Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgical Procedures
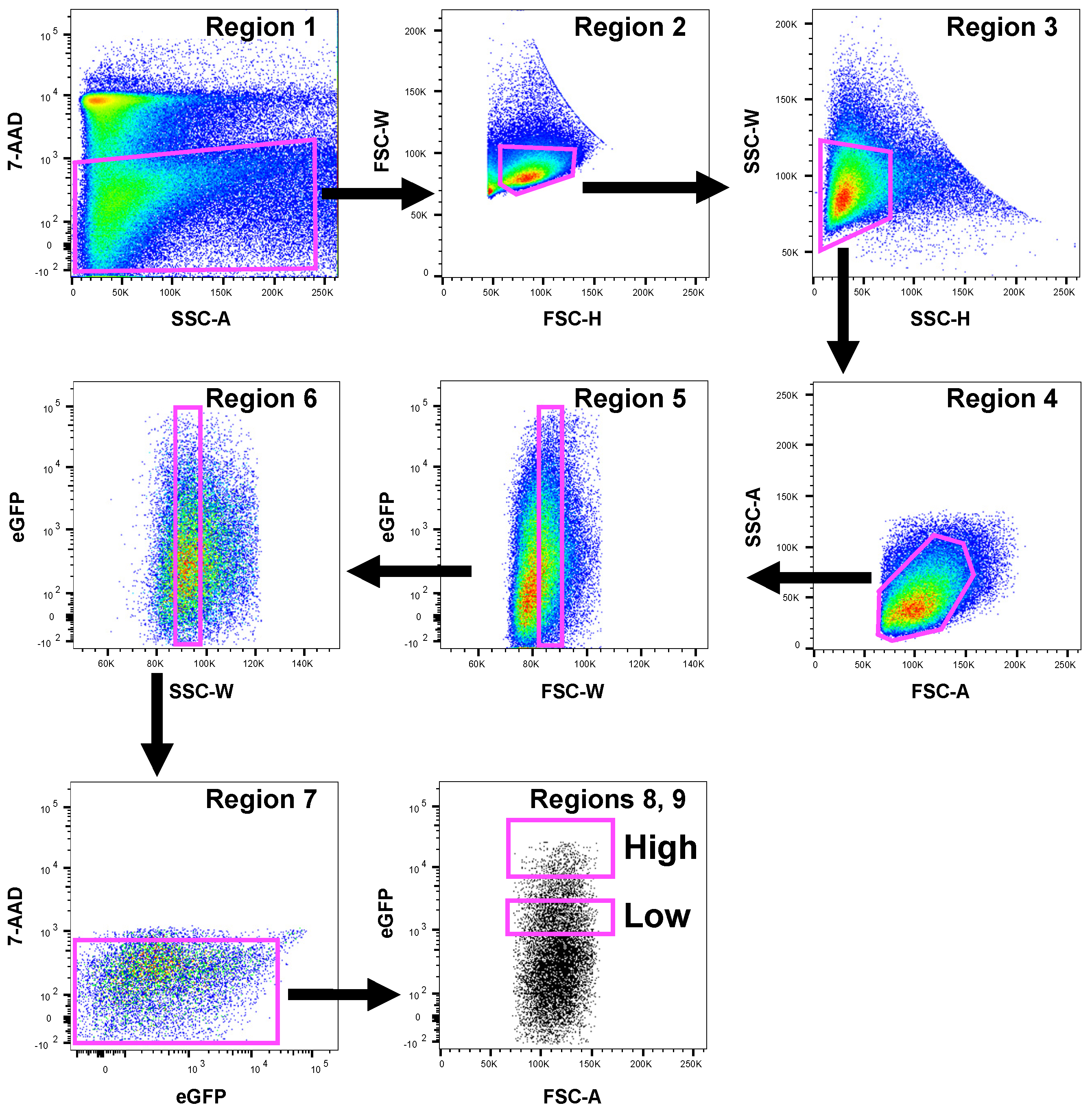
2.2. Sample Preparation for FACS Sorting Analysis
2.3. FACS Analysis and High-Speed Cell Sorting by Flow Cytometry
2.4. Cell Lysates, Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
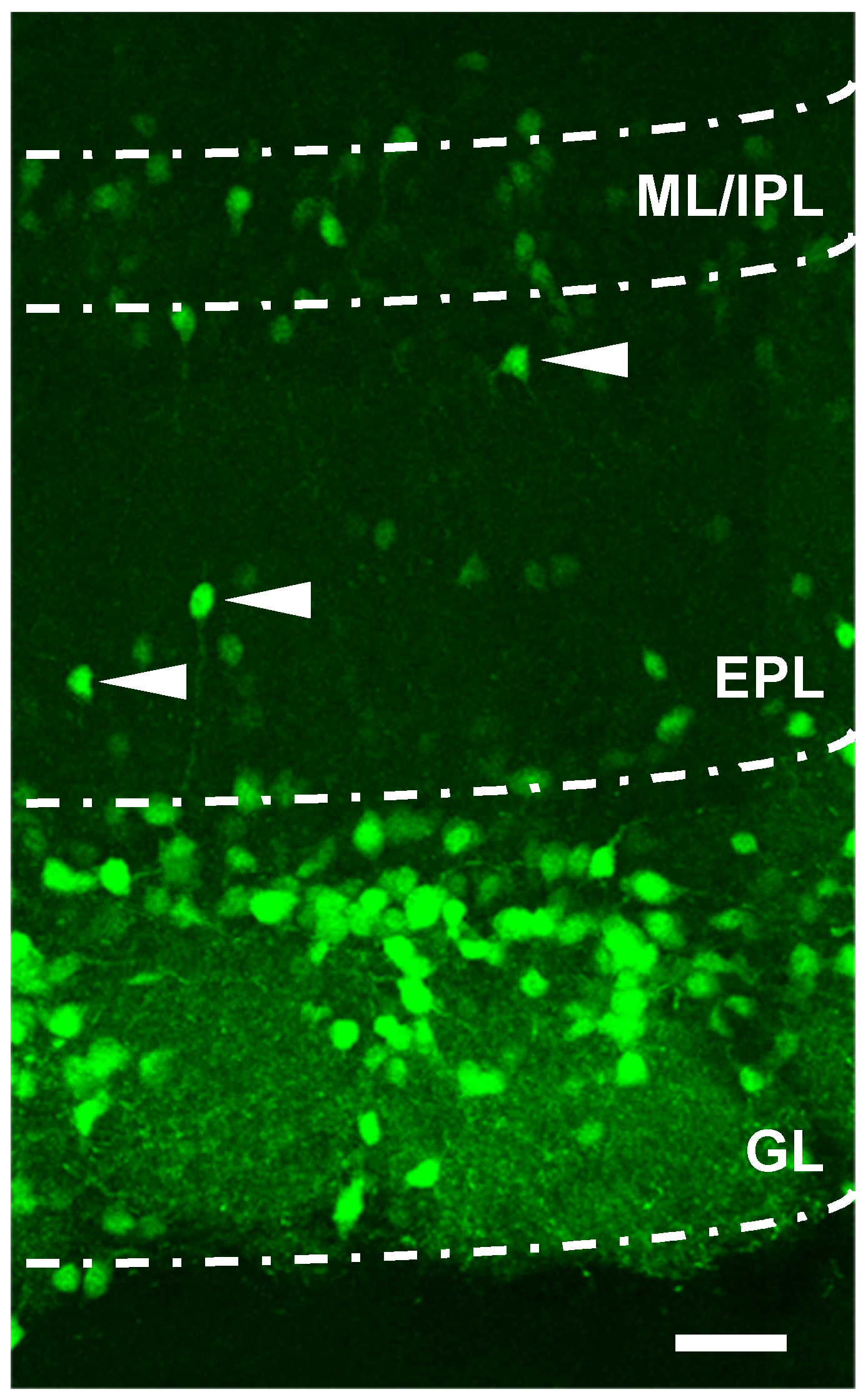
2.5. Confocal Microscopy
2.6. Statistical Analysis
3. Results
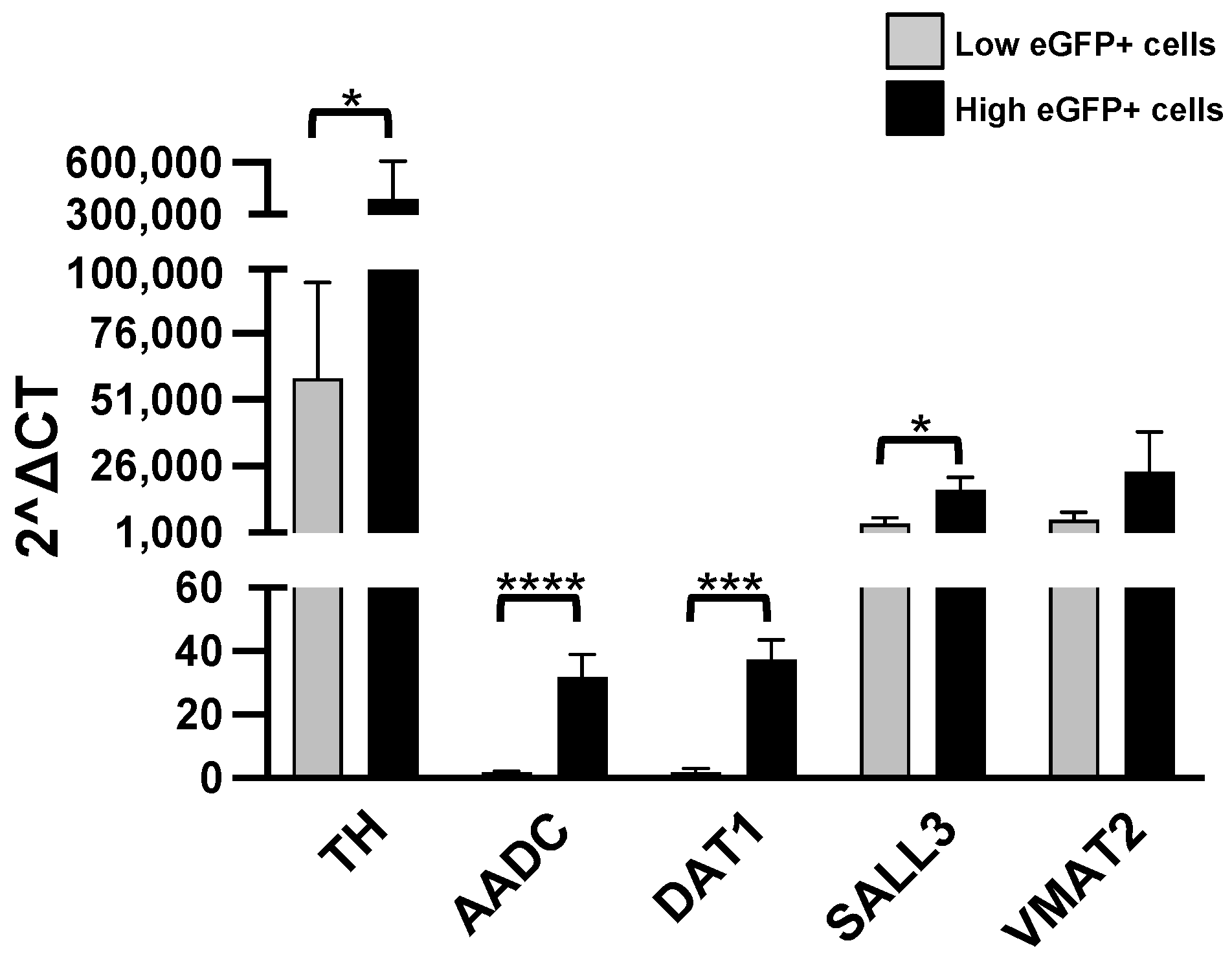
3.1. Analysis of Gene Expression of Different Population of Sorted TH-eGFP Cells from the OB
3.2. Expression of the Markers of Catecholaminergic Neurons
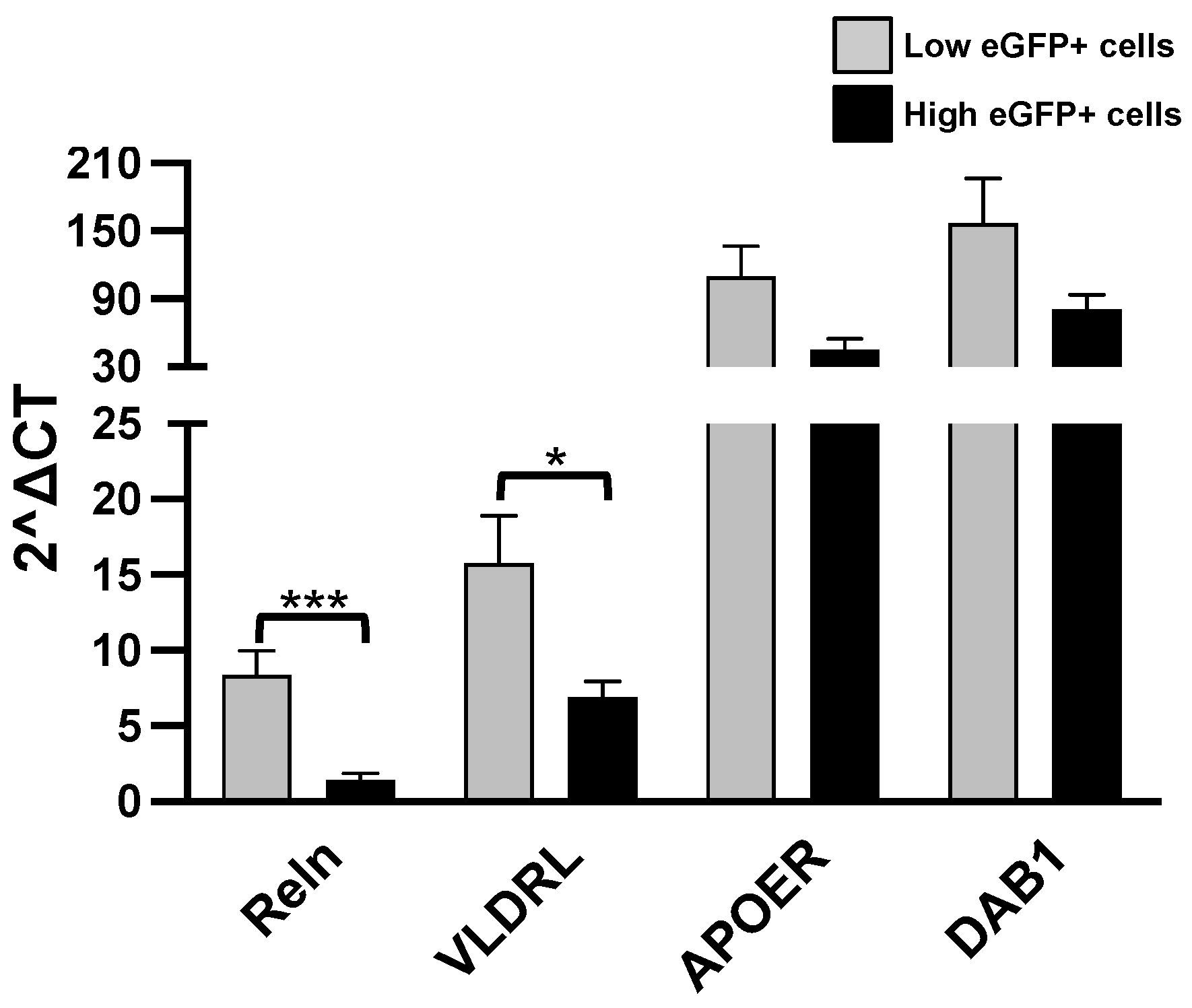
3.3. Expression of Genes Related to Reln Cascade
3.4. Detection of Different Regulation of Transcription Factor, Controlling the Development and the Survival of Dopaminergic Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halasz, N.; Johansson, O.; Hokfelt, T.; Ljungdahl, A.; Goldstein, M. Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J. Neurocytol. 1981, 10, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Pignatelli, A.; Kosaka, K. Heterogeneity of tyrosine hydroxylase expressing neurons in the main olfactory bulb of the mouse. Neurosci. Res. 2020, 157, 15–33. [Google Scholar] [CrossRef]
- Liberia, T.; Blasco-Ibanez, J.M.; Nacher, J.; Varea, E.; Zwafink, V.; Crespo, C. Characterization of a population of tyrosine hydroxylase-containing interneurons in the external plexiform layer of the rat olfactory bulb. Neuroscience 2012, 217, 140–153. [Google Scholar] [CrossRef]
- Capsoni, S.; Fogli Iseppe, A.; Casciano, F.; Pignatelli, A. Unraveling the Role of Dopaminergic and Calretinin Interneurons in the Olfactory Bulb. Front. Neural. Circuits 2021, 15, 718221. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Linster, C.; Cleland, T.A. Dopamine D(2) receptor activation modulates perceived odor intensity. Behav. Neurosci. 2006, 120, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Serguera, C.; Triaca, V.; Kelly-Barrett, J.; Banchaabouchi, M.A.; Minichiello, L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nat. Neurosci. 2008, 11, 949–956. [Google Scholar] [CrossRef]
- Baker, H.; Kawano, T.; Margolis, F.L.; Joh, T.H. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J. Neurosci. 1983, 3, 69–78. [Google Scholar] [CrossRef]
- Cave, J.W.; Akiba, Y.; Banerjee, K.; Bhosle, S.; Berlin, R.; Baker, H. Differential regulation of dopaminergic gene expression by Er81. J. Neurosci. 2010, 30, 4717–4724. [Google Scholar] [CrossRef]
- Parrish-Aungst, S.; Kiyokage, E.; Szabo, G.; Yanagawa, Y.; Shipley, M.T.; Puche, A.C. Sensory experience selectively regulates transmitter synthesis enzymes in interglomerular circuits. Brain Res. 2011, 1382, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Akiba, Y.; Baker, H.; Cave, J.W. Epigenetic control of neurotransmitter expression in olfactory bulb interneurons. Int. J. Dev. Neurosci. 2013, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Hsia, A.Y.; Vincent, J.D.; Lledo, P.M. Dopamine depresses synaptic inputs into the olfactory bulb. J. Neurophysiol. 1999, 82, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M.; Zhou, F.M.; Ciombor, K.J.; Aroniadou-Anderjaska, V.; Hayar, A.; Borrelli, E.; Zimmer, L.A.; Margolis, F.; Shipley, M.T. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J. Neurophysiol. 2001, 86, 2986–2997. [Google Scholar] [CrossRef]
