Friend or Foe: Symbiotic Bacteria in Bactrocera dorsalis–Parasitoid Associations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bactrocera dorsalis and Parasitoid Rearing
2.2. Bacteria Culturing
2.3. Generation of Axenic, Symbiotic, and Mono-Associated Bacterial Lines
2.4. Parasitic Wasp Infection of BMALs and Axenic and Symbiotic Fly Lines
2.5. Effect of Bacterial Symbionts on Host Acceptability for Oviposition by Parasitoid Females
2.6. Parasitoid Emergence and Fitness Assays
2.7. Effect of Bacterial Symbionts Parasitoid Offspring’s Fitness Traits
2.7.1. Parasitoid Progeny Developmental Time
2.7.2. Parasitoid Progeny Body Size
2.7.3. Longevity of Host-Deprived F1 Parasitoid Progeny
2.7.4. Fecundity of F1 Female Parasitoid Progeny
2.8. Data Analysis
3. Results
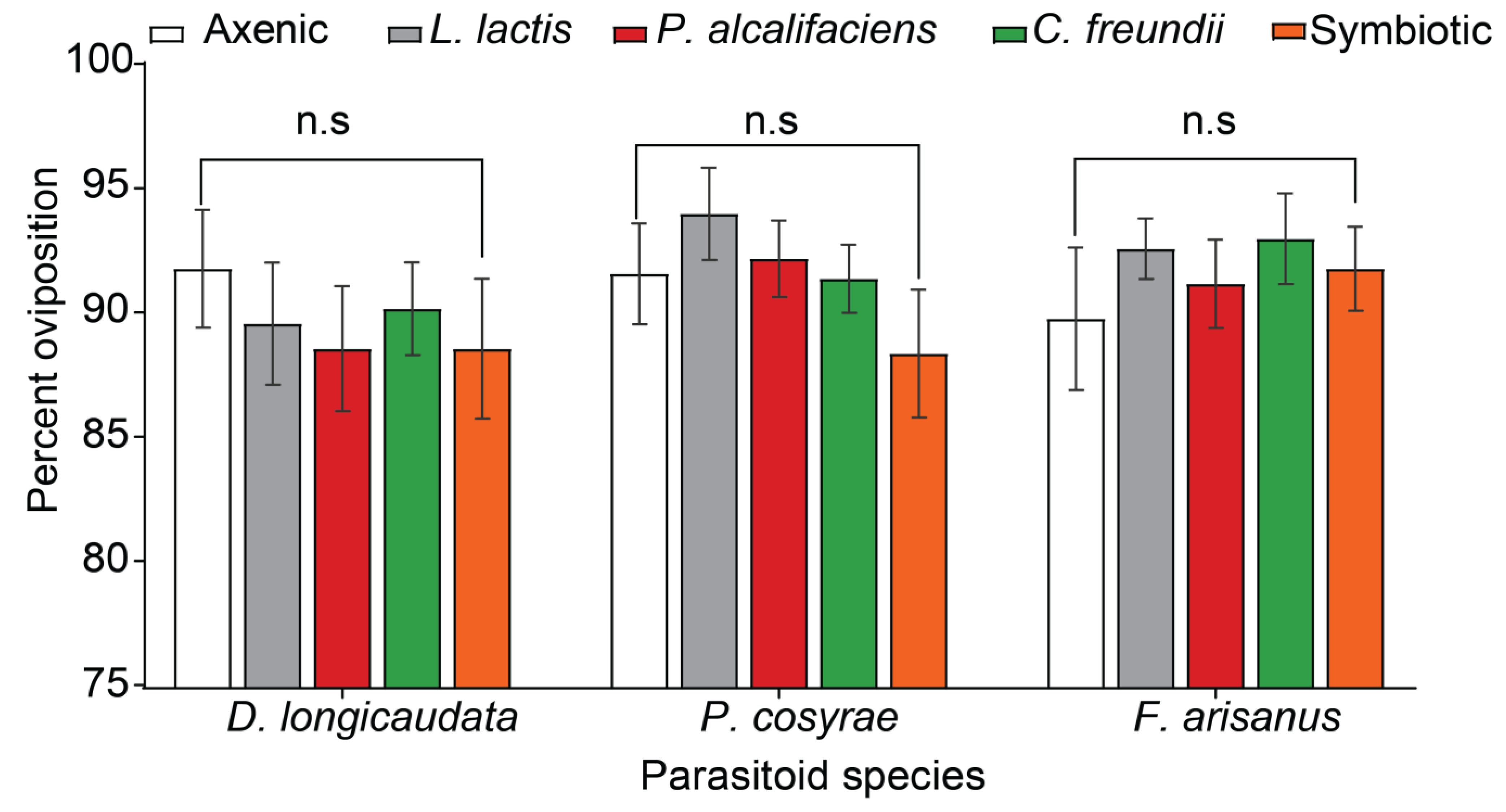
3.1. Effect of Bacterial Symbionts on Host Acceptability for Oviposition by Female Parasitoids
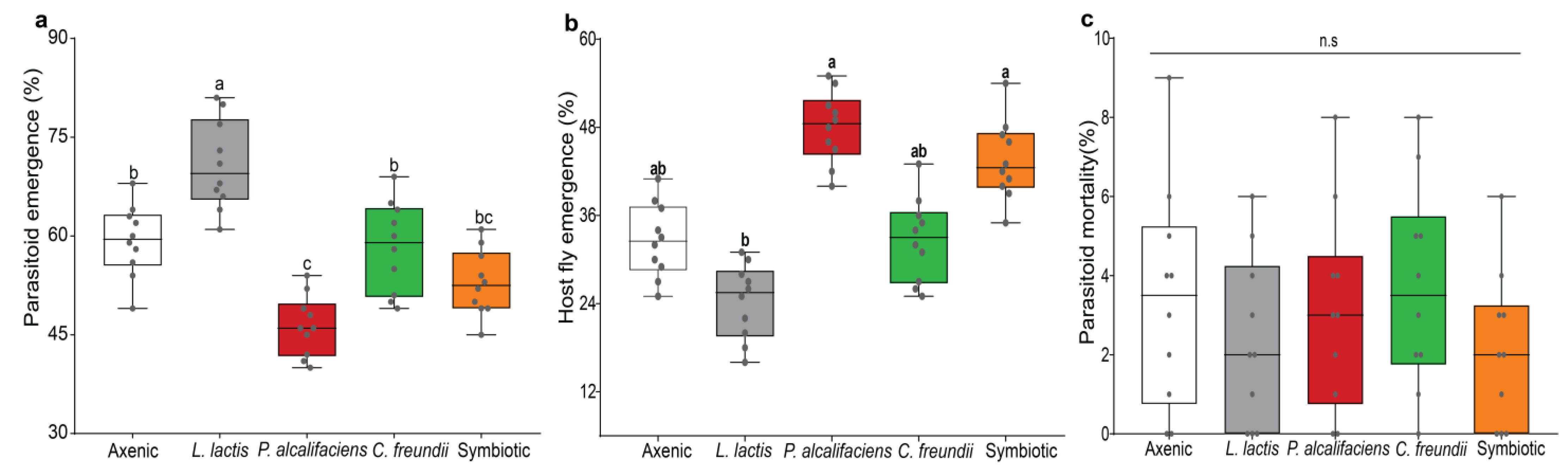
3.2. Effect of Bacterial Symbionts on Host Suitability for Development of the Immature Stages of the Parasitoids
3.3. Effect of Bacterial Symbionts on Parasitoid Offspring’s Fitness Traits
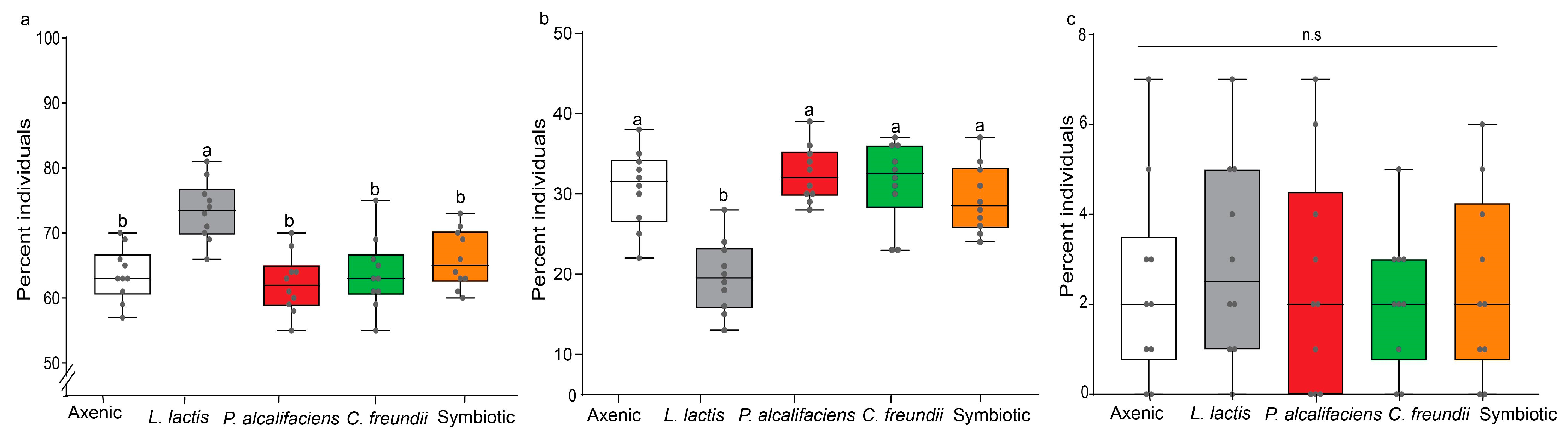
3.3.1. Parasitoid Progeny Sex Ratio
3.3.2. Parasitoid Progeny Developmental Time
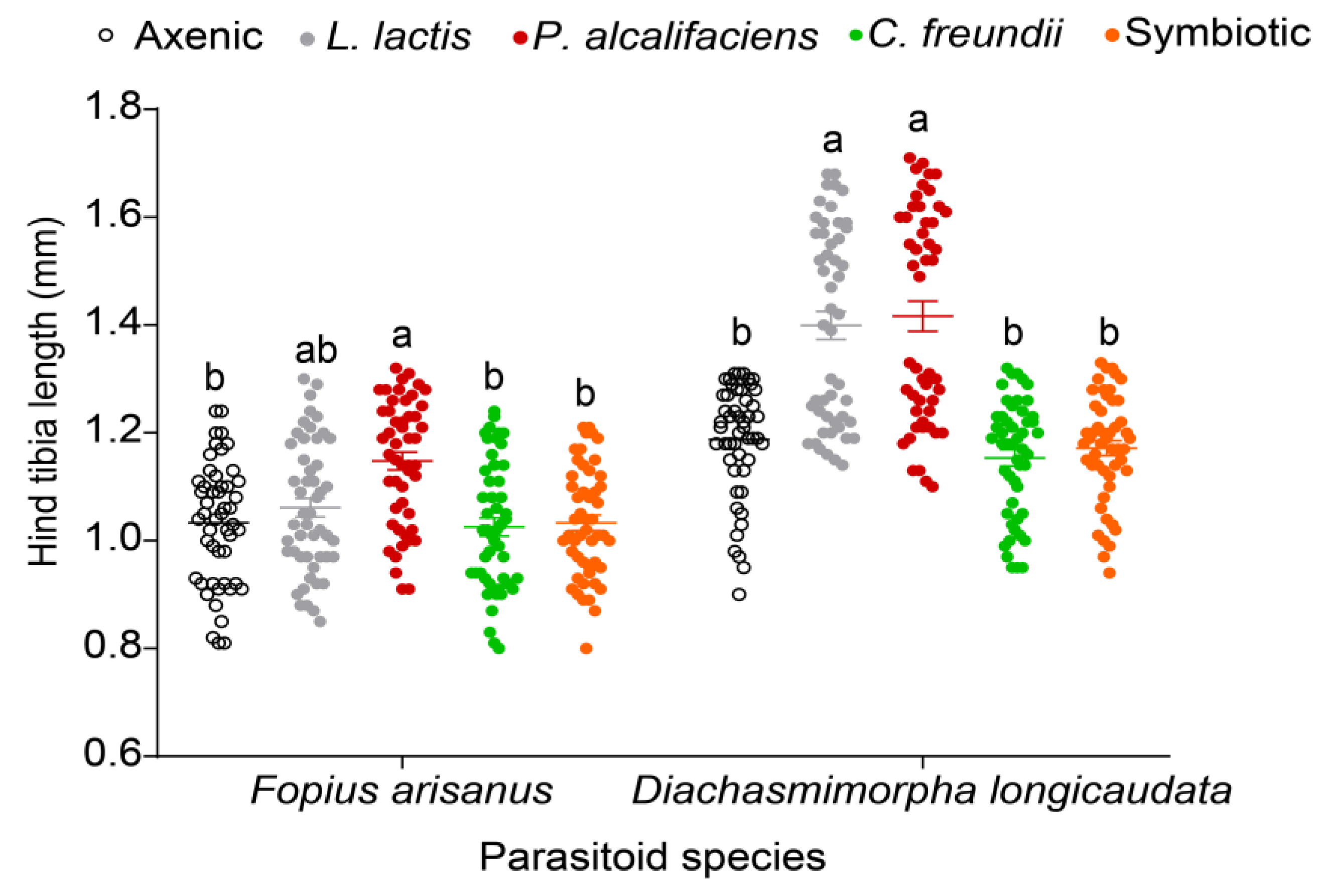
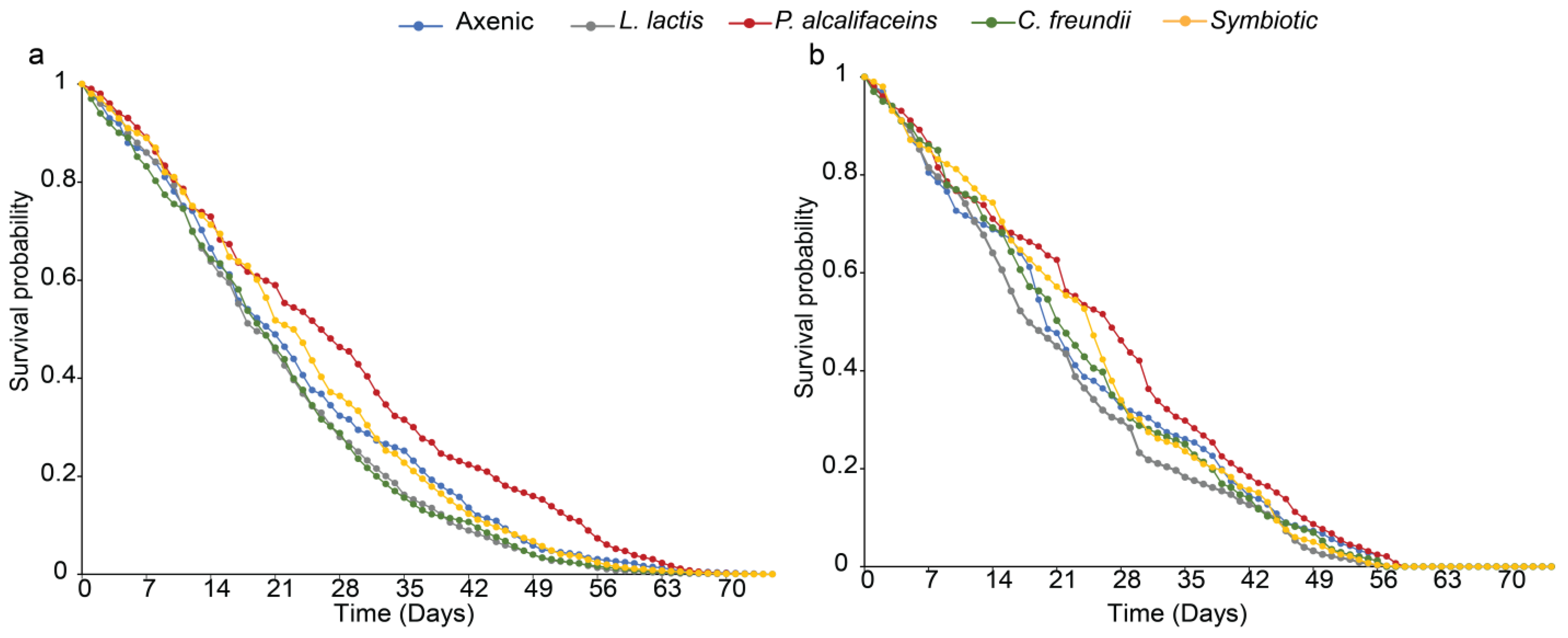
3.3.3. Parasitoid Progeny Body Size and Longevity
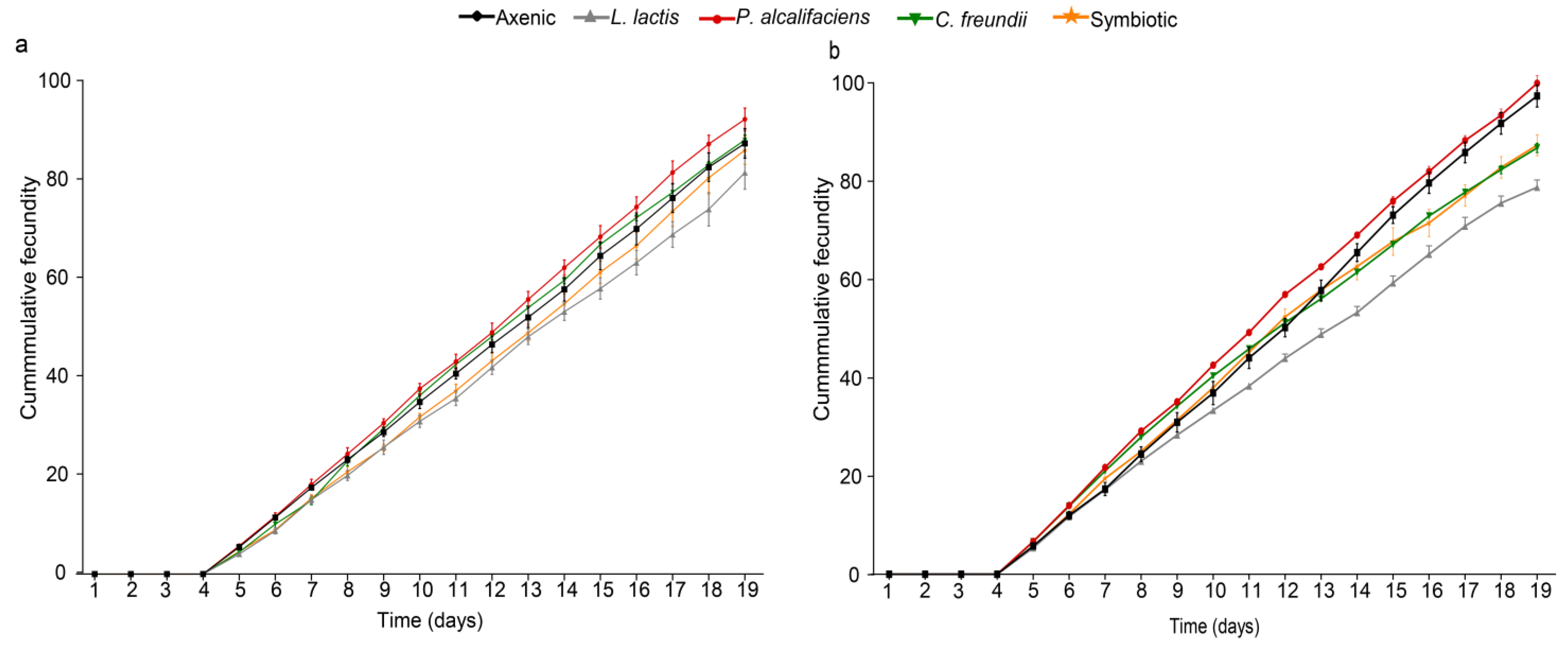
3.3.4. Fecundity of Female Parasitoid Progeny
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekesi, S.; Nderitu, P.W.; Rwomushana, I. Field Infestation, Life History and Demographic Parameters of the Fruit Fly Bactrocera invadens (Diptera: Tephritidae) in Africa. Bull. Entomol. Res. 2006, 96, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.A.; Copeland, R.S.; White, I.M.; Manrakhan, A.; Billah, M.K. A New Invasive Fruit Fly Species from the Bactrocera dorsalis (Hendel) Group Detected in East Africa. Int. J. Trop. Insect Sci. 2003, 23, 355–361. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, L.; Zhang, H. Low Diversity Bacterial Community and the Trapping Activity of Metabolites from Cultivable Bacteria Species in the Female Reproductive System of the Oriental Fruit Fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). Int. J. Mol. Sci. 2012, 13, 6266. [Google Scholar] [CrossRef]
