Alcohol Use and the Risk of Colorectal Liver Metastasis: A Systematic Mapping Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
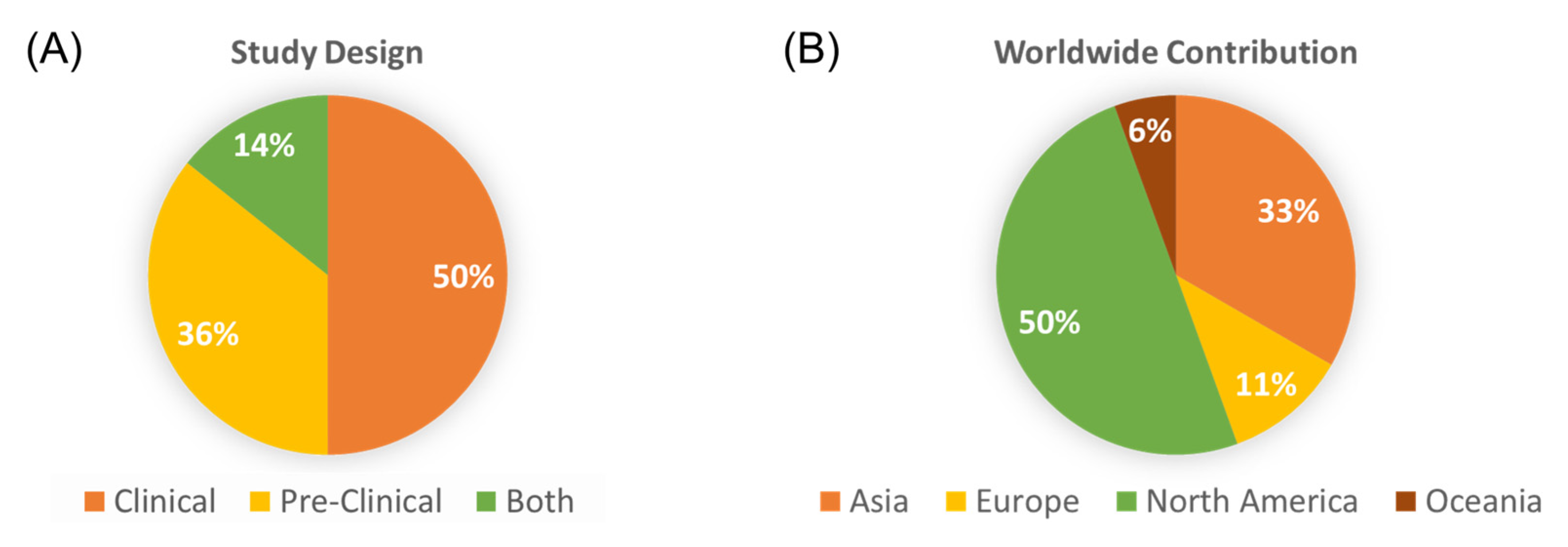
3. Results
3.1. Clinical Studies Evaluating the Role of Alcohol and CRC Outcomes
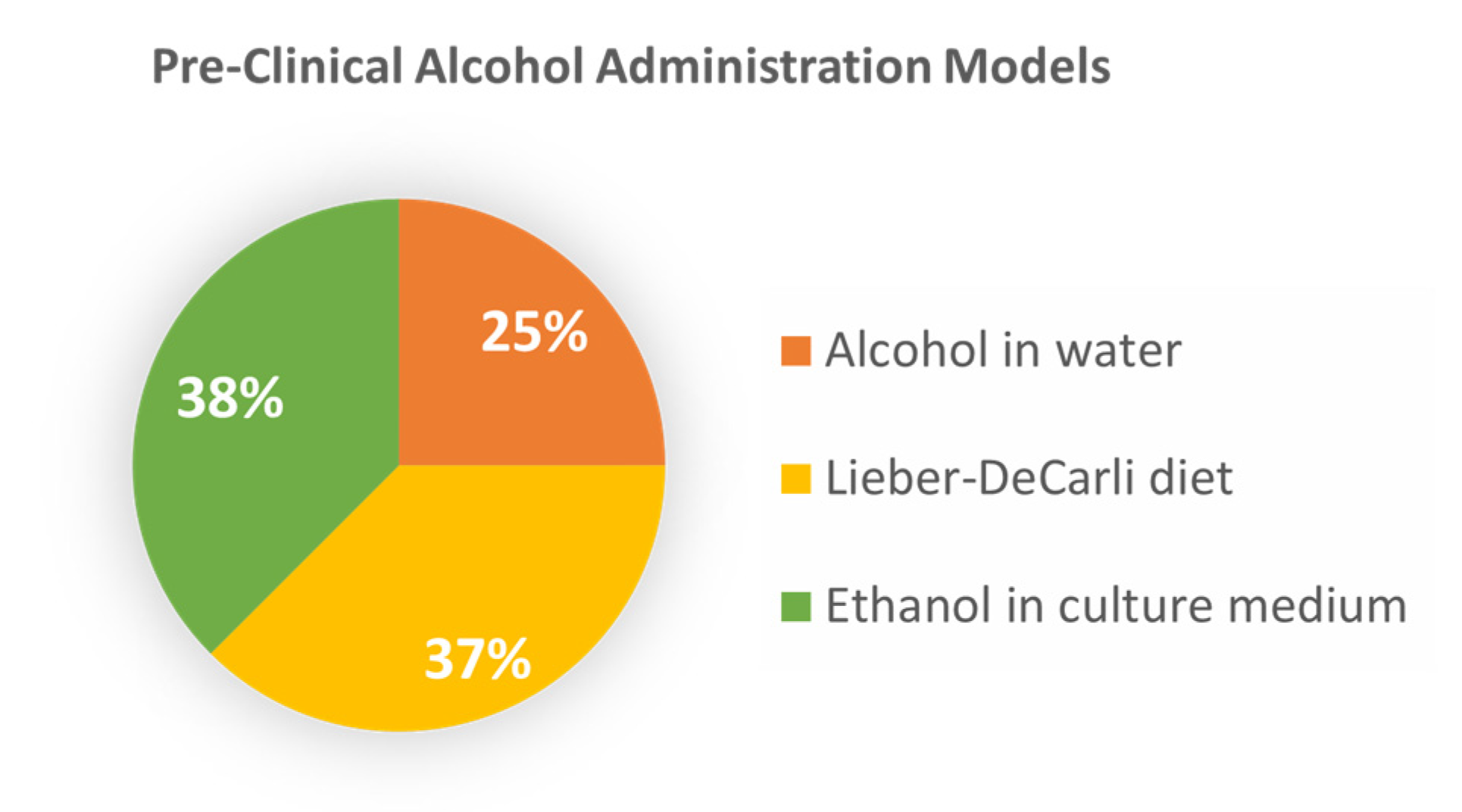
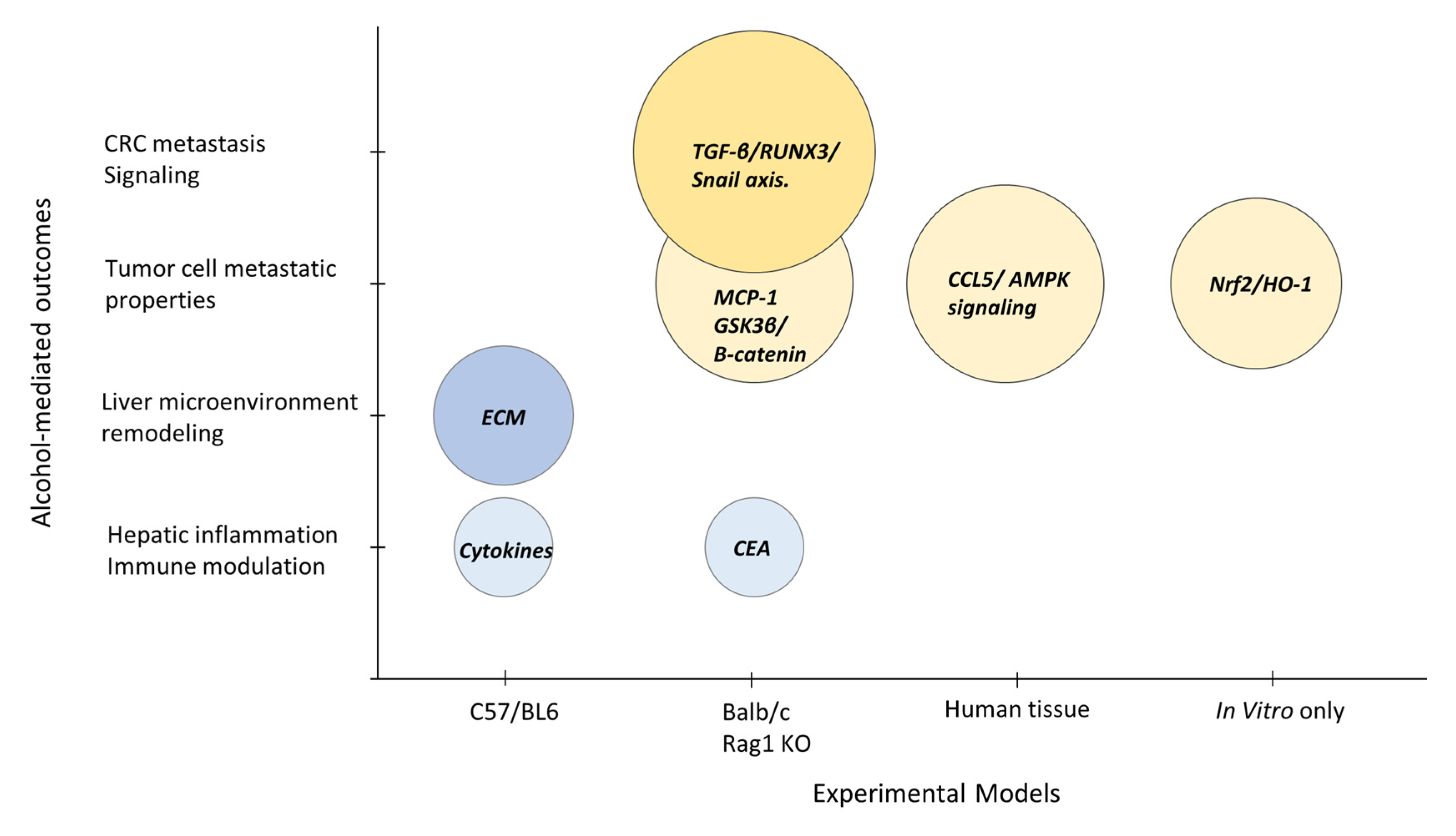
3.2. Preclinical Investigations into the Role of Alcohol in Advanced CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Zarour, L.R.; Anand, S.; Billingsley, K.G.; Bisson, W.H.; Cercek, A.; Clarke, M.F.; Coussens, L.M.; Gast, C.E.; Geltzeiler, C.B.; Hansen, L.; et al. Colorectal cancer liver metastasis: Evolving paradigms and future directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, Y.; Ding, Y.; Chen, K.; Jin, M. Alcohol drinking and the risk of colorectal cancer death: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.R.; Jansen, L.; Walter, V.; Kloor, M.; Roth, W.; Bläker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am. J. Clin. Nutr. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. 