- McGann, J.P. Presynaptic inhibition of olfactory sensory neurons: New mechanisms and potential functions. Chem. Senses 2013, 38, 459–474. [Google Scholar] [CrossRef]
- Liu, S.; Plachez, C.; Shao, Z.; Puche, A.; Shipley, M.T. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J. Neurosci. 2013, 33, 2916–2926. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Benedusi, M.; Ackman, J.; Loturco, J.J.; Belluzzi, O. Functional properties of adult-born juxtaglomerular cells in the mammalian olfactory bulb. Chem. Senses 2005, 30 (Suppl. S1), i119–i120. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Kobayashi, K.; Okano, H.; Belluzzi, O. Functional properties of dopaminergic neurones in the mouse olfactory bulb. J. Physiol. 2005, 564, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Belluzzi, O. Dopaminergic Neurones in the Main Olfactory Bulb: An Overview from an Electrophysiological Perspective. Front. Neuroanat. 2017, 11, 7. [Google Scholar] [CrossRef]
- Smith, R.L.; Baker, H.; Greer, C.A. Immunohistochemical analyses of the human olfactory bulb. J. Comp. Neurol. 1993, 333, 519–530. [Google Scholar] [CrossRef]
- Alizadeh, R.; Hassanzadeh, G.; Soleimani, M.; Joghataei, M.T.; Siavashi, V.; Khorgami, Z.; Hadjighassem, M. Gender and age related changes in number of dopaminergic neurons in adult human olfactory bulb. J. Chem. Neuroanat. 2015, 69, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Altman, J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969, 137, 433–457. [Google Scholar] [CrossRef]
- Betarbet, R.; Zigova, T.; Bakay, R.A.; Luskin, M.B. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int. J. Dev. Neurosci. 1996, 14, 921–930. [Google Scholar] [CrossRef]
- Baker, H.; Liu, N.; Chun, H.S.; Saino, S.; Berlin, R.; Volpe, B.; Son, J.H. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J. Neurosci. 2001, 21, 8505–8513. [Google Scholar] [CrossRef]
- Winner, B.; Cooper-Kuhn, C.M.; Aigner, R.; Winkler, J.; Kuhn, H.G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002, 16, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, A.; Lu, J.; Irving, R.; Feng, G.; Katz, L.C. In vivo imaging of juxtaglomerular neuron turnover in the mouse olfactory bulb. Proc. Natl. Acad. Sci. USA 2006, 103, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.E.; Goldman, J.E. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J. Neurosci. 2007, 27, 4297–4302. [Google Scholar] [CrossRef]
- Lazarini, F.; Gabellec, M.M.; Moigneu, C.; de Chaumont, F.; Olivo-Marin, J.C.; Lledo, P.M. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci. 2014, 34, 14430–14442. [Google Scholar] [CrossRef]
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Ramirez-Amaya, V.; Marrone, D.F.; Gage, F.H.; Worley, P.F.; Barnes, C.A. Integration of new neurons into functional neural networks. J. Neurosci. 2006, 26, 12237–12241. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Belluzzi, O. Neurogenesis in the Adult Olfactory Bulb. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2010; pp. 267–303. [Google Scholar]