- Wang, H.; Jin, L.; Peng, T.; Zhang, H.; Chen, Q.; Hua, Y. Identification of Cultivable Bacteria in the Intestinal Tract of Bactrocera dorsalis from Three Different Populations and Determination of Their Attractive Potential. Pest Manag. Sci. 2014, 70, 80–87. [Google Scholar] [CrossRef]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut Symbiont Enhances Insecticide Resistance in a Significant Pest, the Oriental Fruit Fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Rwomushana, I.; Tanga, C.M. Fruit Fly Species Composition, Distribution and Host Plants with Emphasis on Mango-Infesting Species. In Fruit Fly Research and Development in Africa-Towards a Sustainable Management Strategy to Improve Horticulture; Ekesi, S., Mohamed, S.A., De Meyer, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 71–106. ISBN 9783319432267. [Google Scholar]
- Heve, W.K.; Adjadeh, T.A.; Billah, M.K. Overview and Future Research Needs for Development of Effective Biocontrol Strategies for Management of Bactrocera dorsalis Hendel (Diptera: Tephritidae) in Sub-Saharan Africa. Pest Manag. Sci. 2021, 77, 4224–4237. [Google Scholar]
- Ndlela, S.; Niassy, S.; Mohamed, S.A. Important Alien and Potential Native Invasive Insect Pests of Key Fruit Trees in Sub-Saharan Africa: Advances in Sustainable Pre- and Post-Harvest Management Approaches. CABI Agric. Biosci. 2022 31 2022, 3, 1–46. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ekesi, S.; Hanna, R. Evaluation of the Impact of Diachasmimorpha Longicaudata on Bactrocera invadens and Five African Fruit Fly Species. J. Appl. Entomol. 2008, 132, 789–797. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ekesi, S.; Wharton, R.A.; Lux, S.A.; Overholt, W.A. Old and New Host-Parasitoid Associations: Parasitism of the Native African and Invasive Fruit Flies Species. In Proceedings of the Proceedings of the 5th International Symposium on Biological Control of Arthropods; Mason, P.G., Gillespie, D.R., Vincent, C., Eds.; CABI: Wallingford, UK, 2017; pp. 224–227. [Google Scholar]
- Vargas, R.; Stark, J.D.; Uchida, G.K.; Purcell, M. Opiine Parasitoids (Hymenoptera: Braconidae) of Oriental Fruit Fly (Diptera: Tephritidae) on Kauai Island, Hawaii: Islandwide Relative Abundance and Parasitism Rates in Wild and Orchard Guava Habitats. Environ. Entomol. 1993, 22, 246–253. [Google Scholar] [CrossRef]
- Vargas, R.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional Suppression of Bactrocera Fruit Flies (Diptera: Tephritidae) in the Pacific through Biological Control and Prospects for Future Introductions into Other Areas of the World. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Leblanc, L.; Putoa, R.; Eitam, A. Impact of Introduction of Bactrocera dorsalis (Diptera: Tephritidae) and Classical Biological Control Releases of Fopius Arisanus (Hymenoptera: Braconidae) on Economically Important Fruit Flies in French Polynesia. J. Econ. Entomol 2007, 100, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Nanga Nanga, S.; Hanna, R.; Gnanvossou, D.; Fotso Kuate, A.; Fiaboe, K.K.M.; Djieto-Lordon, C.; Schmidt-Jeffris, R. Fruit Preference, Parasitism, and Offspring Fitness of Fopius arisanus (Hymenoptera: Braconidae) Exposed to Bactrocera dorsalis (Diptera: Tephritidae) Infested Fruit Species. Environ. Entomol. 2019, 48, 1286–1296. [Google Scholar] [CrossRef]
