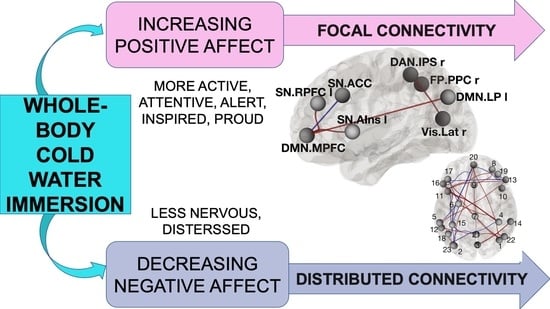
Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participants
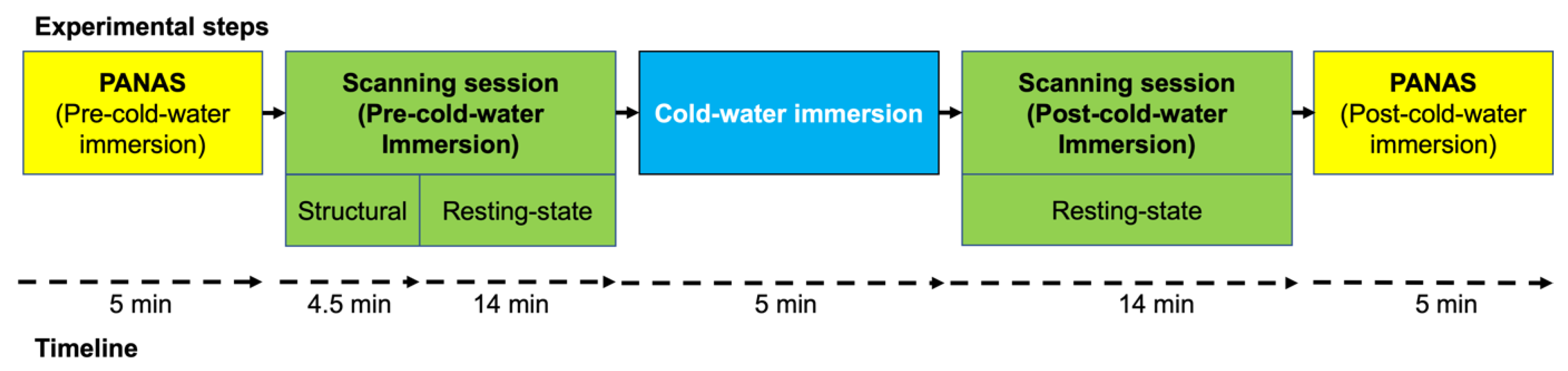
2.3. Experimental Design and Procedures
2.4. Instruments and Measurements
2.4.1. Positive and Negative Affect Schedule (PANAS)
2.4.2. Controlling Physiological Responses to Cold-Water Immersion
2.4.3. MRI Acquisition
2.5. Data Analysis
2.5.1. PANAS