- Cave, J.W.; Baker, H. Adult Neurogenesis in the Subventricular Zone and Migration to the Olfactory Bulb. In Handbook of Olfaction and Gustation; John Wiley & Sons: New York, NY, USA, 2015; pp. 183–208. [Google Scholar]
- Lledo, P.M.; Valley, M. Adult Olfactory Bulb Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018945. [Google Scholar] [CrossRef]
- Malvaut, S.; Saghatelyan, A. The Role of Adult-Born Neurons in the Constantly Changing Olfactory Bulb Network. Neural. Plast. 2016, 2016, 1614329. [Google Scholar] [CrossRef]
- Belluzzi, O.; Benedusi, M.; Ackman, J.; LoTurco, J.J. Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 2003, 23, 10411–10418. [Google Scholar] [CrossRef]
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 6, 507–518. [Google Scholar] [CrossRef]
- Yang, Z. Postnatal subventricular zone progenitors give rise not only to granular and periglomerular interneurons but also to interneurons in the external plexiform layer of the rat olfactory bulb. J. Comp. Neurol. 2008, 506, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, C.; Gheusi, G.; Vincent, J.D.; Lledo, P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002, 22, 2679–2689. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Mori, K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. USA 2005, 102, 9697–9702. [Google Scholar] [CrossRef] [PubMed]
- Bonzano, S.; Bovetti, S.; Fasolo, A.; Peretto, P.; De Marchis, S. Odour enrichment increases adult-born dopaminergic neurons in the mouse olfactory bulb. Eur. J. Neurosci. 2014, 40, 3450–3457. [Google Scholar] [CrossRef]
- Bonzano, S.; Bovetti, S.; Gendusa, C.; Peretto, P.; De Marchis, S. Adult Born Olfactory Bulb Dopaminergic Interneurons: Molecular Determinants and Experience-Dependent Plasticity. Front. Neurosci. 2016, 10, 189. [Google Scholar] [CrossRef]
- McLean, J.H.; Shipley, M.T. Postmitotic, postmigrational expression of tyrosine hydroxylase in olfactory bulb dopaminergic neurons. J. Neurosci. 1988, 8, 3658–3669. [Google Scholar] [CrossRef]
- De Marchis, S.; Bovetti, S.; Carletti, B.; Hsieh, Y.C.; Garzotto, D.; Peretto, P.; Fasolo, A.; Puche, A.C.; Rossi, F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: Implication for intrinsic properties of the subventricular zone progenitor population. J. Neurosci. 2007, 27, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Sudhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef]
- Pignatelli, A.; Ackman, J.B.; Vigetti, D.; Beltrami, A.P.; Zucchini, S.; Belluzzi, O. A potential reservoir of immature dopaminergic replacement neurons in the adult mammalian olfactory bulb. Pflug. Arch. 2009, 457, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, K.S.; Blakemore, L.J.; Bertram, R.; Trombley, P.Q. Spiking and Membrane Properties of Rat Olfactory Bulb Dopamine Neurons. Front. Cell Neurosci. 2020, 14, 60. [Google Scholar] [CrossRef]