- Ndlela, S.; Mohamed, S.A.; Azrag, A.G.A.; Ndegwa, P.N.; Ong’amo, G.O.; Ekesi, S. Interactions between Two Parasitoids of Tephritidae: Diachasmimorpha longicaudata (Ashmead) and Psyttalia cosyrae (Wilkinson) (Hymenoptera: Braconidae), under Laboratory Conditions. Insects 2020, 11, 671. [Google Scholar] [CrossRef]
- Gnanvossou, D.; Hanna, R.; Bokonon-Ganta, A.H.; Ekesi, S.; Mohamed, S.A. Release, Establishment and Spread of the Natural Enemy Fopius arisanus (Hymenoptera: Braconidae) for Control of the Invasive Oriental Fruit Fly Bactrocera dorsalis (Diptera: Tephritidae) in Benin, West Africa. In Fruit Fly Research and Development in Africa—Towards a Sustainable Management Strategy to Improve Horticulture; Springer: Cham, Switzerland, 2016; pp. 575–600. [Google Scholar] [CrossRef]
- Leblanc, L.; Vargas, R.; Putoa, R. From Eradication to Containment: Invasion of French Polynesia by Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) and Releases of Two Natural Enemies: A 17-Year Case Study. In Proceedings of the Proceedings of the Hawaiian Entomological Society; Hawaiian Entomological Society: Honolulu, HI, USA, 2013; pp. 31–43. [Google Scholar]
- Mohamed, S.A.; Ekesi, S.; Hanna, R. Old and New Host-Parasitoid Associations: Parasitism of the Invasive Fruit Fly Bactrocera invadens (Diptera: Tephritidae) and Five African Fruit Fly Species by Fopius arisanus, an Asian Opiine Parasitoid. Biocontrol Sci. Technol. 2010, 20, 183–196. [Google Scholar] [CrossRef]
- Vargas, R.; Leblanc, L.; McKenney, M.; Mackey, B.; Harris, E.; Badji, K. Rearing Fopius arisanus (Sonan) (Hymenoptera: Braconidae) on Mediterranean Fruit Fly and Its Introduction into Senegal against Oriental Fruit Fly (Diptera: Tephritidae). Proc. Hawaiian Entomol. Soc. 2016, 48, 85–94. [Google Scholar]
- Mohamed, S.A.; Ramadan, M.M.; Ekesi, S. In and out of Africa: Parasitoids Used for Biological Control of Fruit Flies. In Fruit Fly Research and Development in Africa-Towards a Sustainable Management Strategy to Improve Horticulture; Ekesi, S., Ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 325–368. ISBN 9783319432267. [Google Scholar]
- da Silva Gonçalves, R.; Manoukis, N.C.; Nava, D.E. Effect of Fopius arisanus Oviposition Experience on Parasitization of Bactrocera dorsalis. BioControl 2017, 62, 595–602. [Google Scholar] [CrossRef]
