An Enhanced 3D Model of Intravascular Lymphatic Valves to Assess Leaflet Apposition and Transvalvular Differences in Wall Distensibility
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
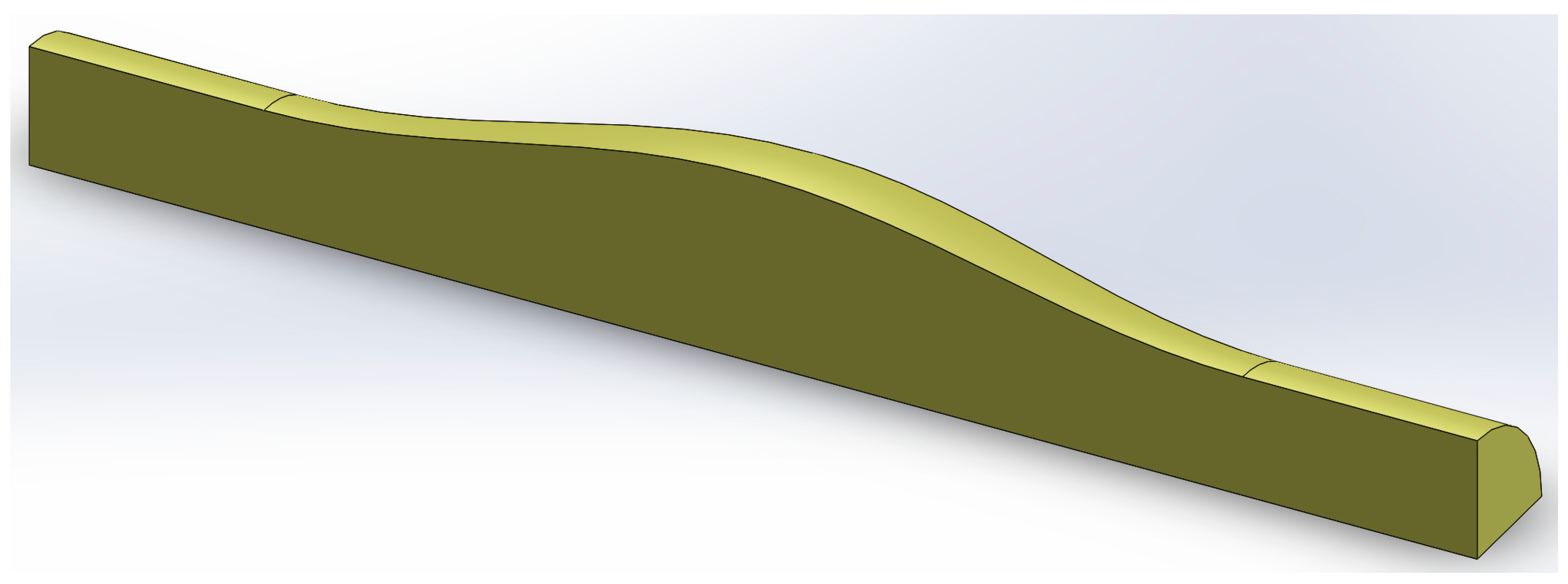
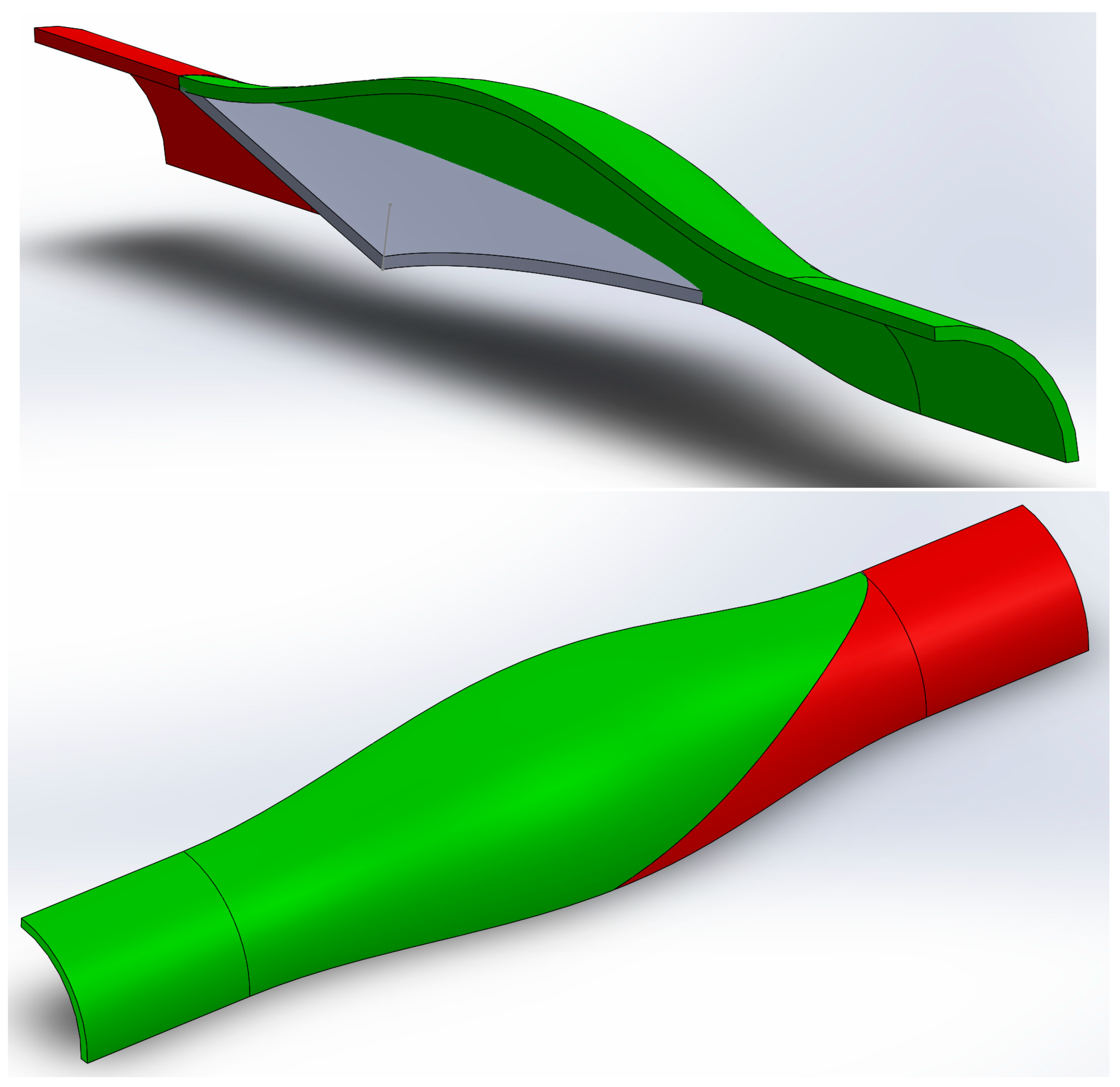
2.1. Numerical
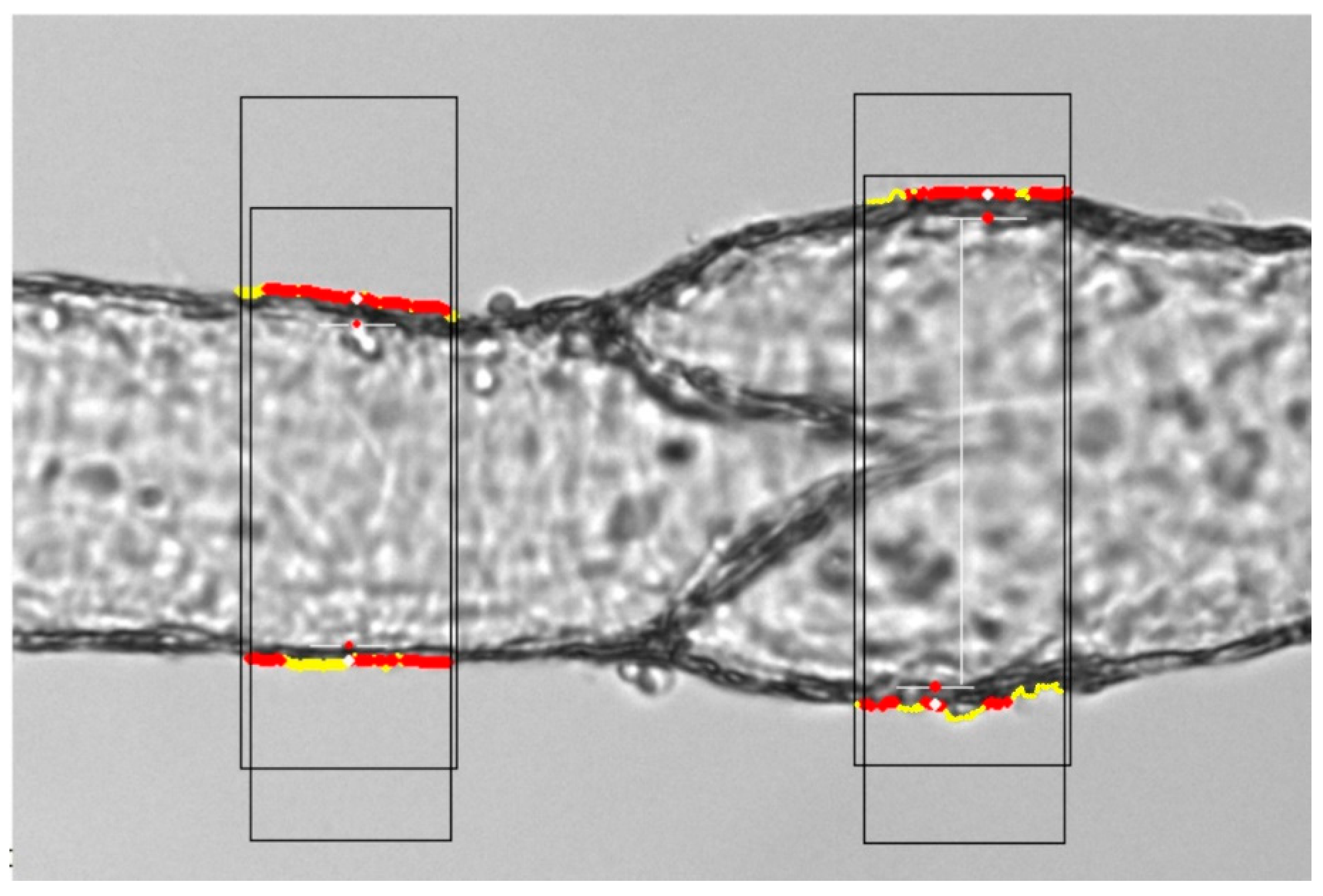
2.2. Experimental
3. Results
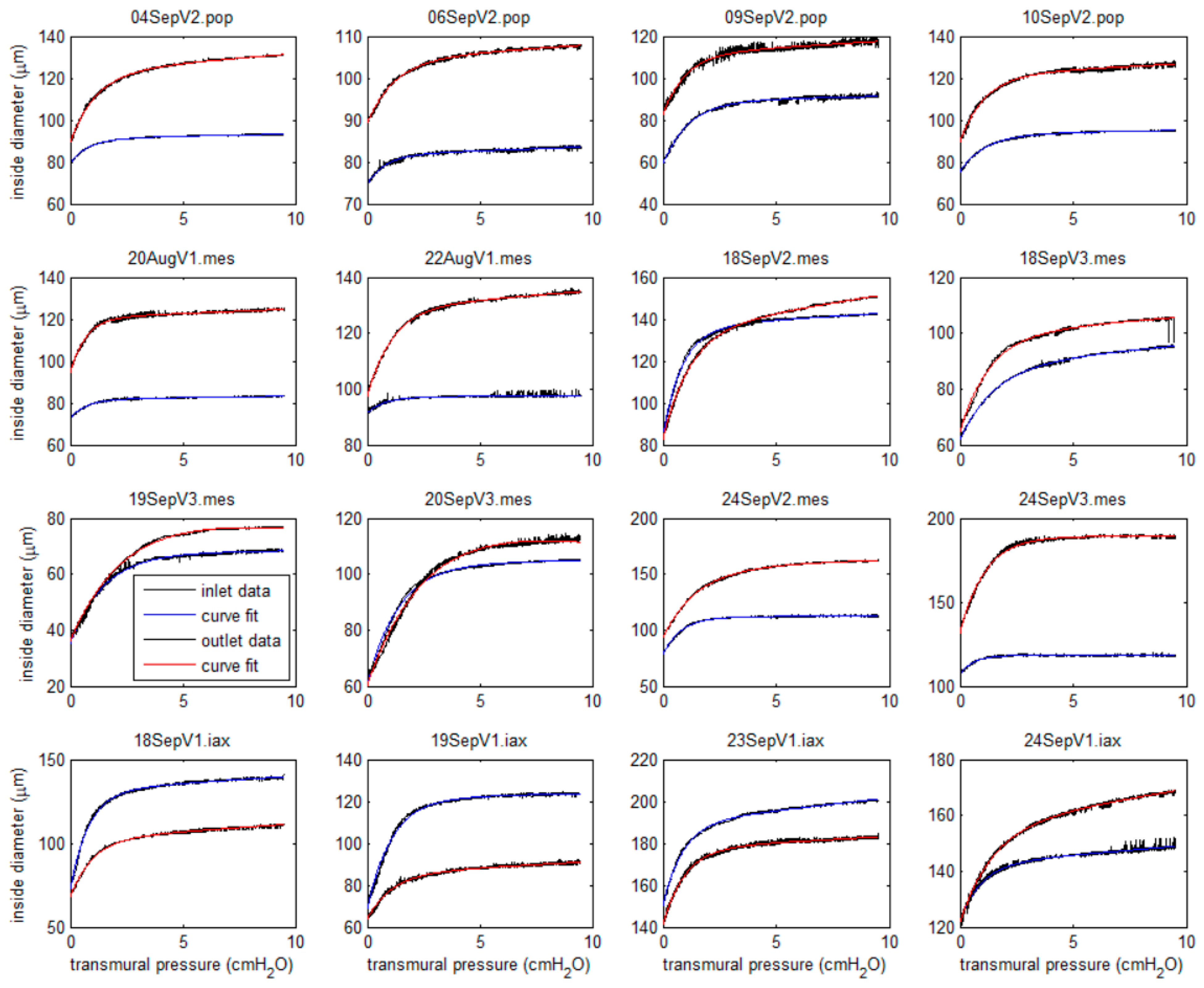
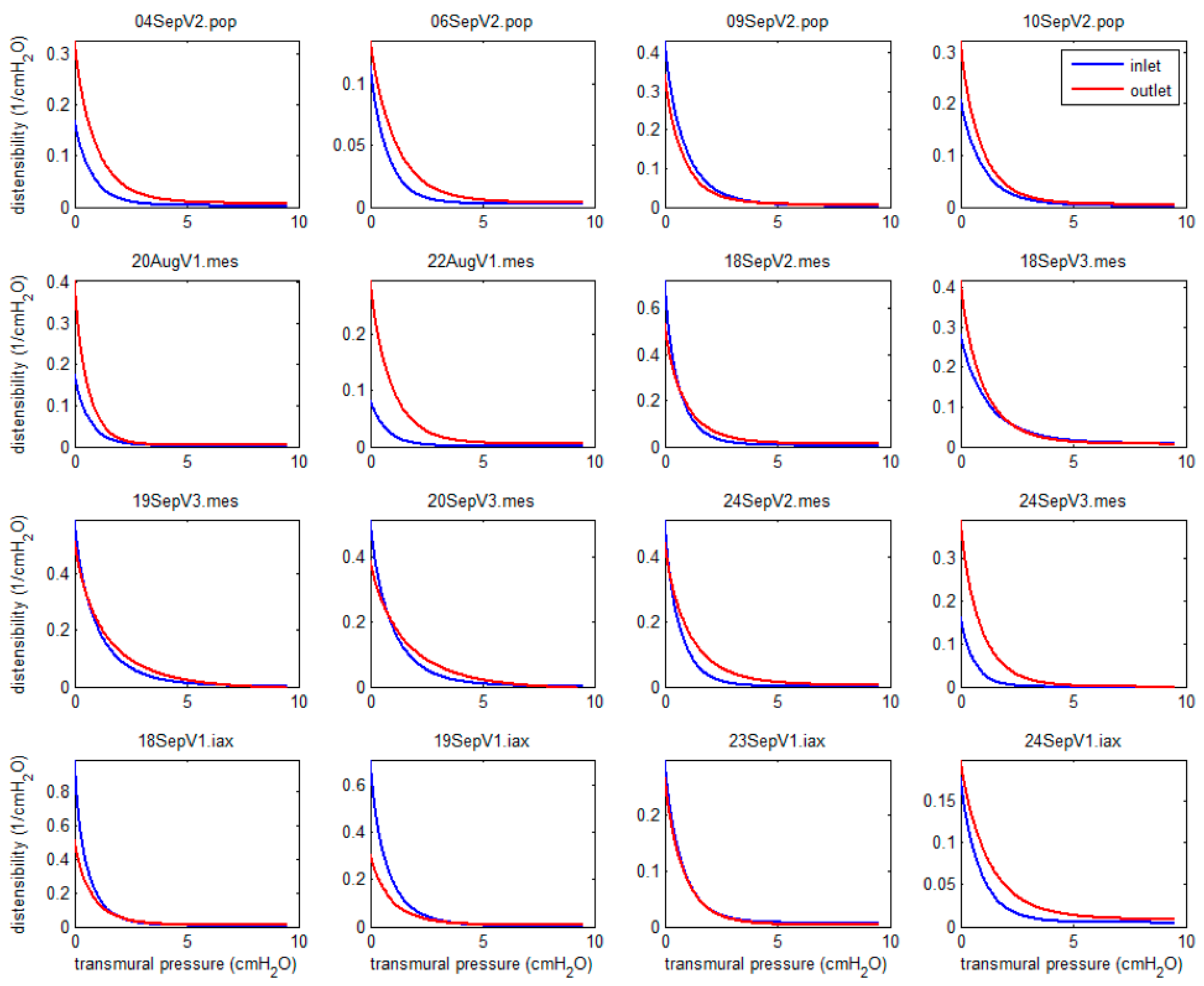
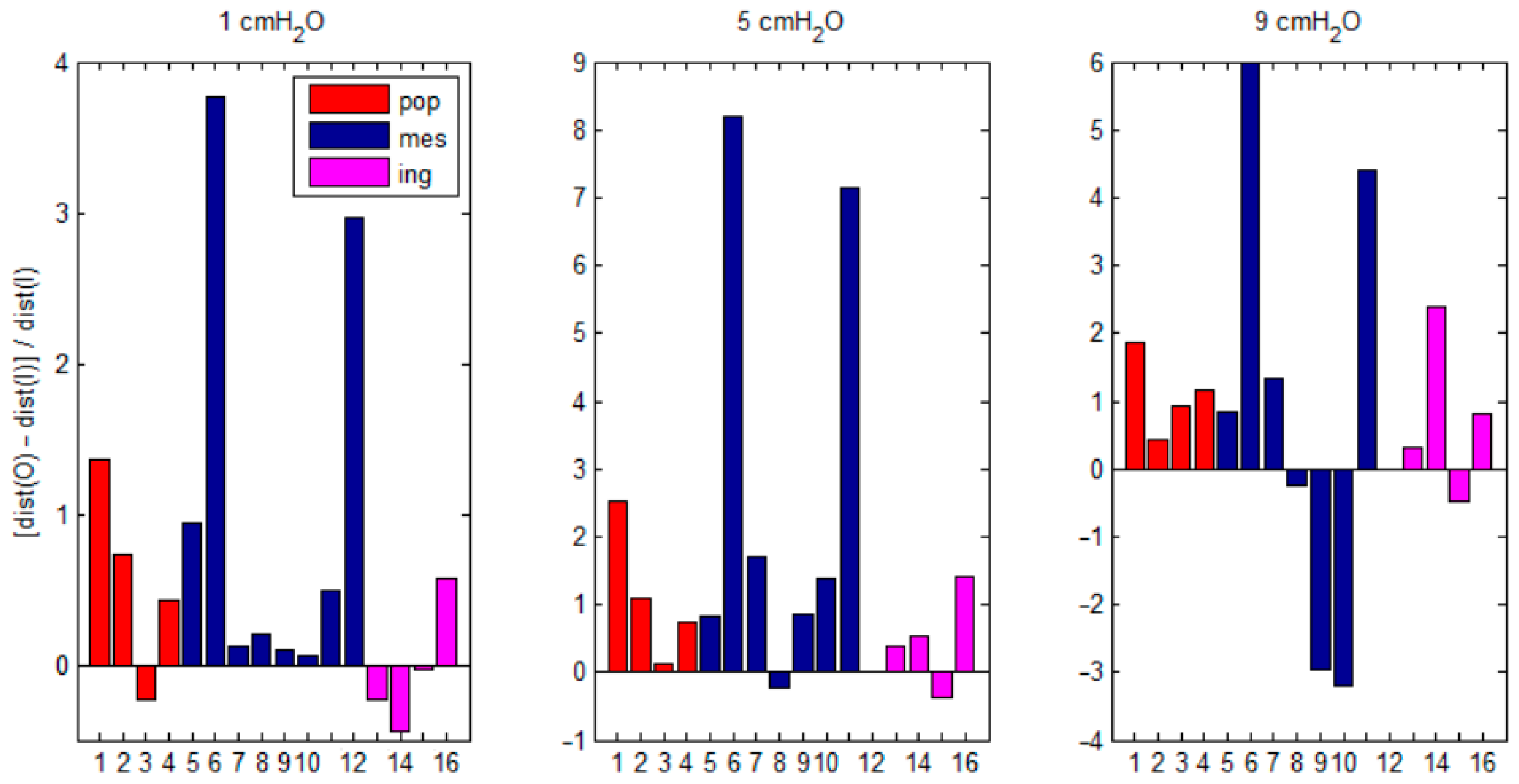
3.1. Experimental
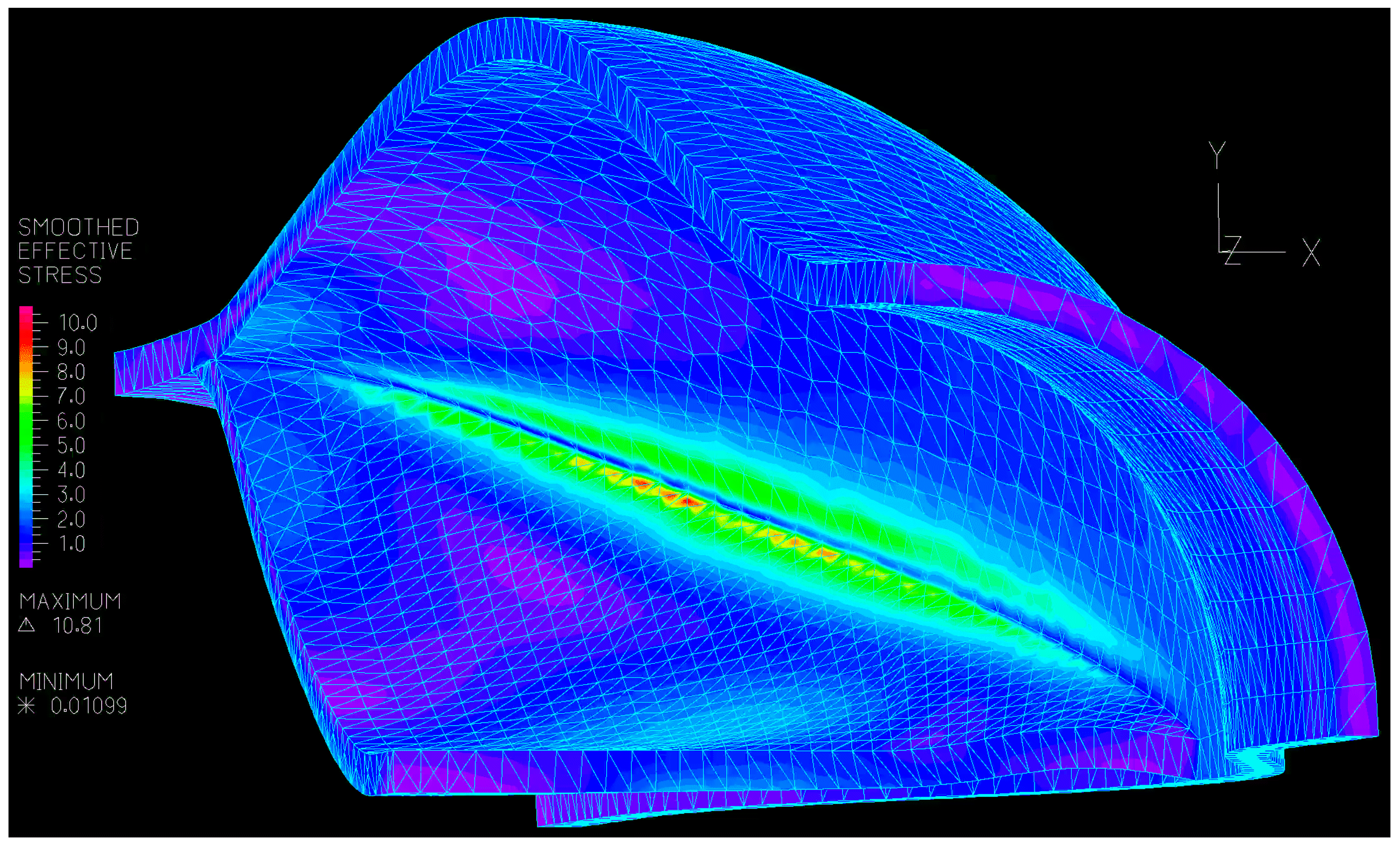
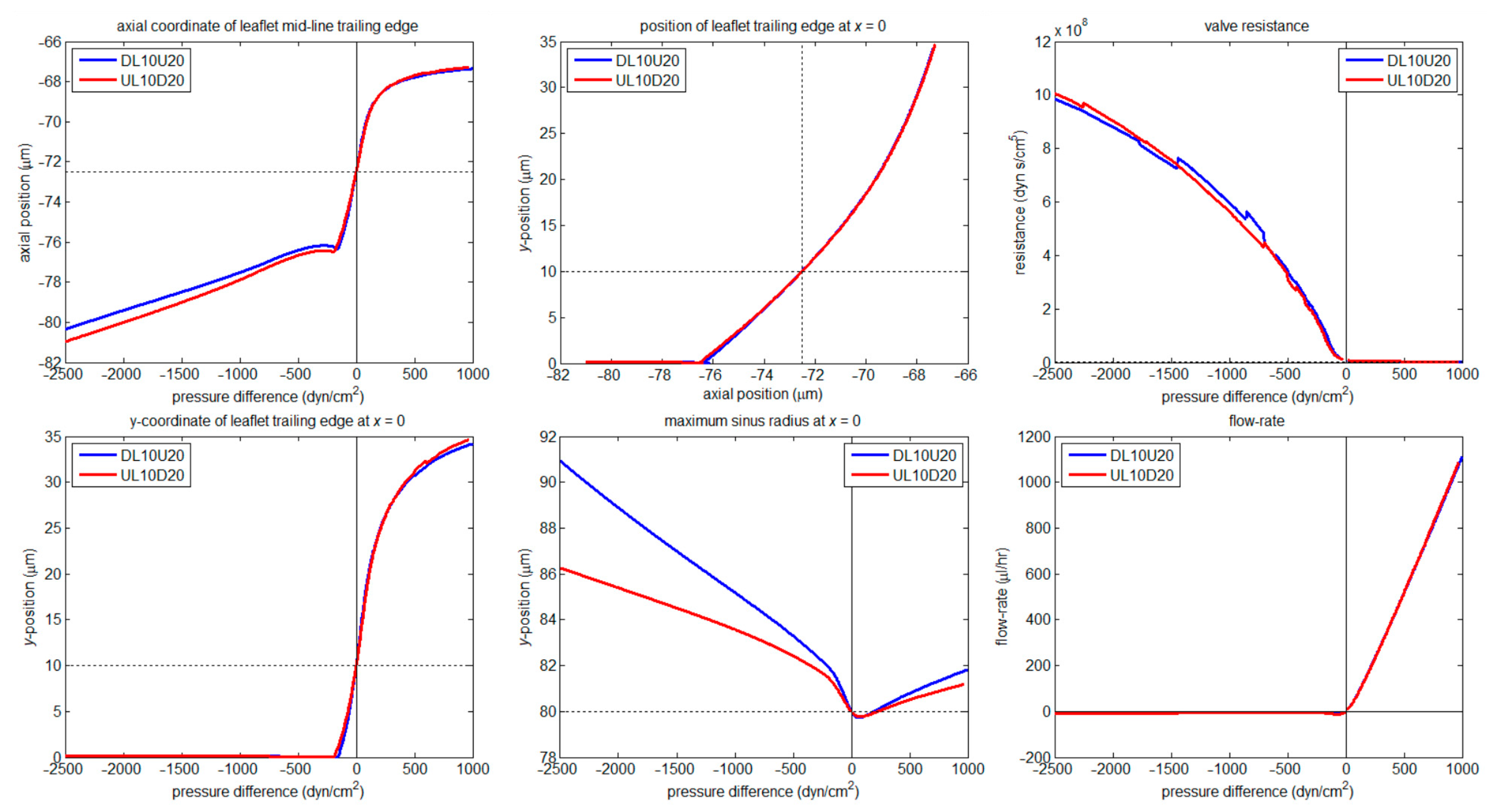
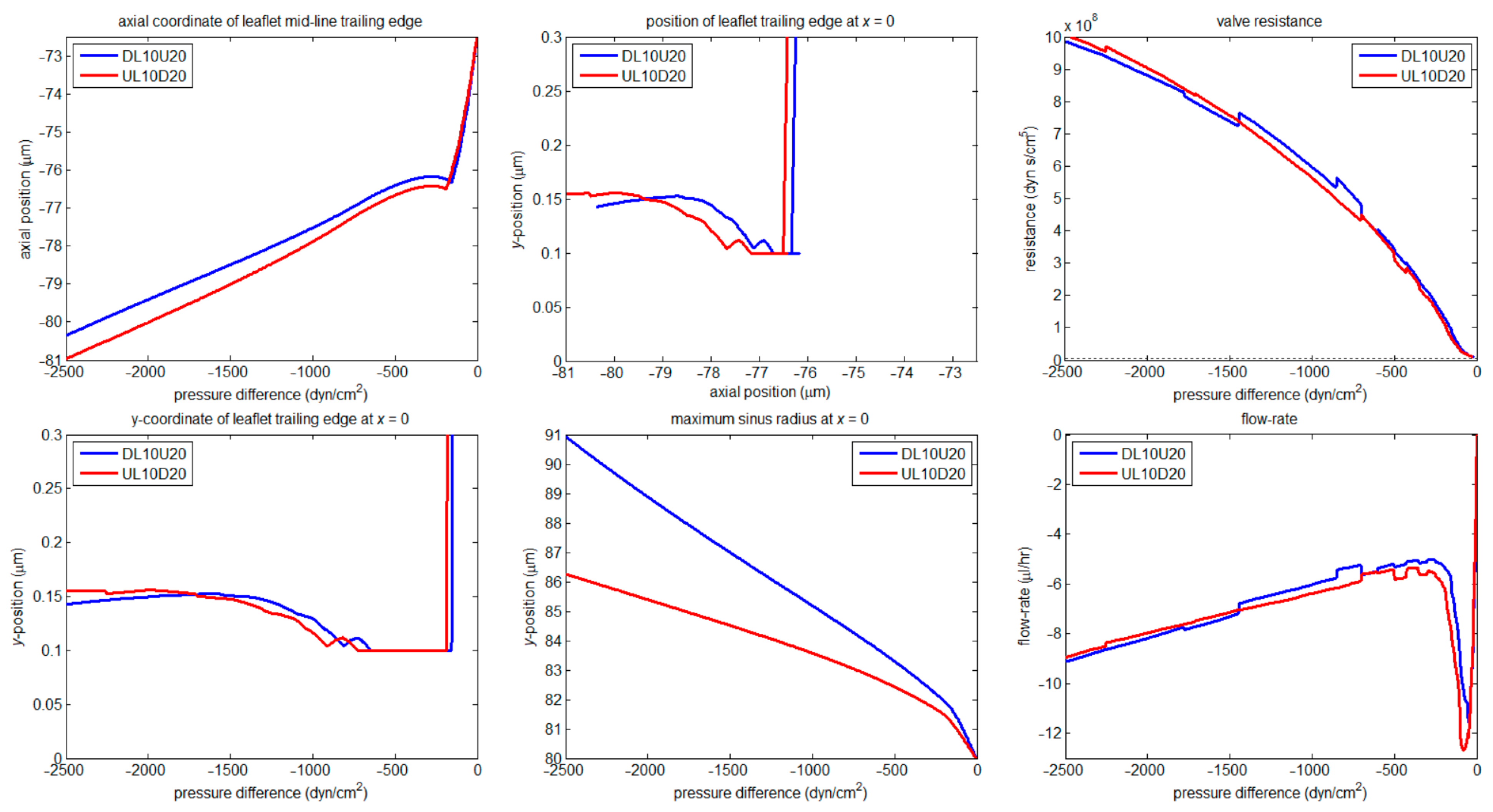
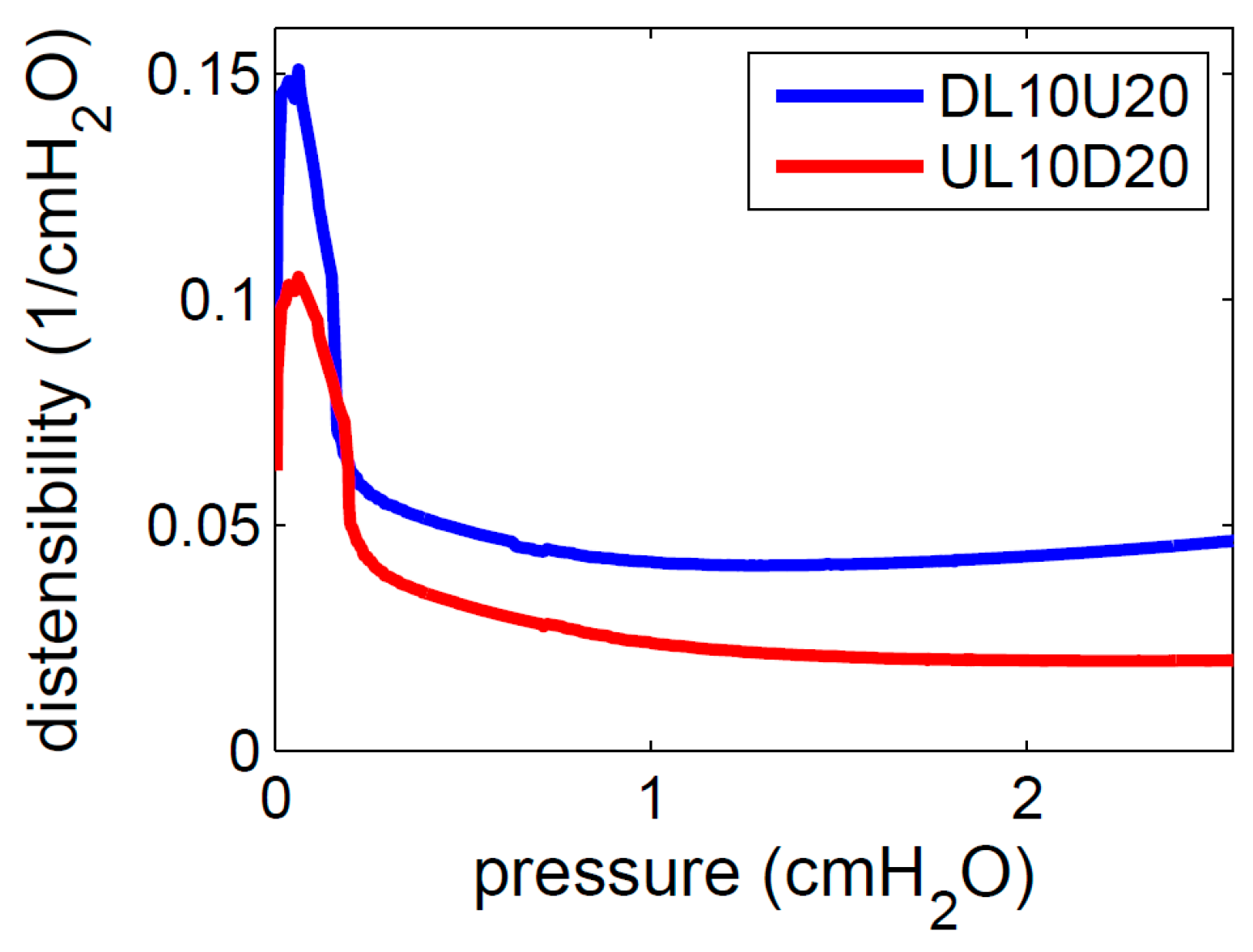
3.2. Numerical
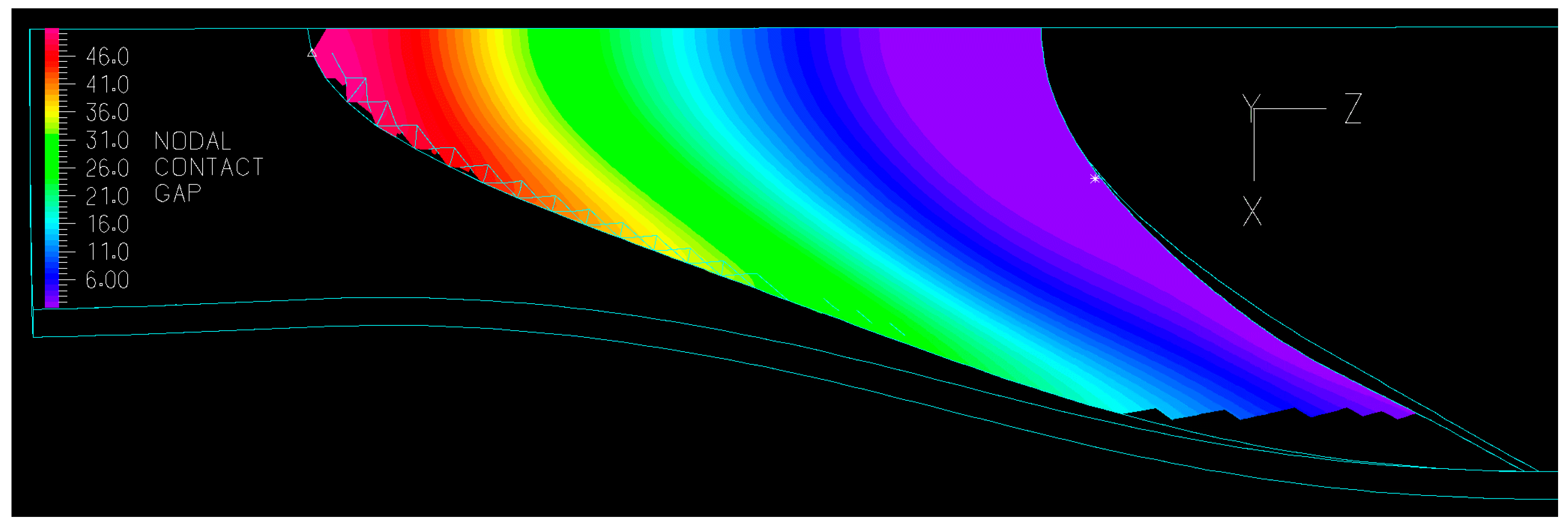
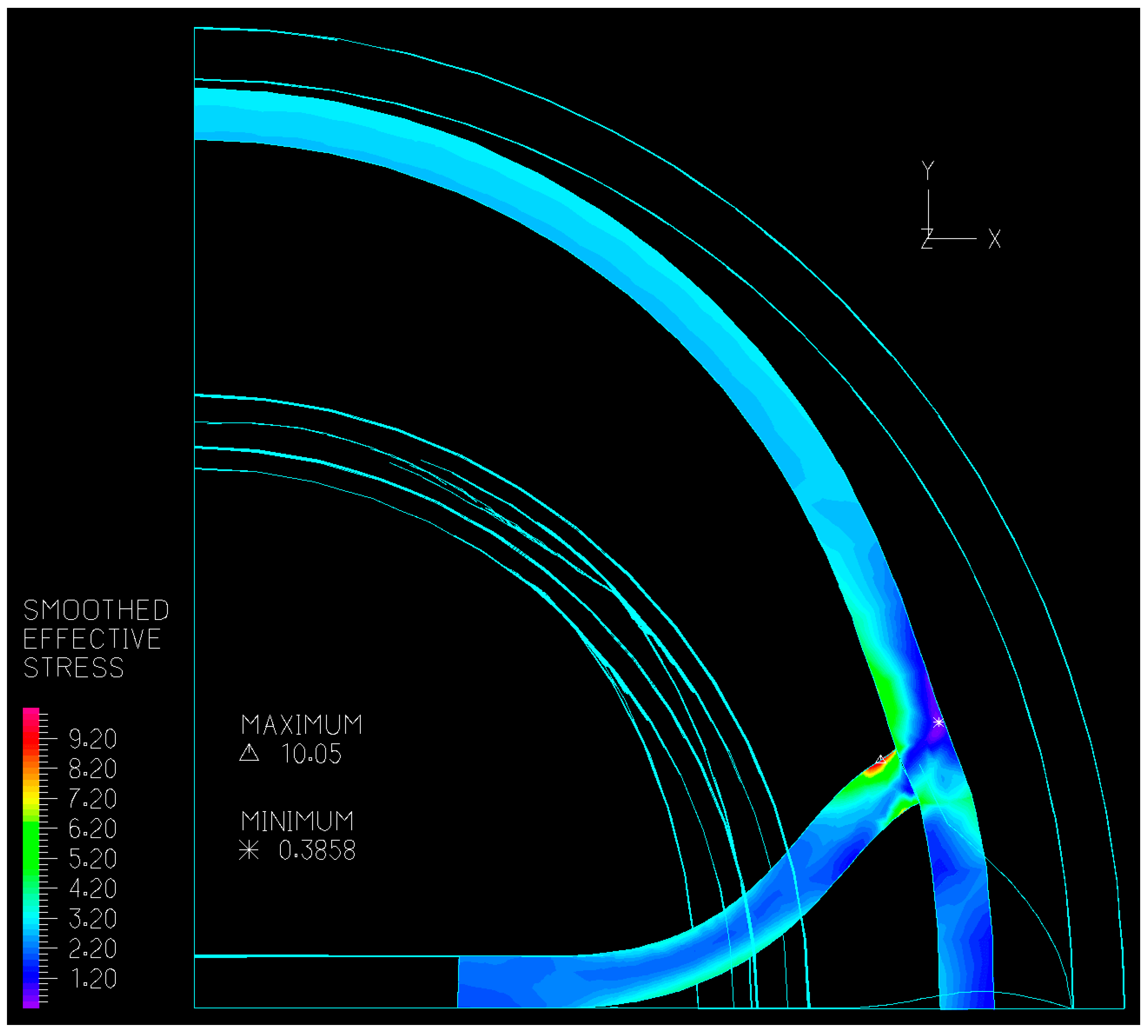
3.2.1. Valve Closure
3.2.2. Wall Stiffness Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, M.N.; Jones, G.T.; van Rij, A.M.; Zhang, M. Micro-venous valves in the superficial veins of the human lower limb. Clin. Anat. 2004, 17, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.C.; Skalak, T.C.; Schmid-Schönbein, G.W. Structure