2019 National Survey on Drug Use and Health. Table 2.20B–Binge Alcohol Use in Past Month among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Percentages, 2018 and 2019. 2019. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics (accessed on 22 July 2022).
- Julien, J.; Ayer, T.; Tapper, E.B.; Barbosa, C.; Dowd, W.; Chhatwal, J. Effect of Increased Alcohol Consumption During COVID-19 Pandemic on Alcohol-related Liver Disease: A Modeling Study. Hepatology 2021, 75, 1480–1490. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
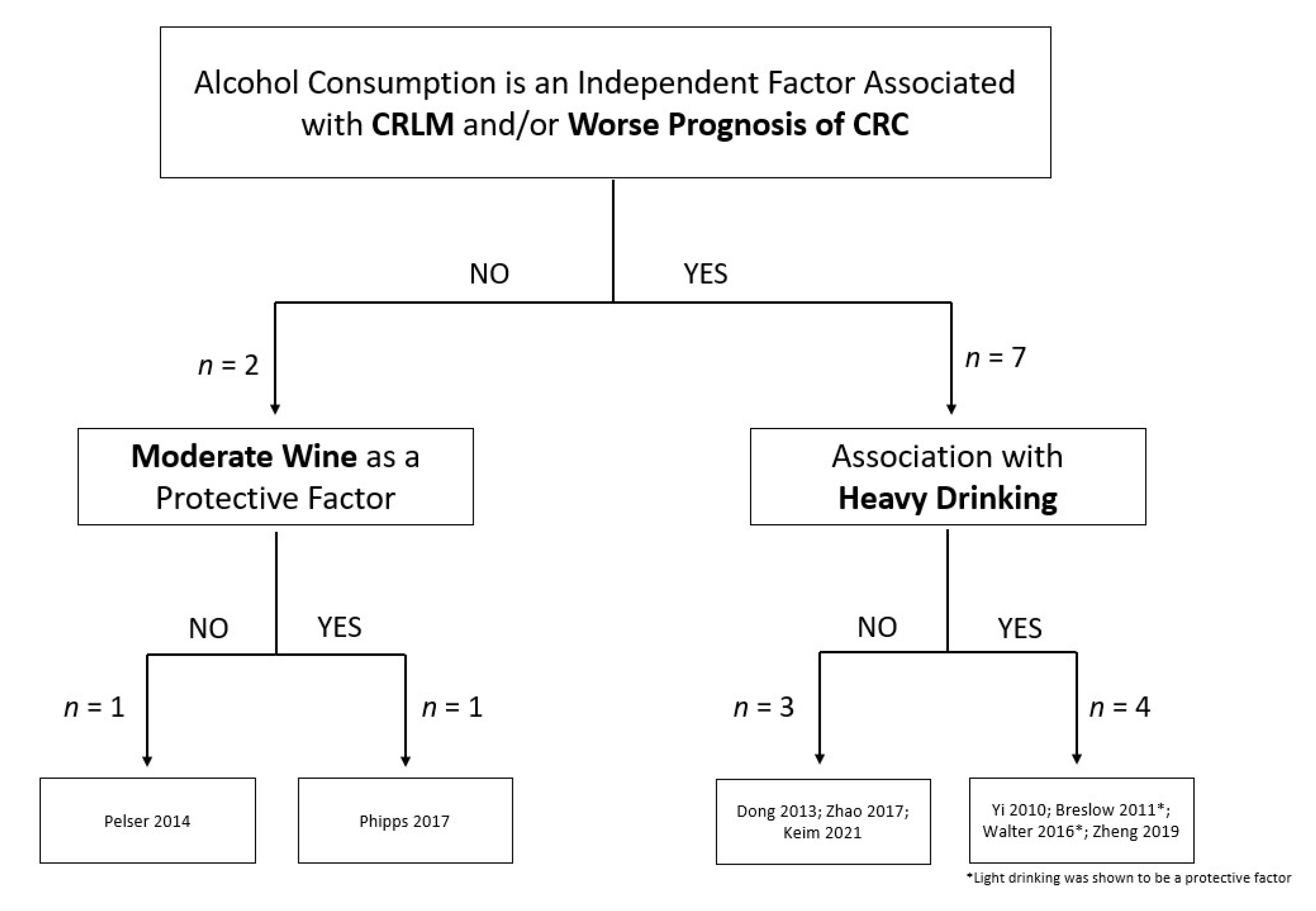
- Dong, H.; Tang, J.; Li, L.-H.; Ge, J.; Chen, X.; Ding, J.; Men, H.-T.; Luo, W.-X.; Du, Y.; Li, C. Serum carbohydrate antigen 19-9 as an indicator of liver metastasis in colorectal carcinoma cases. Asian Pac. J. Cancer Prev. 2013, 14, 909–913. [Google Scholar] [CrossRef]
- Maeda, M.; Nagawa, H.; Maeda, T.; Koike, H.; Kasai, H. Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer 1998, 83, 1483–1488. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Aggarwal, R.; Davidson, B. Intensive follow-up after liver resection for colorectal liver metastases: Results of combined serial tumour marker estimations and computed tomography of the chest and abdomen–a prospective study. Br. J. Cancer 2006, 95, 21–26. [Google Scholar] [CrossRef]