- Gwokyalya, R.; Herren, J.K.; Weldon, C.W.; Khamis, F.M.; Ndlela, S.; Mohamed, S.A. Differential Immune Responses in New and Old Fruit Fly-Parasitoid Associations: Implications for Their Management. Front. Physiol. 2022, 13, 1663. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative Bacterial Symbionts in Aphids Confer Resistance to Parasitic Wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in Resistance to Parasitism in Aphids is Due to Symbionts Not Host Genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef]
- Oliver, K.M.; Noge, K.; Huang, E.M.; Campos, J.M.; Becerra, J.X.; Hunter, M.S. Parasitic Wasp Responses to Symbiont-Based Defense in Aphids. BMC Biol. 2012, 10, 11. [Google Scholar] [CrossRef]
- Asplen, M.K.; Bano, N.; Brady, C.M.; Desneux, N.; Hopper, K.R.; Malouines, C.; Oliver, K.M.; White, J.A.; Heimpel, G.E. Specialisation of Bacterial Endosymbionts That Protect Aphids from Parasitoids. Ecol. Entomol. 2014, 39, 736–739. [Google Scholar] [CrossRef]
- Sochard, C.; Bellec, L.; Simon, J.-C.; Outreman, Y. Influence of “Protective” Symbionts throughout the Different Steps of an Aphid–Parasitoid Interaction. Curr. Zool. 2020, 67, 1–13. [Google Scholar] [CrossRef]
- Leclair, M.; Buchard, C.; Mahéo, F.; Simon, J.C.; Outreman, Y. A Link Between Communities of Protective Endosymbionts and Parasitoids of the Pea Aphid Revealed in Unmanipulated Agricultural Systems. Front. Ecol. Evol. 2021, 9, 187. [Google Scholar]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts Protect Aphids from Parasitic Wasps by Attenuating Herbivore-Induced Plant Volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
- Guay, J.F.; Boudreault, S.; Michaud, D.; Cloutier, C. Impact of Environmental Stress on Aphid Clonal Resistance to Parasitoids: Role of Hamiltonella defensa Bacterial Symbiosis in Association with a New Facultative Symbiont of the Pea Aphid. J. Insect Physiol. 2009, 55, 919–926. [Google Scholar] [CrossRef]
- McLean, A.H.C.; Godfray, H.C.J. Evidence for Specificity in Symbiont-Conferred Protection against Parasitoids. Proc. R. Soc. B Biol. Sci. 2015, 282, 1–8. [Google Scholar] [CrossRef]
- Xie, J.; Butler, S.; Sanchez, G.; Mateos, M. Male Killing Spiroplasma Protects Drosophila melanogaster against Two Parasitoid Wasps. Heredity (Edinb). 2014, 112, 399–408. [Google Scholar] [CrossRef]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Moran, N.A. Evolutionary Genetics of a Defensive Facultative Symbiont of Insects: Exchange of Toxin-Encoding Bacteriophage. Mol. Ecol. 2008, 17, 916–929. [Google Scholar] [CrossRef]
- Moran, N.A.; Degnan, P.H.; Santos, S.R.; Dunbar, H.E.; Ochman, H. The Players in a Mutualistic Symbiosis: Insects, Bacteria, Viruses, and Virulence Genes. Proc. Natl. Acad. Sci. USA 2005, 102, 16919–16926. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Hunter, M.S.; Moran, N.A. Bacteriophages Encode Factors Required for Protection in a Symbiotic Mutualism. Science 2009, 325, 992–994. [Google Scholar] [CrossRef]