of lymphatic valves in the spinotrapezius muscle of the rat. Blood vessels 1987, 24, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.P.; Krouskop, T.A.; Newell, P.H., Jr. Biomechanics of a lymphatic vessel. Blood Vessels 1975, 12, 261–278, Correction in Blood Vessels 1977, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, A.M.; Stewart, R.H.; Laine, G.A.; Dongaonkar, R.M.; Quick, C.M. Lymphangion coordination minimally affects mean flow in lymphatic vessels. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1183–H1189. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Arkill, K.P.; Tabor, G.R.; McHale, N.G.; Winlove, C.P. Modeling flow in collecting lymphatic vessels: One-dimensional flow through a series of contractile elements. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H305–H313. [Google Scholar] [CrossRef]
- Kunert, C.; Baish, J.W.; Liao, S.; Padera, T.P.; Munn, L.L. Mechanobiological oscillators control lymph flow. Proc. Natl. Acad. Sci. USA 2015, 112, 10938–10943. [Google Scholar] [CrossRef]
- Bertram, C.D.; Macaskill, C.; Moore, J.E., Jr. Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. J. Biomech. Eng. 2011, 133, 011008. [Google Scholar] [CrossRef]
- Bertram, C.D.; Macaskill, C.; Moore, J.E., Jr. Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1519–1534. [Google Scholar] [CrossRef]
- Bertram, C.D.; Macaskill, C.; Davis, M.J.; Moore, J.E., Jr. Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech. Model. Mechanobiol. 2014, 13, 401–416. [Google Scholar] [CrossRef]
- Mynard, J.P.; Davidson, M.R.; Penny, D.J.; Smolich, J.J. A simple, versatile valve model for use in lumped parameter and one-dimensional cardiovascular models. Int. J. Numer. Methods Biomed. Eng. 2012, 28, 626–641. [Google Scholar] [CrossRef]
- Contarino, C.; Toro, E.F. A one-dimensional mathematical model of collecting lymphatics coupled with an electro-fluid-mechanical contraction model and valve dynamics. Biomech. Model. Mechanobiol. 2018, 17, 1687–1714. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.J. The Computational Modelling of Collecting Lymphatic Vessels. Ph.D. Thesis, University of Exeter, Exeter, UK, 2008. [Google Scholar]
