M.; Prins, F.; van Lith, J.M.; van der Mast, B.J.; Roelen, D.L.; et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J. Immunol. 2010, 185, 4470–4477. [Google Scholar] [CrossRef]
- Iwatani, Y.; Amino, N.; Tachi, J.; Kimura, M.; Ura, I.; Mori, M.; Miyai, K.; Nasu, M.; Tanizawa, O. Changes of lymphocyte subsets in normal pregnant and postpartum women: Postpartum increase in NK/K (Leu 7) cells. Am. J. Reprod. Immunol. Microbiol. 1988, 18, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Abul, H.; Omu, A.; Al-Rayes, S.; Haines, D.; Whaley, K. Pregnancy-associated changes in peripheral blood lymphocyte subpopulations in normal Kuwaiti women. Gynecol. Obstet. Investig. 2001, 52, 232–236. [Google Scholar] [CrossRef]
- Bhat, N.M.; Mithal, A.; Bieber, M.M.; Herzenberg, L.A.; Teng, N.N. Human CD5+ B lymphocytes (B-1 cells) decrease in peripheral blood during pregnancy. J. Reprod. Immunol. 1995, 28, 53–60. [Google Scholar] [CrossRef]
- Muzzio, D.; Zenclussen, A.C.; Jensen, F. The role of B cells in pregnancy: The good and the bad. Am. J. Reprod. Immunol. 2013, 69, 408–412. [Google Scholar] [CrossRef]
- Tongio, M.M.; Berrebi, A.; Mayer, S. A study of lymphocytotoxic antibodies in multiparous women having had at least four pregnancies. Tissue Antigens 1972, 2, 378–388. [Google Scholar] [CrossRef]
- Taylor, P.V.; Hancock, K.W. Antigenicity of trophoblast and possible antigen-masking effects during pregnancy. Immunology 1975, 28, 973–982. [Google Scholar]
- Canellada, A.; Färber, A.; Zenclussen, A.C.; Gentile, T.; Dokmetjian, J.; Keil, A.; Blois, S.; Miranda, S.; Berod, L.; Gutiérrez, G.; et al. Interleukin regulation of asymmetric antibody synthesized by isolated placental B cells. Am. J. Reprod. Immunol. 2002, 48, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, K.; Bognar, I.; Paal, M.; Szekeres-Bartho, J. A progesterone-induced protein increases the synthesis of asymmetric antibodies. Cell. Immunol. 1996, 167, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Valeff, N.; Muzzio, D.O.; Matzner, F.; Dibo, M.; Golchert, J.; Homuth, G.; Abba, M.C.; Zygmunt, M.; Jensen, F. B cells acquire a unique and differential transcriptomic profile during pregnancy. Genomics 2021, 113, 2614–2622. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Raber, P.; Quinton, R.A.; Ruano, R.; Ikumi, N.; Gray, C.M.; Johnson, E.L.; Chakraborty, R.; Kerr, S.E. Maternal T Cells in the Human Placental Villi Support an Allograft Response during Noninfectious Villitis. J. Immunol. 2020, 204, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Derricott, H.; Jones, R.L.; Greenwood, S.L.; Batra, G.; Evans, M.J.; Heazell, A.E.P. Characterizing Villitis of Unknown Etiology and Inflammation in Stillbirth. Am. J. Pathol. 2016, 186, 952–961. [Google Scholar] [CrossRef]
- Derricott, H.; Jones, R.L.; Heazell, A.E. Investigating the association of villitis of unknown etiology with stillbirth and fetal growth restriction—A systematic review. Placenta 2013, 34, 856–862. [Google Scholar] [CrossRef]
- Lee, J.; Romero, R.; Xu, Y.; Kim, J.S.; Park, J.Y.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S.; Kim, C.J. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: Evidence in support of the chronic nature of maternal anti-fetal rejection. Am. J. Reprod. Immunol. 2011, 66, 510–526. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Xu, C.; Peng, X.; Li, D.; Wang, L.; Du, M. Tissue-resident CD8(+) T memory cells with unique properties are present in human decidua during early pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13254. [Google Scholar] [CrossRef]