- Fytrou, A.; Schofield, P.G.; Kraaijeveld, A.R.; Hubbard, S.F. Wolbachia Infection Suppresses Both Host Defence and Parasitoid Counter-Defence. Proc. R. Soc. B Biol. Sci. 2005, 273, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.C.; Herren, J.K.; Schüpfer, F.; Lemaitre, B. The Role of Lipid Competition for Endosymbiont-Mediated Protection against Parasitoid Wasps in Drosophila. MBio 2016, 7. [Google Scholar] [CrossRef]
- Attia, S.; Renoz, F.; Pons, I.; Louâpre, P.; Foray, V.; Piedra, J.-M.; Sanané, I.; Le Goff, G.; Lognay, G.; Hance, T. The Aphid Facultative Symbiont Serratia symbiotica Influences the Foraging Behaviors and the Life-History Traits of the Parasitoid Aphidius Ervi. Entomol. Gen. 2021. [Google Scholar] [CrossRef]
- Hopper, K.R.; Kuhn, K.L.; Lanier, K.; Rhoades, J.H.; Oliver, K.M.; White, J.A.; Asplen, M.K.; Heimpel, G.E. The Defensive Aphid Symbiont Hamiltonella defensa Affects Host Quality Differently for Aphelinus glycinis versus Aphelinus atriplicis. Biol. Control 2018, 116, 3–9. [Google Scholar]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Beukeboom, L.W.; Mathiopoulos, K.D.; Caceres, C.; Bourtzis, K. The Impact of Fruit Fly Gut Bacteria on the Rearing of the Parasitic Wasp Diachasmimorpha longicaudata. Entomol. Exp. Appl. 2020, 168, 541–559. [Google Scholar] [CrossRef]
- Nyabuga, F.N.; Outreman, Y.; Simon, J.C.; Heckel, D.G.; Weisser, W.W. Effects of Pea Aphid Secondary Endosymbionts on Aphid Resistance and Development of the Aphid Parasitoid Aphidius ervi: A Correlative Study. Entomol. Exp. Appl. 2010, 136, 243–253. [Google Scholar] [CrossRef]
- Pons, I.; Renoz, F.; Noël, C.; Hance, T. New Insights into the Nature of Symbiotic Associations in Aphids: Infection Process, Biological Effects, and Transmission Mode of Cultivable Serratia symbiotica Bacteria. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Schmid, M.; Sieber, R.; Zimmermann, Y.S.; Vorburger, C. Development, Specificity and Sublethal Effects of Symbiont-Conferred Resistance to Parasitoids in Aphids. Funct. Ecol. 2012, 26, 207–215. [Google Scholar] [CrossRef]
- Andongma, A.A.; Wan, L.; Dong, Y.C.; Li, P.; Desneux, N.; White, J.A.; Niu, C.Y. Pyrosequencing Reveals a Shift in Symbiotic Bacteria Populations across Life Stages of Bactrocera dorsalis. Sci. Rep. 2015, 5, 9470. [Google Scholar] [CrossRef]
- Gichuhi, J.; Khamis, F.; Van den Berg, J.; Mohamed, S.; Ekesi, S.; Herren, J.K. Influence of Inoculated Gut Bacteria on the Development of Bactrocera dorsalis and on Its Susceptibility to the Entomopathogenic Fungus, Metarhizium anisopliae. BMC Microbiol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Damodaram, K.J.P.; Ayyasamy, A.; Kempraj, V. Commensal Bacteria Aid Mate-Selection in the Fruit Fly, Bactrocera dorsalis. Microb. Ecol. 2016, 72, 725–729. [Google Scholar] [CrossRef]
