miR-34b/c-5p/CXCL10 Axis Induced by RSV Infection Mediates a Mechanism of Airway Hyperresponsive Diseases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microarray Data
2.2. Differential miRNAs (DEmiRs) and Genes (DEGs) Screening
2.3. Prediction of miR-34b/c-5 Target Genes from DEGs
2.4. Analysis of GO and KEGG Pathway
2.5. Construction of PPI Network and Identification of Hub Genes
2.6. Cell Culture and Viral Preparation
2.7. Transfection of miRNA Mimics and RSV Infection
2.8. Dual-Luciferase Reporter Assay
2.9. Animal Models of RSV-Induced AHR
2.10. Real-Time RT-PCR
2.11. Measurement of Airway Responsiveness to Methacholine
2.12. Hematoxylin-Eosin Staining
2.13. Immunofluorescence
2.14. ELISA
2.15. PMA Induced THP-1 Monocytes to Differentiate into Macrophages
2.16. Chemotaxis Assay
2.17. Statistical Analysis
3. Results
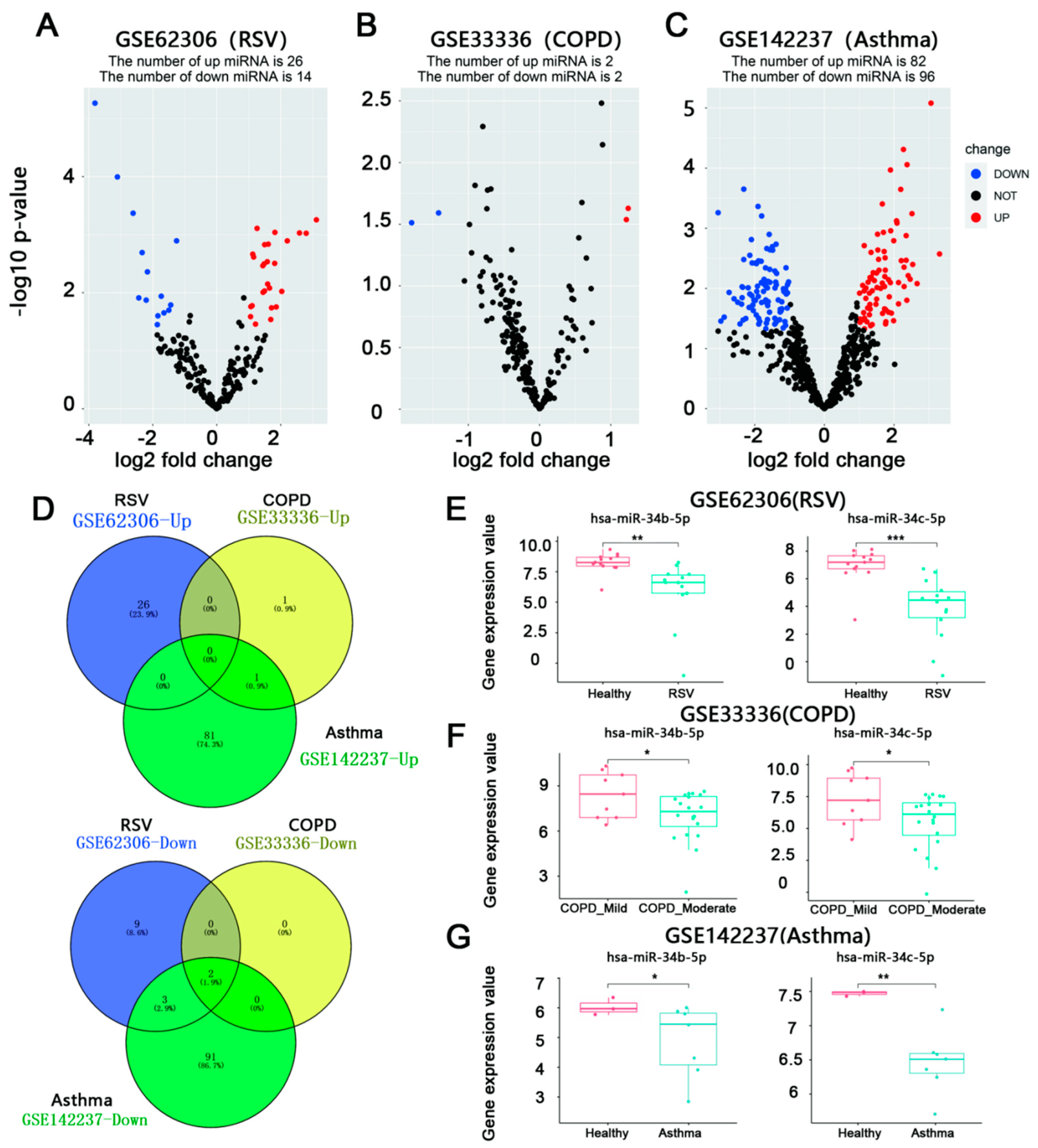
3.1. miR-34b/c-5p Expression Was Downregulated in Patients with RSV Infection Asthma, and COPD
3.2. CXCL10 Is the Most Important Downstream Gene of miR-34b/c-5p
3.3. miR-34b/b-5 P Downregulation Was Accompanied by CXCL10 Activation in Mouse RSV-Infected Models
3.4. CXCL10 Was Consistently Overexpressed in Lung Tissue, While CD14 Was Consistently Over-Expressed in Blood Samples from RSV Infection, Asthma, and COPD
3.5. miR-34b/c-5p Inhibits CXCL10 Expression through Direct Interaction with CXCL10 mRNA
3.6. miR-34b/c-5p Inhibited Macrophages Chemotaxis to RSV-Infected BEAS-2B Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, H.; Nickle, D.; Carter, L. Rhinovirus and asthma: Challenges and opportunities. Rev. Med. Virol. 2021, 31, e2193. [Google Scholar] [CrossRef] [PubMed]
- Priante, E.; Cavicchiolo, M.E.; Baraldi, E. RSV infection and respiratory sequelae. Minerva. Pediatr. 2018, 70, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Niikura, M.; Yang, C.W.T.; Sin, D.D. COVID-19 and COPD. Eur. Respir. J. 2020, 56, 2002108. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; Gustafsson, P.M.; Bjarnason, R.; Lundberg, F.; Schmidt, S.; Sigurbergsson, F.; Kjellman, B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir Crit. Care Med. 2005, 171, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bajinka, O.; Simbilyabo, L.; Tan, Y.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2022, 48, 257–269. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Qiu, K.; Bajinka, O.; Wang, L.; Qin, L.; Tan, Y. RSV Promotes Epithelial Neuroendocrine Phenotype Differentiation through NODAL Signaling Pathway. Biomed. Res. Int. 2021, 2021, 9956078. [Google Scholar] [CrossRef]
- Qin, L.; Qiu, K.; Hu, C.; Wang, L.; Wu, G.; Tan, Y. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J. Med. Microbiol. 2019, 68, 649–656. [Google Scholar] [CrossRef]
- Manti, S.; Piedimonte, G. An overview on the RSV-mediated mechanisms in the onset of non-allergic asthma. Front. Pediatr. 2022, 10, 998296. [Google Scholar] [CrossRef]