- Zhuang, X.; Xia, X.; Liu, L.; Zhang, Y.; Zhang, X.; Wang, C. Expression of Tim-3 in peripheral blood mononuclear cells and placental tissue in unexplained recurrent spontaneous abortion. Med. Baltim. 2018, 97, e12099. [Google Scholar] [CrossRef]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Robertson, S.A.; Davidge, S.T. Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension 2018, 72, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lamprianidou, E.; Daniilidis, M.; Kordella, C.; Zoulia, E.; Nakou, E.; Gerofotis, A.; Vasilaki, A.; Pantos, G.; Kotsianidis, I. The STAT signaling profile at the single cell level reveals novel insights in the association of FOXP3+ T regulatory cells with recurrent spontaneous abortions before and after lymphocyte immunotherapy. Clin. Immunol. 2020, 210, 108261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, X.; Zhang, X.; Pan, Z.; Zhao, W.; Zhang, Y.; Li, J.; Xiao, F.; Wu, H.; Tan, H.; et al. Association between Serum TNF-α Levels and Recurrent Spontaneous Miscarriage: A Meta-analysis. Am. J. Reprod. Immunol. 2016, 75, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi Vahid, S.; Ghaebi, M.; Ahmadi, M.; Nouri, M.; Danaei, S.; Aghebati-Maleki, L.; Mousavi Ardehaie, R.; Yousefi, B.; Hakimi, P.; Hojjat-Farsangi, M.; et al. Altered T-cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. J. Cell. Physiol. 2019, 234, 4924–4933. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Barakonyi, A.; Miko, E.; Polgar, B.; Palkovics, T. The role of gamma/delta T cells in the feto-maternal relationship. Semin. Immunol. 2001, 13, 229–233. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Barakonyi, A.; Polgar, B.; Par, G.; Faust, Z.; Palkovics, T.; Szereday, L. The role of gamma/delta T cells in progesterone-mediated immunomodulation during pregnancy: A review. Am. J. Reprod. Immunol. 1999, 42, 44–48. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Xiong, J.; Liu, J.; Zha, Y.; Kang, Q.; Zhi, P.; Wang, Q.; Wang, H.; Zeng, W.; et al. Activated γδ T Cells With Higher CD107a Expression and Inflammatory Potential During Early Pregnancy in Patients With Recurrent Spontaneous Abortion. Front. Immunol. 2021, 12, 724662. [Google Scholar] [CrossRef]
- Hafeez, N.A.; Fouda Mel, T.; Abdel Gawad, E.R.; Assar, T.; Mansour, A.I. The role of regulatory T cells in preeclampsia. Egypt J. Immunol. 2014, 21, 45–55. [Google Scholar]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Steinborn, A.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Toermer, A.; Meuer, S.; Sohn, C. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin. Immunol. 2008, 129, 401–412. [Google Scholar] [CrossRef]
- Eghbal-Fard, S.; Yousefi, M.; Heydarlou, H.; Ahmadi, M.; Taghavi, S.; Movasaghpour, A.; Jadidi-Niaragh, F.; Yousefi, B.; Dolati, S.; Hojjat-Farsangi, M.; et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell. Physiol. 2019, 234, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Carbone, J.; Gallego, A.; Lanio, N.; Navarro, J.; Orera, M.; Aguaron, A.; Fernandez-Cruz, E.; Sarmiento, E. Quantitative abnormalities of peripheral blood distinct T, B, and natural killer cell subsets and clinical findings in obstetric antiphospholipid syndrome. J. Rheumatol. 2009, 36, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Velasquillo, M.C.; Alcocer-Varela, J.; Alarcón-Segovia, D.; Cabiedes, J.; Sánchez-Guerrero, J. Some patients with primary antiphospholipid syndrome have increased circulating CD5+ B cells that correlate with levels of IgM antiphospholipid antibodies. Clin. Exp. Rheumatol. 1991, 9, 501–505. [Google Scholar]
- Jablonowska, B.; Palfi, M.; Matthiesen, L.; Selbing, A.; Kjellberg, S.; Ernerudh, J. T and B lymphocyte subsets in patients with unexplained recurrent spontaneous abortion: IVIG versus placebo treatment. Am. J. Reprod. Immunol. 2002, 48, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Liu, L.P.; Ding, W.P.; Zhang, L. Functional changes of human peripheral B-lymphocytes in pre-eclampsia. Am. J. Reprod. Immunol. 2009, 61, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Blidaru, I.; Zugun, F.; Cianga, C.; Carasevici, E. Maternal immunophenotypic profile in normal pregnancy and preterm birth. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2002, 107, 343–347. [Google Scholar]
- Selvaggi, L.; Lucivero, G.; Iannone, A.; dell’Osso, A.; Loverro, G.; Antonaci, S.; Bonomo, L.; Bettocchi, S. Analysis of mononuclear cell subsets in pregnancies with intrauterine growth retardation. Evidence of chronic B-lymphocyte activation. J. Perinat. Med. 1983, 11, 213–217. [Google Scholar] [CrossRef]