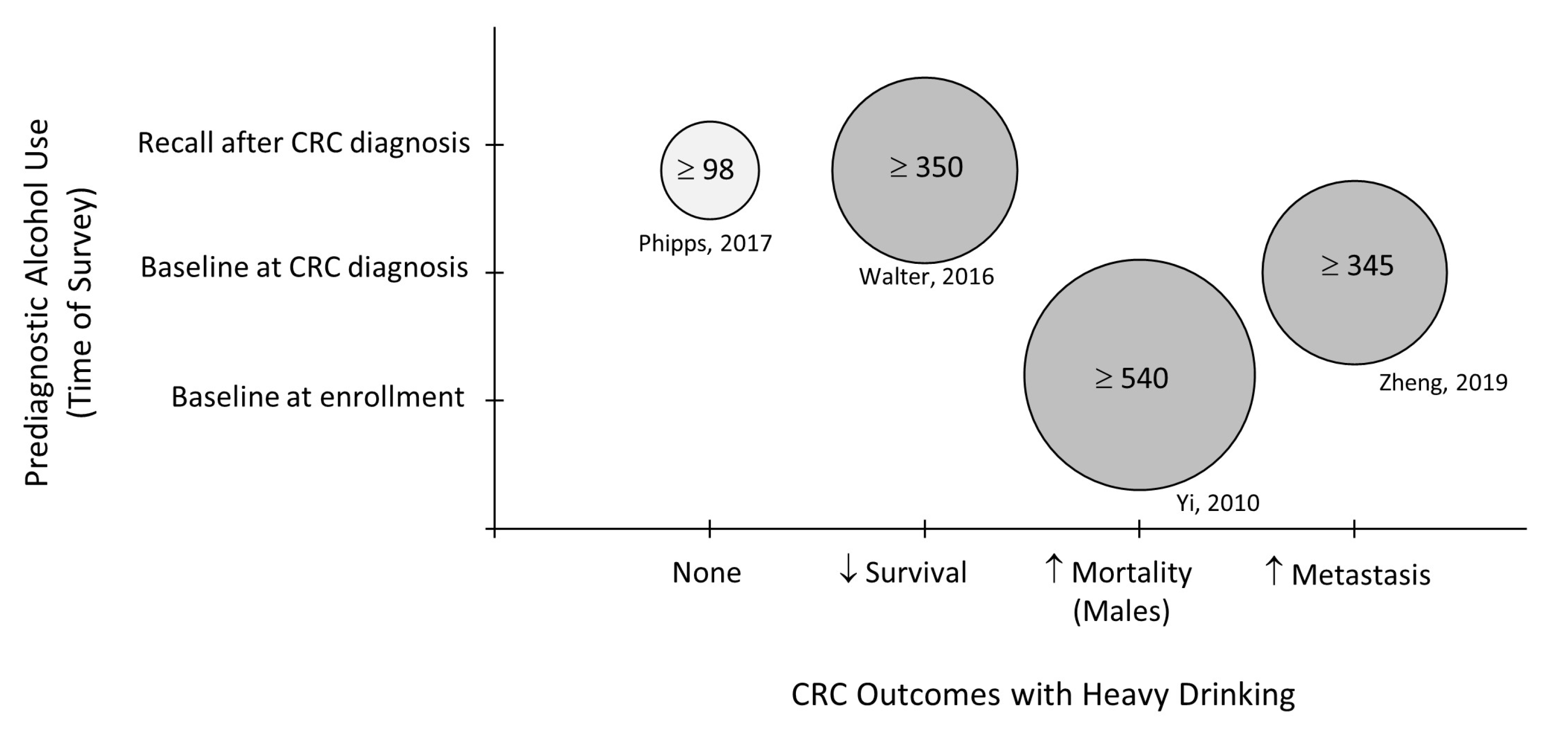
- Walter, V.; Jansen, L.; Ulrich, A.; Roth, W.; Bläker, H.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Alcohol consumption and survival of colorectal cancer patients: A population-based study from Germany. Am. J. Clin. Nutr. 2016, 103, 1497–1506. [Google Scholar] [CrossRef]
- Pelser, C.; Arem, H.; Pfeiffer, R.M.; Elena, J.W.; Alfano, C.M.; Hollenbeck, A.R.; Park, Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014, 120, 1540–1547. [Google Scholar] [CrossRef]
- Zell, J.A.; McEligot, A.J.; Ziogas, A.; Holcombe, R.F.; Anton-Culver, H. Differential effects of wine consumption on colorectal cancer outcomes based on family history of the disease. Nutr. Cancer 2007, 59, 36–45. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Reprint—Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Phipps, A.I.; Robinson, J.R.; Campbell, P.T.; Win, A.K.; Figueiredo, J.C.; Lindor, N.M.; Newcomb, P.A. Prediagnostic alcohol consumption and colorectal cancer survival: The Colon Cancer Family Registry. Cancer 2017, 123, 1035–1043. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Qi, Y.; Chen, L.; Frank, J.A.; Yang, X.H.; Zhang, Z.; Shi, X.; Luo, J. Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Mol. Carcinog. 2016, 55, 1002–1011. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, D.; Cao, R.; Wang, S.; Yu, D.; Liu, Y.; Jiang, Y.; Xu, M.; Luo, J.; Wang, S. Alcohol consumption promotes colorectal carcinoma metastasis via a CCL5-induced and AMPK-pathway-mediated activation of autophagy. Sci. Rep. 2018, 8, 8640. [Google Scholar] [CrossRef]
- Yi, S.-W.; Sull, J.W.; Linton, J.A.; Nam, C.M.; Ohrr, H. Alcohol consumption and digestive cancer mortality in Koreans: The Kangwha Cohort Study. J. Epidemiol. 2010, 20, 204–211. [Google Scholar] [CrossRef]
- Breslow, R.A.; Chen, C.M.; Graubard, B.I.; Mukamal, K.J. Prospective study of alcohol consumption quantity and frequency and cancer-specific mortality in the US population. Am. J. Epidemiol. 2011, 174, 1044–1053. [Google Scholar] [CrossRef]
- Keim, L.M.; Praus, A.W.; Campbell, W.S.; Samson, K.K.; Mohr, A.M.; Tobi, M.; McVicker, B.L. Alcohol Consumption is associated with Increased CEA Levels in Male Patients with Stage IV Colorectal Cancer-A Single-Institution Retrospective Analysis. Grad. Med. Educ. Res. J. 2021, 3, 1–5. [Google Scholar]
- Zheng, K.; Yu, J.; Chen, Z.; Zhou, R.; Lin, C.; Zhang, Y.; Huang, Z.; Yu, L.; Zhao, L.; Wang, Q. Ethanol promotes alcohol-related colorectal cancer metastasis via the TGF-β/RUNX3/Snail axis by inducing TGF-β1 upregulation and RUNX3 cytoplasmic mislocalization. EBioMedicine 2019, 50, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.; De Carli, L. Ethanol dependence and tolerance: A nutritionally controlled experimental model in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1973, 6, 983–991. [Google Scholar] [PubMed]
- Im, H.J.; Kim, H.G.; Lee, J.S.; Kim, H.S.; Cho, J.H.; Jo, I.J.; Park, S.J.; Son, C.G. A Preclinical Model of Chronic Alcohol Consumption Reveals Increased Metastatic Seeding of Colon Cancer Cells in the Liver. Cancer Res. 2016, 76, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.M.; Gould, J.J.; Kubik, J.L.; Talmon, G.A.; Casey, C.A.; Thomas, P.; Tuma, D.J.; McVicker, B.L. Enhanced colorectal cancer metastases in the alcohol-injured liver. Clin. Exp. Metastasis 2017, 34, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.V.; Miller, H.A.; Mahlbacher, G.E.; Saforo, D.; Beverly, L.J.; Arteel, G.E.; Frieboes, H.B. Computational/experimental evaluation of liver metastasis post hepatic injury: Interactions with macrophages and transitional ECM. Sci. Rep. 2019, 9, 15077. [Google Scholar] [CrossRef]
- Cernigliaro, C.; D’Anneo, A.; Carlisi, D.; Giuliano, M.; Marino Gammazza, A.; Barone, R.; Longhitano, L.; Cappello, F.; Emanuele, S.; Distefano, A. Ethanol-mediated stress promotes autophagic survival and aggressiveness of colon cancer cells via activation of Nrf2/HO-1 pathway. Cancers 2019, 11, 505. [Google Scholar] [CrossRef]
- Young, M.; Russell, W.T. An Investigation into the Statistics of Cancer in Different Trades and Professions; Medical Research Council Special Report Series, No. 99; Her Majesty’s Stationery Office: London, UK, 1926. [Google Scholar]
- International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 96, p. 3.