- Shi, T.; Li, N.; He, Y.; Feng, J.; Mei, Z.; Du, Y.; Jie, Z. Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb. Pathog. 2021, 156, 104867. [Google Scholar] [CrossRef]
- Glaser, L.; Coulter, P.J.; Shields, M.; Touzelet, O.; Power, U.F.; Broadbent, L. Airway Epithelial Derived Cytokines and Chemokines and Their Role in the Immune Response to Respiratory Syncytial Virus Infection. Pathogens 2019, 8, 106. [Google Scholar] [CrossRef]
- Nuriev, R.; Johansson, C. Chemokine regulation of inflammation during respiratory syncytial virus infection. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Taka, S.; Tzani-Tzanopoulou, P.; Wanstall, H.; Papadopoulos, N.G. MicroRNAs in Asthma and Respiratory Infections: Identifying Common Pathways. Allergy Asthma Immunol. Res. 2020, 12, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zeng, D.; Zheng, J.; Zhao, D. MicroRNAs: Mediators and Therapeutic Targets to Airway Hyper Reactivity After Respiratory Syncytial Virus Infection. Front. Microbiol. 2018, 9, 2177. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets–update. Nucleic. Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Savarimuthu Francis, S.M.; Davidson, M.R.; Tan, M.E.; Wright, C.M.; Clarke, B.E.; Duhig, E.E.; Bowman, R.V.; Hayward, N.K.; Fong, K.M.; Yang, I.A. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. BMC Genomics 2014, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, Y.; Liang, Y.; Wu, W.; Chang, C.; Chen, D.; Chen, S.; Gao, J.; Chen, G.; Yi, L.; et al. Epithelial miR-206 targets CD39/extracellular ATP to upregulate airway IL-25 and TSLP in type 2-high asthma. JCI Insight 2021, 6, e148103. [Google Scholar]
- Yu, J.; Peterson, D.R.; Baran, A.M.; Bhattacharya, S.; Wylie, T.N.; Falsey, A.R.; Mariani, T.J.; Storch, G.A. Host Gene Expression in Nose and Blood for the Diagnosis of Viral Respiratory Infection. J. Infect. Dis. 2019, 219, 1151–1161. [Google Scholar] [CrossRef]
- Inchley, C.S.; Sonerud, T.; Fjærli, H.O.; Nakstad, B. Nasal mucosal microRNA expression in children with respiratory syncytial virus infection. BMC Infect. Dis. 2015, 15, 150. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic. Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Tang, Z.; Qi, M.; Liu, D.; Bajinka, O.; Tan, Y. Dispersion and utilization of lipid droplets mediates respiratory syncytial virus-induced airway hy-perresponsiveness. Pediatr. Allergy Immunol. 2022, 33, e13651. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Y.; Xu, Z.; Zhang, Z.; Zheng, Y.; Qi, F. Identification of prognostic genes in adrenocortical carcinoma microenvironment based on bioinformatic methods. Cancer Med. 2020, 9, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Z.; Pan, L.; Zhang, Y. MicroRNA-34/449 family and viral infections. Virus Res. 2019, 260, 1–6. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, S.; Sun, Y.; Li, S.; Ning, Y.; Dong, Y.; Shang, Y.; Bai, C. MicroRNA-34/449 targets IGFBP-3 and attenuates airway remodeling by suppressing Nur77-mediated autophagy. Cell Death Dis. 2017, 8, e2998. [Google Scholar] [CrossRef]