- Akami, M.; Ren, X.-M.; Qi, X.; Mansour, A.; Gao, B.; Cao, S.; Niu, C.-Y. Symbiotic Bacteria Motivate the Foraging Decision and Promote Fecundity and Survival of Bactrocera dorsalis (Diptera: Tephritidae). BMC Microbiol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Akami, M.; Andongma, A.A.; Zhengzhong, C.; Nan, J.; Khaeso, K.; Jurkevitch, E.; Niu, C.-Y.Y.; Yuval, B. Intestinal Bacteria Modulate the Foraging Behavior of the Oriental Fruit Fly Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2019, 14, e0210109. [Google Scholar] [CrossRef]
- Khan, M.; Seheli, K.; Bari, M.A.; Sultana, N.; Khan, S.A.; Sultana, K.F.; Hossain, M.A. Potential of a Fly Gut Microbiota Incorporated Gel-Based Larval Diet for Rearing Bactrocera dorsalis (Hendel). BMC Biotechnol. 2019, 19, 94. [Google Scholar] [CrossRef]
- Khaeso, K.; Andongma, A.A.; Akami, M.; Souliyanonh, B.; Zhu, J.; Krutmuang, P.; Niu, C.-Y. Assessing the Effects of Gut Bacteria Manipulation on the Development of the Oriental Fruit Fly, Bactrocera dorsalis (Diptera; Tephritidae). Symbiosis 2017, 74, 97–105. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, P.; Wang, B.; Liu, X.; Lin, J.; Hua, R.; Zhang, H.; Yi, C.; Song, X.; Ji, Q.; et al. Manipulation of Gut Symbionts for Improving the Sterile Insect Technique: Quality Parameters of Bactrocera dorsalis (Diptera: Tephritidae) Genetic Sexing Strain Males After Feeding on Bacteria-Enriched Diets. J. Econ. Entomol. 2021, 114, 560–570. [Google Scholar] [CrossRef]
- Ekesi, S.; Mohamed, S.A. Mass Rearing and Quality Control Parameters for Tephritid Fruit Flies of Economic Importance in Africa. In Wide Spectra of Quality Control; Akyar, I., Ed.; IntechOpen: London, UK, 2011; pp. 387–410. ISBN 978-953-307-683-6. [Google Scholar]
- Mohamed, S.A.; Overholt, W.A.; Wharton, R.A.; Lux, S.A.; Eltoum, E.M. Host Specificity of Psyttalia cosyrae (Hymenoptera: Braconidae) and the Effect of Different Host Species on Parasitoid Fitness. Biol. Control 2003, 28, 155–163. [Google Scholar] [CrossRef]
- Chang, C.L. Fruit Fly Liquid Larval Diet Technology Transfer and Update. J. Appl. Entomol. 2009, 133, 164–173. [Google Scholar] [CrossRef]
- Ekesi, S.; Mohamed, S.A.; Chang, C.L. A Liquid Larval Diet for Rearing Bactrocera invadens and Ceratitis fasciventris (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S90–S98. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 8 November 2022).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. Version 2.38. 2015. Available online: https://CRAN.R-project.org/package=survival (accessed on 8 November 2022).