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
- Nelson, D.E.; Jarman, D.W.; Rehm, J.; Greenfield, T.K.; Rey, G.; Kerr, W.C.; Miller, P.; Shield, K.D.; Ye, Y.; Naimi, T.S. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am. J. Public Health 2013, 103, 641–648. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, H.; Yang, H.; Lin, J. A pooled analysis of alcohol intake and colorectal cancer. Int. J. Clin. Exp. Med. 2015, 8, 6878. [Google Scholar]
- Paschos, K.A.; Majeed, A.W.; Bird, N.C. Natural history of hepatic metastases from colorectal cancer-pathobiological pathways with clinical significance. World J. Gastroenterol. 2014, 20, 3719. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual dimorphism in colon cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef]
- Northcote, J.; Livingston, M. Accuracy of self-reported drinking: Observational verification of ‘last occasion’ drink estimates of young adults. Alcohol Alcohol 2011, 46, 709–713. [Google Scholar] [CrossRef]
- Bloomfield, K.; Stockwell, T.; Gmel, G.; Rehn, N. International comparisons of alcohol consumption. Alcohol Res. Health 2003, 27, 95. [Google Scholar]
- Castaldelli-Maia, J.M.; Segura, L.E.; Martins, S.S. The concerning increasing trend of alcohol beverage sales in the US during the COVID-19 pandemic. Alcohol 2021, 96, 37–42. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| English language studies | Non-English |
| Studies from 2010 to 2021 | Studies published prior to 2010 |
| Alcohol | Reviews and meta-analyses |
| Alcohol-associated liver disease | Reports with overlapping data |
| Colorectal cancer mortality | Overall survival |
| Colorectal cancer liver metastasis | Exclusion of Stage IV CRC data |
| Clinical-based studies (prospective, retrospective, population studies) | No access to full text |
| Preclinical studies (animal, cell culture) | |
| Access to full text |
| Reference | Country | Study Design | Subject (No.) | Sex (% Male) | Alcohol Use | Outcomes |
|---|---|---|---|---|---|---|
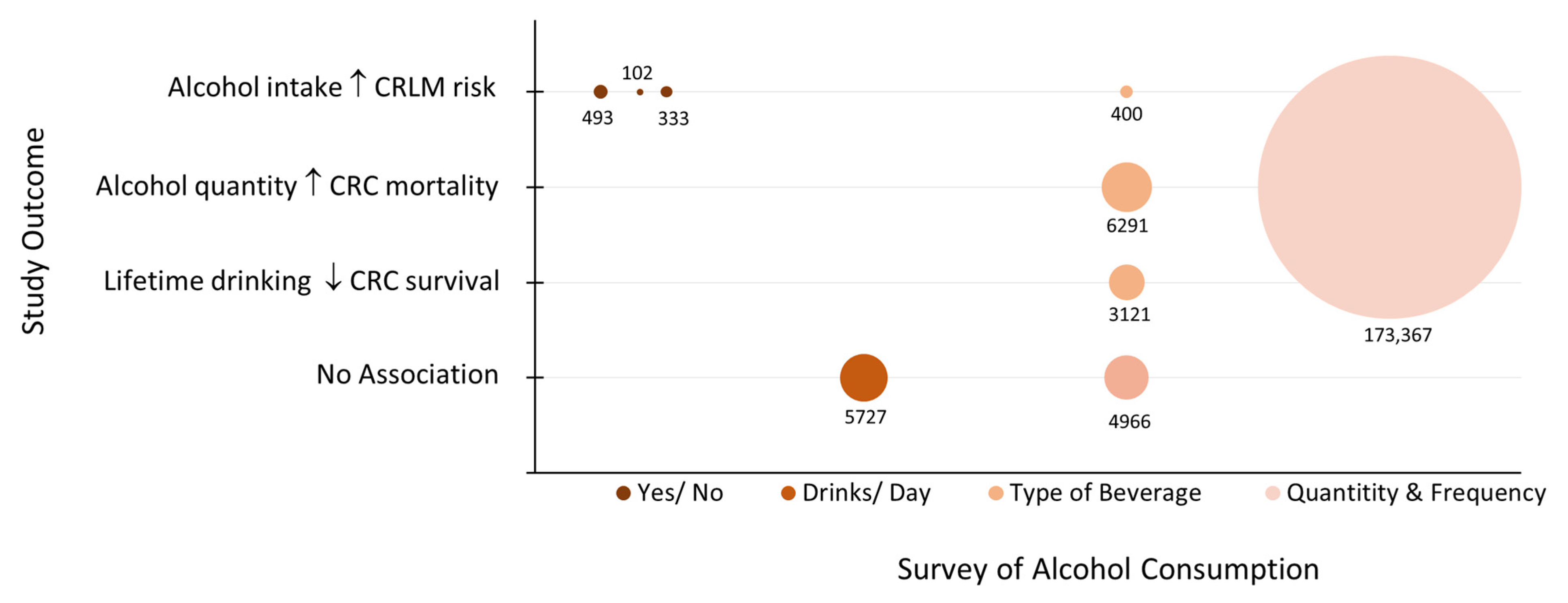
| Yi, 2010 [19] | Korea | Prospective Cohort | 6291 | 42.8 | Grams/week low to high Beverage type | Risk of CRC mortality postively associates with increasing alcohol consumption in men. |
| Breslow, 2011 [20] | USA | Prospective Pooled Survey Data | 173,367 a | 52.3 | Drinks/day Frequency Light to heavy | Higher-quantity drinking associates with increased risk of CRC mortality in women. |
| Dong, 2013 [9] | China | Retrospective | 493 | 63.4 | Yes/No | Alcohol use is an independent risk factor for CRLM. |
| Pelser, 2014 [13] | USA | Retrospective | 5727 | 68.4 | Drinks/day Moderate to Heavy | No association between alcohol intake and CRC mortality. |
| Walter, 2016 [12] | Germany | Prospective Cohort | 3121 | 59.4 | Grams/day Light to heavy Beverage type | Lifetime heavy drinking associates with poorer CRC survival. |
| Phipps, 2017 [16] | USA Canada, Australia | Prospective Pooled Survey Data | 4966 | 52.0 | Servings/week Beverage type | No associatation between beer or liquor consumption and CRC survival. |