- Saino-Saito, S.; Sasaki, H.; Volpe, B.T.; Kobayashi, K.; Berlin, R.; Baker, H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J. Comp. Neurol. 2004, 479, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Farbman, A.I. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience 1993, 52, 115–134. [Google Scholar] [CrossRef]
- Brunjes, P.C.; Smith-Crafts, L.K.; McCarty, R. Unilateral odor deprivation: Effects on the development of olfactory bulb catecholamines and behavior. Brain Res. 1985, 354, 1–6. [Google Scholar] [CrossRef]
- Cho, J.Y.; Min, N.; Franzen, L.; Baker, H. Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J. Comp. Neurol. 1996, 369, 264–276. [Google Scholar] [CrossRef]
- Stone, D.M.; Grillo, M.; Margolis, F.L.; Joh, T.H.; Baker, H. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J. Comp. Neurol. 1991, 311, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Wood, J.G. Functional consequences of unilateral olfactory deprivation: Time-course and age sensitivity. Neuroscience 1992, 49, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Saino-Saito, S. Visualization of spatiotemporal differentiation of dopaminergic interneurons in adult mouse olfactory bulb using transgenic mice. Anat Sci Int 2008, 83, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Kiyokage, E.; Pan, Y.Z.; Shao, Z.; Kobayashi, K.; Szabo, G.; Yanagawa, Y.; Obata, K.; Okano, H.; Toida, K.; Puche, A.C.; et al. Molecular identity of periglomerular and short axon cells. J. Neurosci. 2010, 30, 1185–1196. [Google Scholar] [CrossRef]
- Pignatelli, A.; Gambardella, C.; Borin, M.; Fogli Iseppe, A.; Belluzzi, O. Pacemaker currents in dopaminergic neurons of the mice olfactory bulb. Electrophysiol. –Plants Heart 2012, 25–50. [Google Scholar]
- Huisman, E.; Uylings, H.B.; Hoogland, P.V. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov. Disord. 2004, 19, 687–692. [Google Scholar] [CrossRef]
- Cave, J.W.; Wang, M.; Baker, H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front. Neurosci. 2014, 8, 16. [Google Scholar] [CrossRef]
- Alizadeh, R.; Ramezanpour, F.; Mohammadi, A.; Eftekharzadeh, M.; Simorgh, S.; Kazemiha, M.; Moradi, F. Differentiation of human olfactory system-derived stem cells into dopaminergic neuron-like cells: A comparison between olfactory bulb and mucosa as two sources of stem cells. J. Cell Biochem. 2019, 120, 19712–19720. [Google Scholar] [CrossRef]
- Sawamoto, K.; Nakao, N.; Kobayashi, K.; Matsushita, N.; Takahashi, H.; Kakishita, K.; Yamamoto, A.; Yoshizaki, T.; Terashima, T.; Murakami, F.; et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 6423–6428. [Google Scholar] [CrossRef]
- Matsushita, N.; Okada, H.; Yasoshima, Y.; Takahashi, K.; Kiuchi, K.; Kobayashi, K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J. Neurochem. 2002, 82, 295–304. [Google Scholar] [CrossRef]