- Desneux, N.; Asplen, M.K.; Brady, C.M.; Heimpel, G.E.; Hopper, K.R.; Luo, C.; Monticelli, L.; Oliver, K.M.; White, J.A. Intraspecific Variation in Facultative Symbiont Infection among Native and Exotic Pest Populations: Potential Implications for Biological Control. Biol. Control 2018, 116, 27–35. [Google Scholar] [CrossRef]
- Cayetano, L.; Vorburger, C. Symbiont-Conferred Protection against Hymenopteran Parasitoids in Aphids: How General Is It? Ecol. Entomol. 2015, 40, 85–93. [Google Scholar] [CrossRef]
- Luo, C.; Gatti, J.L.; Monticelli, L.S.; Poirié, M.; Desneux, N.; Zhao, H.; Hu, Z. An Increased Risk of Parasitism Mediated by the Facultative Symbiont Regiella insecticola. J. Pest Sci. 2020, 93, 737–745. [Google Scholar] [CrossRef]
- Charnov, E.L.; Los-Den Hartogh, R.L.; Jones, W.T.; Van Den Assem, J. Sex Ratio Evolution in a Variable Environment. Nature 1981, 289, 27–33. [Google Scholar] [CrossRef]
- Charnov, E.L. The Theory of Sex Allocation; Princeton University Press: Princeton, NJ, USA, 1982; Volume 18, ISBN 9780691083124. [Google Scholar]
- King, B.H.; Napoleon, M.E. Using Effects of Parasitoid Size on Fitness to Test a Host Quality Model Assumption with the Parasitoid Wasp Spalangia endius. Can. J. Zool. 2006, 84, 1678–1682. [Google Scholar] [CrossRef]
- King, B.H. Host-Size-Dependent Sex Ratios among Parasitoid Wasps: Does Host Growth Matter ? Oecologia 1989, 78, 420–426. [Google Scholar]
- Ode, P.J.; Hardy, I.C.W. Parasitoid Sex Ratios and Biological Control. In Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 253–291. ISBN 9781405163477. [Google Scholar]
- Harvey, J.A.; de Haan, L.; Verdeny-Vilalta, O.; Visser, B.; Gols, R. Reproduction and Offspring Sex Ratios Differ Markedly among Closely Related Hyperparasitoids Living in the Same Microhabitats. J. Insect Behav. 2019, 32, 243–251. [Google Scholar] [CrossRef]
- Arthur, A.P.; Wylie, H.G. Effects of Host Size on Sex Ration, Development Time and Size of Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Entomophaga 1959, 4, 297–301. [Google Scholar] [CrossRef]
- Harvey, J.A.; Kadash, K.; Strand, R. Micheal Differences in Larval Feeding Behavior Correlate with Altered Developmental Strategies in Two Parasitic Wasps: Implications for the Size-Fitness Hypothesis. Oikos 2000, 88, 621–629. [Google Scholar]
- Mackauer, M.; Sequeira, R. Patterns of Development in Insect Parasites. In Parasites and Pathogens of Insects; Beckage, N.E., Thompson, S.N., Federici, B.A., Eds.; Academic Press: San Diego, CA, USA, 1993; Volume 1, p. 12. ISBN 9780120844418. [Google Scholar]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology, 1st ed.Princeton University Press: Princeton, NJ, USA, 1994; Volume 67, ISBN 9780691000473. [Google Scholar]