- Li, H.; Mei, Y.; Maimon, N.; Padera, T.P.; Baish, J.W.; Munn, L.L. The effects of valve leaflet mechanics on lymphatic pumping assessed using numerical simulations. Sci. Rep. 2019, 9, 10649. [Google Scholar] [CrossRef] [PubMed]
- Elich, H.; Barrett, A.; Shankar, V.; Fogelson, A.L. Pump efficacy in a two-dimensional, fluid–structure interaction model of a chain of contracting lymphangions. Biomech. Model. Mechanobiol. 2021, 20, 1941–1968. [Google Scholar] [CrossRef]
- Wilson, J.T.; Wang, W.; Hellerstedt, A.H.; Zawieja, D.C.; Moore, J.E., Jr. Confocal image-based computational modeling of nitric oxide transport in a rat mesenteric lymphatic vessel. J. Biomech. Eng. 2013, 135, 051005, Erratum in J. Biomech. Eng. 2013, 135, 117001. https://doi.org/10.1115/1.4025336. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; van Loon, R.; Wang, W.; Zawieja, D.C.; Moore, J.E., Jr. Determining the combined effect of the lymphatic valve leaflets and sinus on resistance to forward flow. J. Biomech. 2015, 48, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Edgar, L.T.; Prabhakar, S.; Horner, M.; van Loon, R.; Moore, J.E., Jr. A fully coupled fluid-structure interaction model of the secondary lymphatic valve. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.J.; Sazonov, I.; Zawieja, D.C.; Moore, J.E., Jr.; van Loon, R. Integrated geometric and mechanical analysis of an image-based lymphatic valve. J. Biomech. 2017, 64, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.D. Modelling secondary lymphatic valves with a flexible vessel wall: How geometry and material properties combine to provide function. Biomech. Model. Mechanobiol. 2020, 19, 2081–2098. [Google Scholar] [CrossRef]
- Ballard, M.; Wolf, K.T.; Nepiyushchikh, Z.; Dixon, J.B.; Alexeev, A. Probing the effect of morphology on lymphatic valve dynamic function. Biomech. Model. Mechanobiol. 2018, 17, 1343–1356. [Google Scholar] [CrossRef]