- Gustincich, S.; Feigenspan, A.; Wu, D.K.; Koopman, L.J.; Raviola, E. Control of dopamine release in the retina: A transgenic approach to neural networks. Neuron 1997, 18, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Belluzzi, O. Cholinergic modulation of dopaminergic neurons in the mouse olfactory bulb. Chem. Senses 2008, 33, 331–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Faini, G.; Del Bene, F.; Albadri, S. Reelin functions beyond neuronal migration: From synaptogenesis to network activity modulation. Curr. Opin. Neurobiol. 2021, 66, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Miyata, T.; Nakajima, K.; Yagyu, K.; Seike, M.; Ikenaka, K.; Yamamoto, H.; Mikoshiba, K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 1995, 14, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chai, X.; Forster, E.; Frotscher, M. Reelin is a positional signal for the lamination of dentate granule cells. Development 2004, 131, 5117–5125. [Google Scholar] [CrossRef]
- Hack, I.; Bancila, M.; Loulier, K.; Carroll, P.; Cremer, H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat. Neurosci. 2002, 5, 939–945. [Google Scholar] [CrossRef]
- Hartfuss, E.; Forster, E.; Bock, H.H.; Hack, M.A.; Leprince, P.; Luque, J.M.; Herz, J.; Frotscher, M.; Gotz, M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development 2003, 130, 4597–4609. [Google Scholar] [CrossRef]
- Kim, H.M.; Qu, T.; Kriho, V.; Lacor, P.; Smalheiser, N.; Pappas, G.D.; Guidotti, A.; Costa, E.; Sugaya, K. Reelin function in neural stem cell biology. Proc. Natl. Acad. Sci. USA 2002, 99, 4020–4025. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Homayouni, R.; Keshvara, L.; Rice, D.S.; Sheldon, M.; Curran, T. Reelin is a ligand for lipoprotein receptors. Neuron 1999, 24, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hiesberger, T.; Trommsdorff, M.; Howell, B.W.; Goffinet, A.; Mumby, M.C.; Cooper, J.A.; Herz, J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 1999, 24, 481–489. [Google Scholar] [CrossRef]
- Howell, B.W.; Hawkes, R.; Soriano, P.; Cooper, J.A. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 1997, 389, 733–737. [Google Scholar] [CrossRef]
- Trommsdorff, M.; Gotthardt, M.; Hiesberger, T.; Shelton, J.; Stockinger, W.; Nimpf, J.; Hammer, R.E.; Richardson, J.A.; Herz, J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 1999, 97, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Sheldon, M.; D’Arcangelo, G.; Nakajima, K.; Goldowitz, D.; Curran, T. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development 1998, 125, 3719–3729. [Google Scholar] [CrossRef]
- Martin-Lopez, E.; Blanchart, A.; De Carlos, J.A.; Lopez-Mascaraque, L. Dab1 (Disable homolog-1) reelin adaptor protein is overexpressed in the olfactory bulb at early postnatal stages. PLoS ONE 2011, 6, e26673. [Google Scholar] [CrossRef]
- Perez-Garcia, C.G.; Tissir, F.; Goffinet, A.M.; Meyer, G. Reelin receptors in developing laminated brain structures of mouse and human. Eur. J. Neurosci. 2004, 20, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, S.; Hack, I.; Zucker, B.; Brunne, B.; Junghans, D. Reelin together with ApoER2 regulates interneuron migration in the olfactory bulb. PLoS ONE 2012, 7, e50646. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.M.; Strasser, V.; Andrade, N.; Duit, S.; Hofbauer, R.; Schneider, W.J.; Nimpf, J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008, 27, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto-Torii, K.; Torii, M.; Sarkisian, M.R.; Bartley, C.M.; Shen, J.; Radtke, F.; Gridley, T.; Sestan, N.; Rakic, P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 2008, 60, 273–284. [Google Scholar] [CrossRef]