- Chen, G.; Wilson, R.; Cumming, G.; Walker, J.J.; McKillop, J.H. Immunological changes in pregnancy-induced hypertension. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 53, 21–25. [Google Scholar] [CrossRef]
- Reece, E.A.; Gabrielli, S.; Cullen, M.T.; Zheng, X.-Z.; Hobbins, J.C.; Nigel Harris, E. Recurrent adverse pregnancy outcome and antiphospholipid antibodies. Am. J. Obstet. Gynecol. 1990, 163, 162–169. [Google Scholar] [CrossRef]
- Zhou, C.C.; Zhang, Y.; Irani, R.A.; Zhang, H.; Mi, T.; Popek, E.J.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 2008, 14, 855–862. [Google Scholar] [CrossRef]
- Jensen, F.; Wallukat, G.; Herse, F.; Budner, O.; El-Mousleh, T.; Costa, S.D.; Dechend, R.; Zenclussen, A.C. CD19+CD5+ cells as indicators of preeclampsia. Hypertension 2012, 59, 861–868. [Google Scholar] [CrossRef]
- Beard, R.W.; Braude, P.; Mowbray, J.F.; Underwood, J.L. Protective antibodies and spontaneous abortion. Lancet 1983, 2, 1090. [Google Scholar] [CrossRef] [PubMed]
- Malan Borel, I.; Gentile, T.; Angelucci, J.; Pividori, J.; Guala, M.C.; Binaghi, R.A.; Margni, R.A. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 1991, 20, 129–140. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Gentile, T.; Kortebani, G.; Mazzolli, A.; Margni, R. Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 2001, 45, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gelabert, A.; Balasch, J.; Ercilla, G.; Vanrell, J.A.; Vives, J.; González-Merlo, J.; Castillo, R. Abortion may sensitize the mother to HLA antigens. Tissue Antigens 1981, 17, 353–356. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.W.; King, A. Human Implantation: Cell Biology and Immunology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Nair, R.R.; Verma, P.; Singh, K. Immune-endocrine crosstalk during pregnancy. Gen. Comp. Endocrinol. 2017, 242, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, Y.M.; Cheng, X.Y.; Zhu, T.F.; Chen, Z.F.; Yao, H.; Su, L.X. Dendritic cells derived from preeclampsia patients influence Th1/Th17 cell differentiation in vitro. Int. J. Clin. Exp. Med. 2014, 7, 5303–5309. [Google Scholar]
- Panda, B.; Panda, A.; Ueda, I.; Abrahams, V.M.; Norwitz, E.R.; Stanic, A.K.; Young, B.C.; Ecker, J.L.; Altfeld, M.; Shaw, A.C.; et al. Dendritic cells in the circulation of women with preeclampsia demonstrate a pro-inflammatory bias secondary to dysregulation of TLR receptors. J. Reprod. Immunol. 2012, 94, 210–215. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Sun, F.; Chen, C.; Ye, J.; Li, D.; Qian, J.; Du, M. Th17/Treg-cell balance in the peripheral blood of pregnant females with a history of recurrent spontaneous abortion receiving progesterone or cyclosporine A. Exp. Ther. Med. 2021, 21, 37. [Google Scholar] [CrossRef]
- Nagaeva, O.; Jonsson, L.; Mincheva-Nilsson, L. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am. J. Reprod. Immunol. 2002, 48, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, A.; Harrison, L.C. Gamma delta T cells as mediators of mucosal tolerance: The autoimmune diabetes model. Immunol. Rev. 2000, 173, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L. Pregnancy and gamma/delta T cells: Taking on the hard questions. Reprod. Biol. Endocrinol. 2003, 1, 120. [Google Scholar] [CrossRef] [PubMed]
- Polgar, B.; Barakonyi, A.; Xynos, I.; Szekeres-Bartho, J. The role of gamma/delta T cell receptor positive cells in pregnancy. Am. J. Reprod. Immunol. 1999, 41, 239–244. [Google Scholar] [CrossRef]
- Barakonyi, A.; Polgar, B.; Szekeres-Bartho, J. The role of gamma/delta T-cell receptor-positive cells in pregnancy: Part II. Am. J. Reprod. Immunol. 1999, 42, 83–87. [Google Scholar]
- Miko, E.; Szereday, L.; Barakonyi, A.; Jarkovich, A.; Varga, P.; Szekeres-Bartho, J. Immunoactivation in preeclampsia: Vdelta2+ and regulatory T cells during the inflammatory stage of disease. J. Reprod. Immunol. 2009, 80, 100–108. [Google Scholar] [CrossRef]
- Carter, A.M. Animal models of human placentation—A review. Placenta 2007, 28, S41–S47. [Google Scholar] [CrossRef]