- Wolf, K.T.; Dixon, J.B.; Alexeev, A. Fluid pumping of peristaltic vessel fitted with elastic valves. J. Fluid Mech. 2021, 918, A28-1–A28-26. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.A.; Clarke, N. Computational phlebology: The simulation of a vein valve. J. Biol. Phys. 2006, 32, 507–521. [Google Scholar] [CrossRef]
- Rahbar, E.; Weimer, J.; Gibbs, H.; Yeh, A.T.; Bertram, C.D.; Davis, M.J.; Hill, M.A.; Zawieja, D.C.; Moore, J.E., Jr. Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat. Res. Biol. 2012, 10, 152–163. [Google Scholar] [CrossRef]
- Davis, M.J.; Rahbar, E.; Gashev, A.A.; Zawieja, D.C.; Moore, J.E., Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H48–H60. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 2005, 12, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Hazel, A.L.; Boyle, J. Solvers for large-displacement fluid-structure interaction problems: Segregated vs. monolithic approaches. Comput. Mech. 2007, 43, 91–101. [Google Scholar] [CrossRef]
- Venugopal, A.M.; Stewart, R.H.; Laine, G.A.; Quick, C.M. Nonlinear lymphangion pressure-volume relationship minimizes edema. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H876–H882. [Google Scholar] [CrossRef]