- Keilani, S.; Sugaya, K. Reelin induces a radial glial phenotype in human neural progenitor cells by activation of Notch-1. BMC Dev. Biol. 2008, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, R.; Ohtsuka, T.; Shimojo, H.; Imayoshi, I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 2008, 11, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Givogri, M.I.; de Planell, M.; Galbiati, F.; Superchi, D.; Gritti, A.; Vescovi, A.; de Vellis, J.; Bongarzone, E.R. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 2006, 28, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Stump, G.; Durrer, A.; Klein, A.L.; Lutolf, S.; Suter, U.; Taylor, V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech. Dev. 2002, 114, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kele, J.; Simplicio, N.; Ferri, A.L.; Mira, H.; Guillemot, F.; Arenas, E.; Ang, S.L. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 2006, 133, 495–505. [Google Scholar] [CrossRef]
- Roybon, L.; Deierborg, T.; Brundin, P.; Li, J.Y. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur. J. Neurosci. 2009, 29, 232–243. [Google Scholar] [CrossRef]
- Brill, M.S.; Ninkovic, J.; Winpenny, E.; Hodge, R.D.; Ozen, I.; Yang, R.; Lepier, A.; Gascon, S.; Erdelyi, F.; Szabo, G.; et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009, 12, 1524–1533. [Google Scholar] [CrossRef]
- Winpenny, E.; Lebel-Potter, M.; Fernandez, M.E.; Brill, M.S.; Gotz, M.; Guillemot, F.; Raineteau, O. Sequential generation of olfactory bulb glutamatergic neurons by Neurog2-expressing precursor cells. Neural Dev. 2011, 6, 12. [Google Scholar] [CrossRef]
- Shaker, T.; Dennis, D.; Kurrasch, D.M.; Schuurmans, C. Neurog1 and Neurog2 coordinately regulate development of the olfactory system. Neural Dev. 2012, 7, 28. [Google Scholar] [CrossRef]
- Kimura, N.; Nakashima, K.; Ueno, M.; Kiyama, H.; Taga, T. A novel mammalian T-box-containing gene, Tbr2, expressed in mouse developing brain. Brain Res. Dev. Brain Res. 1999, 115, 183–193. [Google Scholar] [CrossRef]
- Simon, H.H.; Saueressig, H.; Wurst, W.; Goulding, M.D.; O’Leary, D.D. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 2001, 21, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lee, Y.M.; Guy, V.C.; Freed, C.R. Embryonic stem cells with GFP knocked into the dopamine transporter yield purified dopamine neurons in vitro and from knock-in mice. Stem Cells 2009, 27, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, A.; Gruss, P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J. Neurosci. 1994, 14, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Dellovade, T.L.; Pfaff, D.W.; Schwanzel-Fukuda, M. Olfactory bulb development is altered in small-eye (Sey) mice. J. Comp. Neurol. 1998, 402, 402–418. [Google Scholar] [CrossRef]
- Kohwi, M.; Osumi, N.; Rubenstein, J.L.; Alvarez-Buylla, A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 2005, 25, 6997–7003. [Google Scholar] [CrossRef]