- Wang, X.G.; Messing, R.H. Fitness Consequences of Body-Size-Dependent Host Species Selection in a Generalist Ectoparasitoid. Behav. Ecol. Sociobiol. 2004, 56, 513–522. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Wei, K.; Wang, X.; Yang, Z.; Zhang, Y. Relationships between Body Size and Parasitic Fitness and Offspring Performance of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae). PLoS ONE 2016, 11, e0156831. [Google Scholar] [CrossRef] [PubMed]
- Sagarra, L.A.A.; Vincent, C.; Stewart, R.K.K. Body Size as an Indicator of Parasitoid Quality in Male and Female Anagyrus kamali (Hymenoptera: Encyrtidae). Bull. Entomol. Res. 2001, 91, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Monticelli, L.; Meng, L.; Li, D.; Fan, J.; Zhao, H.; Hu, Z. Effect of the Endosymbiont Regiella insecticola on an Aphid Parasitoid. Entomol. Gen. 2017, 36, 301–308. [Google Scholar] [CrossRef]


| Developmental Time Days (Mean ± SEM) | ||||
|---|---|---|---|---|
| Parasitoid Species | Fly Line | Male | Female | Pooled |
| Fopius arisanus | Axenic | 19.96 ± 0.109 a | 21.39 ± 0.118 a | 20.76 ± 0.133 ab |
| L. lactis | 20.30 ± 0.147 ab | 21.86 ± 0.083 b | 21.05 ± 0.174 bc | |
| P. alcalifaciens | 20.69 ± 0.077 b | 21.80 ± 0.074 b | 21.16 ± 0.210 c | |
| C. freundii | 19.99 ± 0.115 a | 21.29 ± 0.122 a | 20.78 ± 0.086 ab | |
| Symbiotic | 19.93 ± 0.078 a | 21.12 ± 0.084 a | 20.55 ± 0.118 a | |
| Diachasmimorpha longicaudata | Axenic | 14.97 ± 0.977 a | 17.65 ± 0.096 a | 16.97 ± 0.107 a |
| L. lactis | 16.91 ± 0.220 b | 18.57 ± 0.243 c | 17.89 ± 0.224 b | |
| P. alcalifaciens | 16.75 ± 0.101 ab | 18.18 ± 0.048 bc | 17.61 ± 0.052 b | |
| C. freundii | 15.81 ± 0.113 ab | 17.56 ± 0.096 a | 16.81 ± 0.106 a | |
| Symbiotic | 16.08 ± 0.117 ab | 17.72 ± 0.097 ab | 16.99 ± 0.096 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwokyalya, R.; Weldon, C.W.; Herren, J.K.; Gichuhi, J.; Makhulu, E.E.; Ndlela, S.; Mohamed, S.A. Friend or Foe: Symbiotic Bacteria in Bactrocera dorsalis–Parasitoid Associations. Biology 2023, 12, 274. https://doi.org/10.3390/biology12020274
Gwokyalya R, Weldon CW, Herren JK, Gichuhi J, Makhulu EE, Ndlela S, Mohamed SA. Friend or Foe: Symbiotic Bacteria in Bactrocera dorsalis–Parasitoid Associations. Biology. 2023; 12(2):274. https://doi.org/10.3390/biology12020274
Chicago/Turabian StyleGwokyalya, Rehemah, Christopher W. Weldon, Jeremy Keith Herren, Joseph Gichuhi, Edward Edmond Makhulu, Shepard Ndlela, and Samira Abuelgasim Mohamed. 2023. "Friend or Foe: Symbiotic Bacteria in Bactrocera dorsalis–Parasitoid Associations" Biology 12, no. 2: 274. https://doi.org/10.3390/biology12020274
APA StyleGwokyalya, R., Weldon, C. W., Herren, J. K., Gichuhi, J., Makhulu, E. E., Ndlela, S., & Mohamed, S. A. (2023). Friend or Foe: Symbiotic Bacteria in Bactrocera dorsalis–Parasitoid Associations. Biology, 12(2), 274. https://doi.org/10.3390/biology12020274