- Sooranna, S.R.; Oteng-Ntim, E.; Meah, R.; Ryder, T.A.; Bajoria, R. Characterization of human placental explants: Morphological, biochemical and physiological studies using first and third trimester placenta. Hum. Reprod. 1999, 14, 536–541. [Google Scholar] [CrossRef]
- Couture, C.; Brien, M.E.; Boufaied, I.; Duval, C.; Soglio, D.D.; Enninga, E.A.L.; Cox, B.; Girard, S. Proinflammatory changes in the maternal circulation, maternal-fetal interface, and placental transcriptome in preterm birth. Am. J. Obstet. Gynecol. 2022, 228, 332-e1. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Galaz, J.; Miller, D.; Farias-Jofre, M.; Liu, Z.; Arenas-Hernandez, M.; Garcia-Flores, V.; Shaffer, Z.; Greenberg, J.M.; Theis, K.R.; et al. The immunobiology of preterm labor and birth: Intra-amniotic inflammation or breakdown of maternal-fetal homeostasis. Reproduction 2022, 164, R11–R45. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Xu, Y.; Pusod, E.; Romero, R.; Pique-Regi, R.; Gomez-Lopez, N. Preparation of single-cell suspensions from the human placenta. Nat. Protoc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Garcia-Flores, V.; Romero, R.; Galaz, J.; Pique-Regi, R.; Gomez-Lopez, N. Single-Cell Immunobiology of the Maternal-Fetal Interface. J. Immunol. 2022, 209, 1450–1464. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.; Liu, Y.; Garmire, L.X. Single cell transcriptome research in human placenta. Reproduction 2020, 160, R155–R167. [Google Scholar] [CrossRef] [PubMed]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 2019, 8, e52004. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.; Campe, K.J.; Nowak, D.; Schumacher, A.; Plenagl, S.; Langwisch, S.; Tiegs, G.; Reinhold, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Parhamifar, L.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.; Farhangrazi, Z.; Hunter, A. Particulate systems for targeting of macrophages: Basic and therapeutic concepts. J. Innate Immun. 2012, 4, 509–528. [Google Scholar] [CrossRef]
- Miranda, S.; Litwin, S.; Barrientos, G.; Szereday, L.; Chuluyan, E.; Bartho, J.; Arck, P.; Blois, S. Dendritic cells therapy confers a protective microenvironment in murine pregnancy. Scand. J. Immunol. 2006, 64, 493–499. [Google Scholar] [CrossRef]
- Schumacher, A.; Wafula, P.O.; Teles, A.; El-Mousleh, T.; Linzke, N.; Zenclussen, M.L.; Langwisch, S.; Heinze, K.; Wollenberg, I.; Casalis, P.A. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS ONE 2012, 7, e42301. [Google Scholar] [CrossRef]
- Hackstein, H.; Taner, T.; Zahorchak, A.F.; Morelli, A.E.; Logar, A.J.; Gessner, A.; Thomson, A.W. Rapamycin inhibits IL-4—Induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood 2003, 101, 4457–4463. [Google Scholar] [CrossRef]
- Eskandarian, M.; Moazzeni, S.M. Uterine dendritic cells modulation by mesenchymal stem cells provides a protective microenvironment at the feto-maternal interface: Improved pregnancy outcome in abortion-prone mice. Cell J. Yakhteh 2019, 21, 274. [Google Scholar]
- Fuchisawa, A.; Van Eeden, S.; Magee, L.A.; Whalen, B.; Leung, P.C.K.; Russell, J.A.; Walley, K.R.; Von Dadelszen, P. Neutrophil apoptosis in preeclampsia, do steroids confound the relationship? J. Obstet. Gynaecol. Res. 2004, 30, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Einenkel, R.; Ehrhardt, J.; Hartmann, K.; Krüger, D.; Muzzio, D.O.; Zygmunt, M. Hormonally controlled ILC antigen presentation potential is reduced during pregnancy. Reproduction 2020, 160, 155–169. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. https://doi.org/10.3390/biology12030402
Weng J, Couture C, Girard S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology. 2023; 12(3):402. https://doi.org/10.3390/biology12030402
Chicago/Turabian StyleWeng, Jessica, Camille Couture, and Sylvie Girard. 2023. "Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy" Biology 12, no. 3: 402. https://doi.org/10.3390/biology12030402
APA StyleWeng, J., Couture, C., & Girard, S. (2023). Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology, 12(3), 402. https://doi.org/10.3390/biology12030402