- Fujita, M.; Shimada, S.; Nishimura, T.; Uhl, G.R.; Tohyama, M. Ontogeny of dopamine transporter mRNA expression in the rat brain. Brain Res. Mol. Brain Res. 1993, 19, 222–226. [Google Scholar] [CrossRef]
- Weihe, E.; Depboylu, C.; Schutz, B.; Schafer, M.K.; Eiden, L.E. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell. Mol. Neurobiol. 2006, 26, 659–678. [Google Scholar] [CrossRef]
- Diaz-Guerra, E.; Pignatelli, J.; Nieto-Estevez, V.; Vicario-Abejon, C. Transcriptional regulation of olfactory bulb neurogenesis. Anat. Rec. 2013, 296, 1364–1382. [Google Scholar] [CrossRef]
- Heng, X.; Breer, H.; Zhang, X.; Tang, Y.; Li, J.; Zhang, S.; Le, W. Sall3 correlates with the expression of TH in mouse olfactory bulb. J. Mol. Neurosci. 2012, 46, 293–302. [Google Scholar] [CrossRef]
- Harrison, S.J.; Parrish, M.; Monaghan, A.P. Sall3 is required for the terminal maturation of olfactory glomerular interneurons. J. Comp. Neurol. 2008, 507, 1780–1794. [Google Scholar] [CrossRef]
- Koizumi, H.; Higginbotham, H.; Poon, T.; Tanaka, T.; Brinkman, B.C.; Gleeson, J.G. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat. Neurosci. 2006, 9, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ocbina, P.J.; Dizon, M.L.; Shin, L.; Szele, F.G. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol. Cell Neurosci. 2006, 33, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef]
- Cockerham, R.; Liu, S.; Cachope, R.; Kiyokage, E.; Cheer, J.F.; Shipley, M.T.; Puche, A.C. Subsecond Regulation of Synaptically Released Dopamine by COMT in the Olfactory Bulb. J. Neurosci. 2016, 36, 7779–7785. [Google Scholar] [CrossRef]
- Schott, B.H.; Frischknecht, R.; Debska-Vielhaber, G.; John, N.; Behnisch, G.; Duzel, E.; Gundelfinger, E.D.; Seidenbecher, C.I. Membrane-Bound Catechol-O-Methyl Transferase in Cortical Neurons and Glial Cells is Intracellularly Oriented. Front. Psychiatry 2010, 1, 142. [Google Scholar] [CrossRef] [PubMed]
| Gene | Average ± of Gene Expression of L-GFP Group, [N] | Average ± of Gene Expression of H-GFP Group, [N] | p-Value |
|---|---|---|---|
| **** AADC (aromatic L-amino acid decarboxylase) | 1.87 ± 0.36, [14] | 31.95 ± 6.94, [23] | <0.0001 |
| ApoEr2 apolipopritein E receptor 2 | 109.90 ± 26.48, [19] | 45.26 ± 9.27, [18] | 0.13 |
| ARX aristaless-related homeobox | 289.80 ± 123.40, [9] | 131.10 ± 44.53, [9] | 0.6 |
| COMT catechol-O-methyltransferase | 1.70 ± 0.29, [3] | 1.99 ± 0.51, [3] | 0.66 |
| CXCL12 C-X-C motif chemokine ligand 12 | 0.09 ± 0.08, [8] | 0.007 ± 0.002, [8] | 0.59 |
| DAB1 DAB adaptor protein 1 | 156.80 ± 39.59, [17] | 80.62 ± 12.95, [20] | 0.22 |
| *** DAT dopamine membrane transporter | 1.93 ± 1.09, [4] | 37.48 ± 6.06 [8] | 0.0005 |
| DCX doublecortin | 26.96 ± 11.76, [11] | 60.91 ± 22.82, [15] | 0.33 |
| DLX1 distal-less homeobox 1 | 23.25 ± 5.12, [22] | 17.66 ± 4.80, [19] | 0.57 |
| DLX2 distal-less homeobox 1 | 23.79 ± 5.63, [13] | 13.14 ± 3.01, [13] | 0.10 |
| DLX5 distal-less homeobox 5 | 40.30 ± 10.75, [13] | 18.73 ± 3.85, [13] | 0.26 |
| EGR1 early growth response 1 | 63.11 ± 48.14, [13] | 144.6 ± 98.11, [14] | 0.62 |
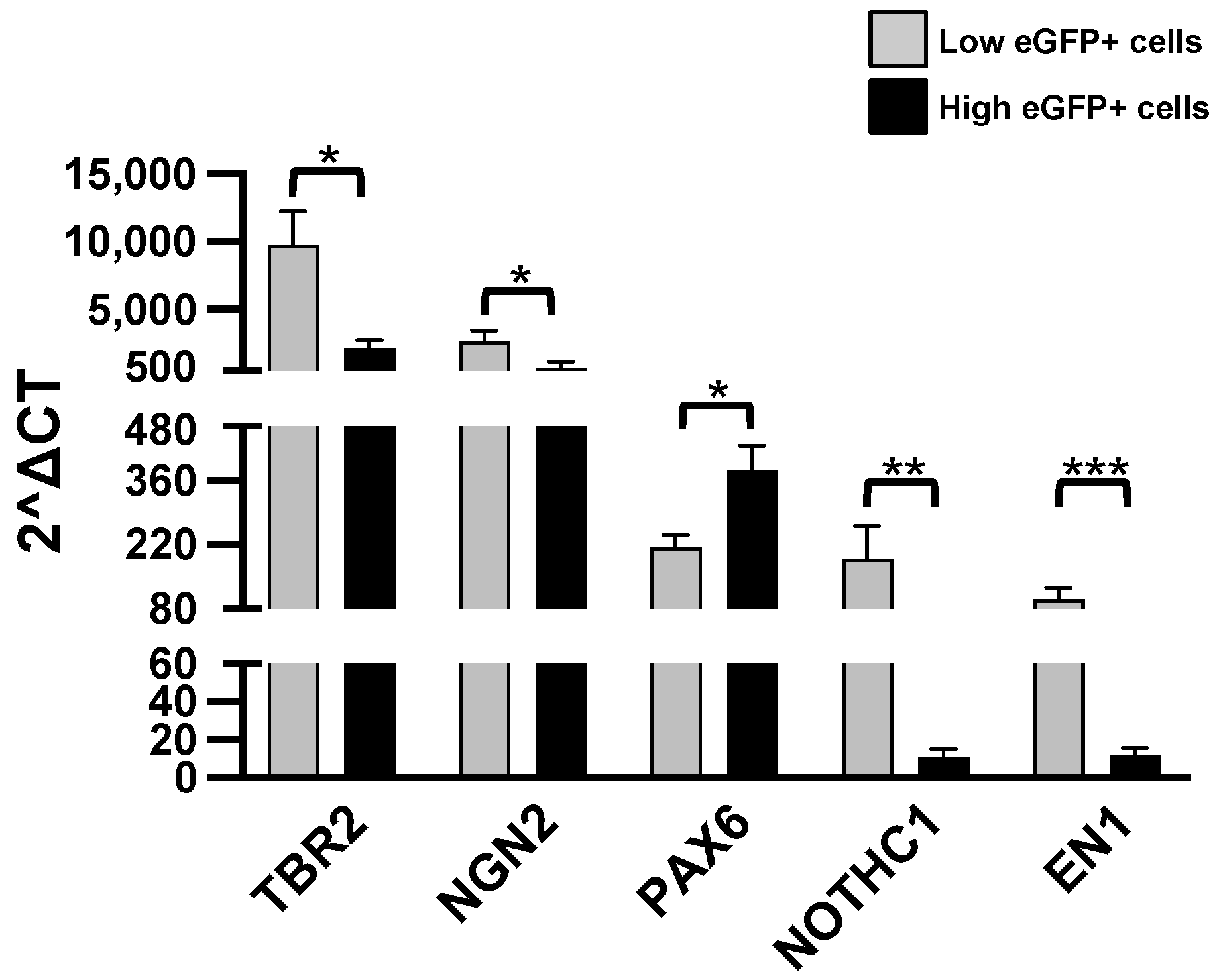