- Petrova, T.V.; Karpanen, T.; Norrmén, C.; Mellor, R.; Tamakoshi, T.; Finegold, D.; Ferrell, R.; Kerjaschki, D.; Mortimer, P.; Ylä-Herttuala, S.; et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004, 10, 974–981. [Google Scholar] [CrossRef]
- Davis, M.J.; Scallan, J.P.; Castorena-Gonzalez, J.A.; Kim, H.J.; Ying, L.H.; Pin, Y.K.; Angeli, V. Multiple aspects of lymphatic dysfunction in an ApoE−/− mouse model of hypercholesterolemia. Front. Physiol. 2023, 13, 1098408. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Kistner, R.L.; Eklof, B.; Kessler, D. Mechanism of venous valve closure and role of the valve in circulation: A new concept. J. Vasc. Surg. 2003, 38, 955–961. [Google Scholar] [CrossRef]
- Ackroyd, J.S.; Pattison, M.; Browse, N.L. A study of the mechanical properties of fresh and preserved human femoral vein wall and valve cusps. Br. J. Surg. 1985, 72, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E., Jr.; Bertram, C.D. Lymphatic system flows. Annu. Rev. Fluid Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertram, C.D.; Davis, M.J. An Enhanced 3D Model of Intravascular Lymphatic Valves to Assess Leaflet Apposition and Transvalvular Differences in Wall Distensibility. Biology 2023, 12, 379. https://doi.org/10.3390/biology12030379
Bertram CD, Davis MJ. An Enhanced 3D Model of Intravascular Lymphatic Valves to Assess Leaflet Apposition and Transvalvular Differences in Wall Distensibility. Biology. 2023; 12(3):379. https://doi.org/10.3390/biology12030379
Chicago/Turabian StyleBertram, Christopher D., and Michael J. Davis. 2023. "An Enhanced 3D Model of Intravascular Lymphatic Valves to Assess Leaflet Apposition and Transvalvular Differences in Wall Distensibility" Biology 12, no. 3: 379. https://doi.org/10.3390/biology12030379
APA StyleBertram, C. D., & Davis, M. J. (2023). An Enhanced 3D Model of Intravascular Lymphatic Valves to Assess Leaflet Apposition and Transvalvular Differences in Wall Distensibility. Biology, 12(3), 379. https://doi.org/10.3390/biology12030379