- Daugaard, I.; Knudsen, A.; Kjeldsen, T.E.; Hager, H.; Hansen, L.L. The association between miR-34 dysregulation and distant metastases formation in lung adenocarcinoma. Exp. Mol. Pathol. 2017, 102, 484–491. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, W.K.; Lee, E.B.; Son, J.W.; Kim, D.S.; Park, J.Y. Combined Effect of Metastasis-Related MicroRNA, miR-34 and miR-124 Family, Methylation on Prognosis of Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, e13–e20. [Google Scholar] [CrossRef]
- Solberg, O.D.; Ostrin, E.J.; Love, M.I.; Peng, J.C.; Bhakta, N.R.; Hou, L.; Nguyen, C.; Solon, M.; Nguyen, C.; Barczak, A.J.; et al. Airway epithelial miRNA expression is altered in asthma. Am. J. Respir. Crit. Care Med. 2012, 186, 965–974. [Google Scholar] [CrossRef]
- Gao, H.X.; Su, Y.; Zhang, A.L.; Xu, J.W.; Fu, Q.; Yan, L. MiR-34c-5p plays a protective role in chronic obstructive pulmonary disease via targeting CCL22. Exp. Lung Res. 2019, 45, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Du, X.; Yang, Y.; Yuan, L.; Yang, M.; Qin, L.; Wang, L.; Zhou, K.; Xiang, Y.; Qu, X.; et al. miRNA-34b/c regulates mucus secretion in RSV-infected airway epithelial cells by targeting FGFR1. J. Cell Mol. Med. 2021, 25, 10565–10574. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, Y.; Xiao, G.; Yang, M.; Yuan, L.; Qin, L.; He, R.; Wang, L.; Wu, M.; Wu, S.; et al. Respiratory syncytial virus infection-induced mucus secretion by down-regulation of miR-34b/c-5p expression in airway epithelial cells. J. Cell Mol. Med. 2020, 24, 12694–12705. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Ammit, A.J.; Kaur, D.; Black, J.L.; Wardlaw, A.J.; Hughes, J.M.; Bradding, P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. Crit. Care Med. 2005, 171, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Puthothu, B.; Forster, J.; Heinzmann, A.; Krueger, M. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Dis. Markers 2006, 22, 303–308. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Tang, Z.; Bajinka, O.; Dai, P.; Wu, G.; Qin, L.; Tan, Y. miR-34b/c-5p/CXCL10 Axis Induced by RSV Infection Mediates a Mechanism of Airway Hyperresponsive Diseases. Biology 2023, 12, 317. https://doi.org/10.3390/biology12020317
Liu D, Tang Z, Bajinka O, Dai P, Wu G, Qin L, Tan Y. miR-34b/c-5p/CXCL10 Axis Induced by RSV Infection Mediates a Mechanism of Airway Hyperresponsive Diseases. Biology. 2023; 12(2):317. https://doi.org/10.3390/biology12020317
Chicago/Turabian StyleLiu, Dan, Zhongxiang Tang, Ousman Bajinka, Pei Dai, Guojun Wu, Ling Qin, and Yurong Tan. 2023. "miR-34b/c-5p/CXCL10 Axis Induced by RSV Infection Mediates a Mechanism of Airway Hyperresponsive Diseases" Biology 12, no. 2: 317. https://doi.org/10.3390/biology12020317
APA StyleLiu, D., Tang, Z., Bajinka, O., Dai, P., Wu, G., Qin, L., & Tan, Y. (2023). miR-34b/c-5p/CXCL10 Axis Induced by RSV Infection Mediates a Mechanism of Airway Hyperresponsive Diseases. Biology, 12(2), 317. https://doi.org/10.3390/biology12020317