| *** EN1 engrailed 1 | 99.94 ± 25.34, [13] | 12.04 ± 3.69, [12] | 0.0003 |
| FEZF1 FEZ family zinc finger 1 | 24.53 ± 13.74, [4] | 10.52 ± 4.39, [7] | 0.25 |
| GSH2 glutathione synthetase 2 | 26.99 ± 7.22, [19] | 15.67 ± 4.02, [14] | 0.70 |
| HES1 hairy and enhancer of split 1 | 24.34 ± 7.18, [11] | 14.49 ± 3.46, [12] | 0.22 |
| LMX1A LIM homeobox transcription factor 1 alpha | 32.30 ± 14.26, [12] | 177.2 ± 80.04, [12] | 0.11 |
| MASH1-ASCL1 achaete-scute family bHLH transcription factor 1 | 5.91 ± 1.03, [21] | 11.67 ± 3.37, [20] | 0.27 |
| MEIS2 Homeobox protein Meis2 | 134.8 ± 30.79, [11] | 350.30 ± 103.90, [14] | 0.34 |
| MYST-4 K(lysine) acetyltransferase 6B | 68.78 ± 18.46, [15] | 30.03 ± 5.69, [15] | 0.40 |
| NEUROD1 neuronal differentiation 1 | 1328.00 ± 860.6, [10] | 2650 ± 1205, [13] | 0.48 |
| * NGN2 neurogenin 2 | 2668 ± 824.70, [11] | 717.80 ± 452.8 [10] | 0.036 |
| ** NOTCH1 notch receptor 1 | 189.6 ± 71.15, [16] | 10.79 ± 4.41, [14] | 0.0079 |
| * NURR1 Nuclear Receptor Subfamily 4 Group A Member 2 | 16.78 ± 2.72, [8] | 34.68 ± 6.56, [9] | 0.02 |
| * PAX6 paired box gene 6 | 214.6 ± 26.74, [7] | 384.6 ± 52.59, [7] | 0.01 |
| PTX3 pentraxin-related gene | 14.80 ± 3.49, [11] | 16.39 ± 6.47, [13] | 0.46 |
| *** Reln Reelin | 8.37 ± 1.59, [16] | 1.42 ± 0.43, [9] | 0.0006 |
| * SALL3 spalt-like transcription factor 3 | 4263.00 ± 2151.00, [11] | 17,117 ± 4625, [16] | 0.0109 |
| SHH sonic hedgehog | 48.95 ± 14.28, [11] | 100.9 ± 28.88, [17] | 0.46 |
| SLIT2 slit guidance ligand 2 | 7.60 ± 2.09, [19] | 6.48 ± 1.29, [10] | 0.78 |
| TBR1 T-box brain transcription factor 1 | 1325 ± 487.8, [11] | 1356 ± 679.5, [8] | 0.27 |
| * TBR2 T-box brain transcription factor 1 | 9768 ± 2452, [15] | 2200 ± 563.6, [13] | 0.04 |
| * TH tyrosine hydroxylase | 59,131 ± 36,008, [21] | 392,278 ± 217,515, [22] | 0.0268 |
| TNR tenascin-R | 58.34 ± 16.92, [15] | 20.32 ± 5.98, [11] | 0.20 |
| * VLDR very-low-density lipoprotein receptor | 15.75 ± 3.16, [17] | 6.91 ± 1.00, [18] | 0.032 |
| VMAT2 vesicular dopamine transporter | 5915 ± 2789, [6] | 24,002 ± 14,880, [5] | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casciano, F.; Bianchi, N.; Borin, M.; Vellani, V.; Secchiero, P.; Bergamini, C.M.; Capsoni, S.; Pignatelli, A. Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb. Biology 2023, 12, 367. https://doi.org/10.3390/biology12030367
Casciano F, Bianchi N, Borin M, Vellani V, Secchiero P, Bergamini CM, Capsoni S, Pignatelli A. Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb. Biology. 2023; 12(3):367. https://doi.org/10.3390/biology12030367
Chicago/Turabian StyleCasciano, Fabio, Nicoletta Bianchi, Mirta Borin, Vittorio Vellani, Paola Secchiero, Carlo M. Bergamini, Simona Capsoni, and Angela Pignatelli. 2023. "Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb" Biology 12, no. 3: 367. https://doi.org/10.3390/biology12030367
APA StyleCasciano, F., Bianchi, N., Borin, M., Vellani, V., Secchiero, P., Bergamini, C. M., Capsoni, S., & Pignatelli, A. (2023). Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb. Biology, 12(3), 367. https://doi.org/10.3390/biology12030367