| Zhao, 2017 [18] | USA, China | Retrospective | 102 | 67.0 | Yes/No | Alcohol use increases risk of advanced TNM stage, metastasis, and poorer prognosis. |
| Zheng, 2019 [22] | China | Retrospective | 400 | 61.5 | Grams/week Beverage type | Higher and frequent alcohol intake associates with CRC metastasis. |
| Keim, 2021 [21] | USA | Retrospective | 333 | 58.0 | Yes/No | Alcohol use in males associates with the risk of advanced CRC disease. |
| Reference | Experimental Model | Species/CRC Cell | Alcohol Treatment | Outcomes |
|---|---|---|---|---|
| Im, 2016 [24] | Animal | C57BL/6 mice MC38 | Water/20% alcohol, 4–7 wks | Alcohol accelerates CRLM; alcohol altered hepatic microenvironment, inactivated immune surveillance. |
| Xu, 2016 [17] | Animal Cell culture | Balb/c mice HCT116, DLD-1, HT29, SW480 | Water/2% alcohol, 4 wks 100–400 mg/dl ethanol | Alcohol increases the migration, metastasis of CRC cells; modulation of GSK3β/β-catenin/MCP-1 pathway. |
| Mohr, 2017 [25] | Animal | Rag1 KO mice LS174T | Lieber–DeCarli diet, 4–9 wks | Enhanced rate and burden of CRLM in alcohol-affected livers; CEA-mediated inflammatory mechanisms. |
| Zhao, 2017 [18] | Human tissue Cell culture | CRC biopsies HT29, DLD-1, RKO, SW480 | Abstainers vs. drinkers 200 mg/dl ethanol | Alcohol use increases CCL5 expression in patient tumor tissue; CCL5 mediates CRC cell migration via autophagy and AMPK signaling. |
| Cernigliaro, 2019 [27] | Cell culture | HCT116, HT29, Caco-2 | 30–300 mM ethanol | Ethanol treatment leads to activation of Nrf2/HO-1 pathway; CRC cell survival and aggressive phenotype. |
| Hudson, 2019 [26] | Animal | C57BL/6 mice | Lieber–DeCarli diet, 6 wks | Modeling of M2 macrophages and ECM in metastasis. |
| Zheng, 2019 [22] | Animal Cell culture | Balb/c mice HT29, HCT116, LS174T, RKO, SW620,CT26 | Lieber–DeCarli diet 100–200 mg/dl ethanol | Alcohol promotes CRLM via the TGF-β/RUNX3/Snail axis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, R.; Zakaria, J.; Glenn, E.; Richard, H.; Rimawi, A.; Tobi, M.; McVicker, B. Alcohol Use and the Risk of Colorectal Liver Metastasis: A Systematic Mapping Review. Biology 2023, 12, 257. https://doi.org/10.3390/biology12020257
Sapkota R, Zakaria J, Glenn E, Richard H, Rimawi A, Tobi M, McVicker B. Alcohol Use and the Risk of Colorectal Liver Metastasis: A Systematic Mapping Review. Biology. 2023; 12(2):257. https://doi.org/10.3390/biology12020257
Chicago/Turabian StyleSapkota, Roshan, Joseph Zakaria, Emily Glenn, Heather Richard, Ahmad Rimawi, Martin Tobi, and Benita McVicker. 2023. "Alcohol Use and the Risk of Colorectal Liver Metastasis: A Systematic Mapping Review" Biology 12, no. 2: 257. https://doi.org/10.3390/biology12020257
APA StyleSapkota, R., Zakaria, J., Glenn, E., Richard, H., Rimawi, A., Tobi, M., & McVicker, B. (2023). Alcohol Use and the Risk of Colorectal Liver Metastasis: A Systematic Mapping Review. Biology, 12(2), 257. https://doi.org/10.3390/biology12020257