2.5.2. Measuring Physiological Responses of Cold-water Immersion
2.5.3. fMRI Data Pre-Processing
2.5.4. fMRI Data Denoising
3. Results
3.1. Increased Cardio-Respiratory Activity Resulting from CWI
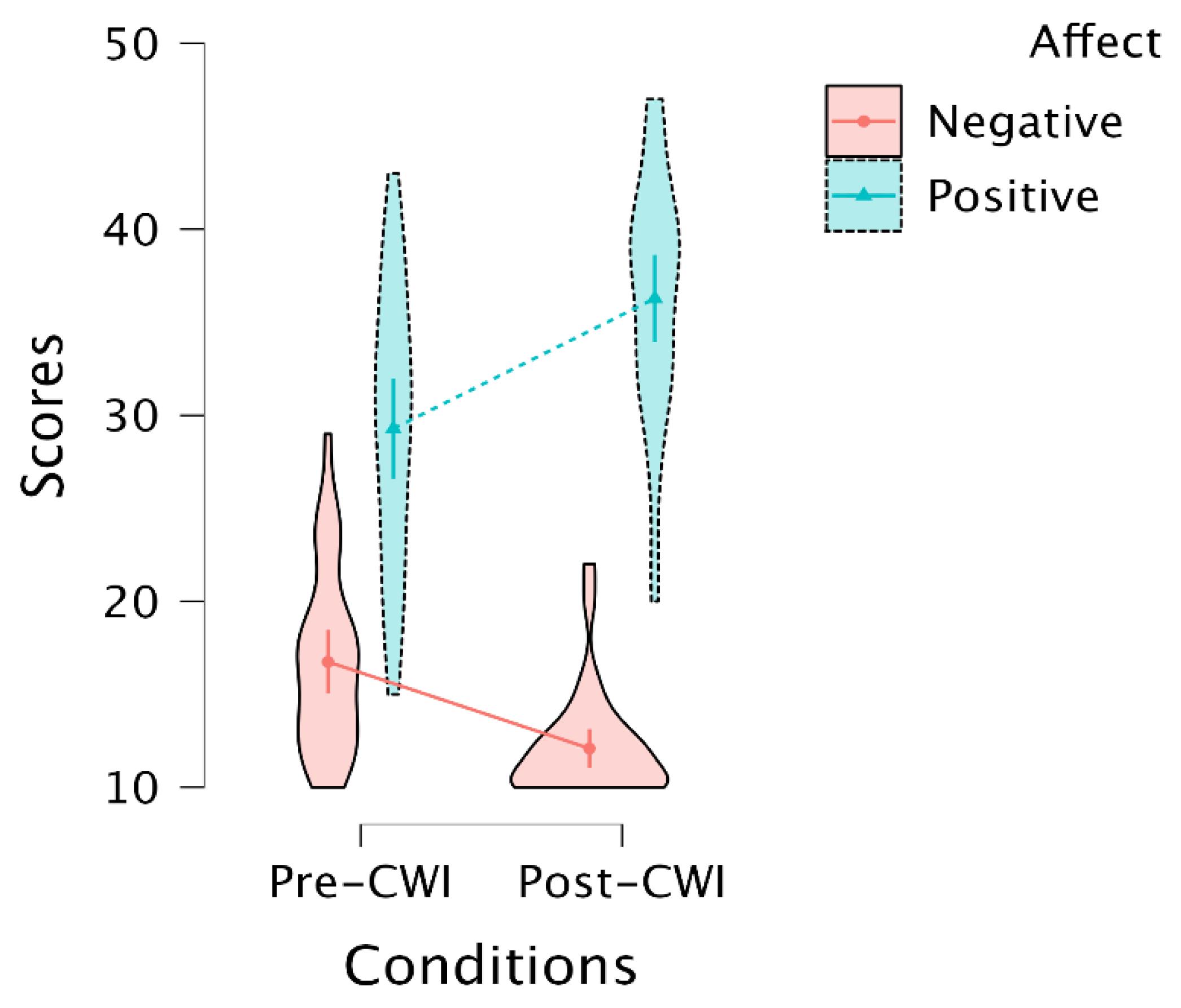
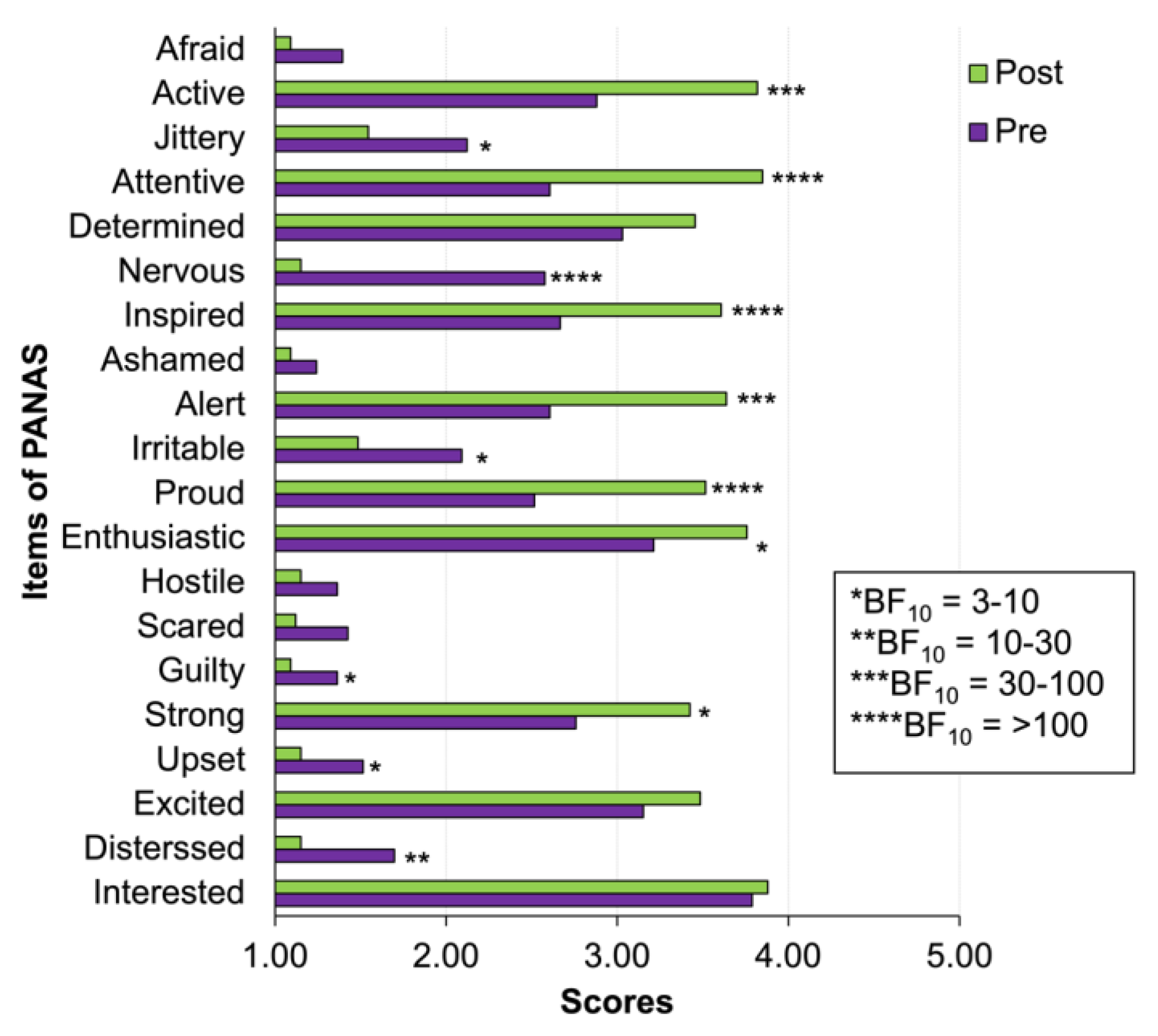
3.2. Self-Report of Increasing Positive and Decreasing Negative Affect after CWI
3.3. Changes in Functional Connections in Post-CWI Compared to Pre-CWI
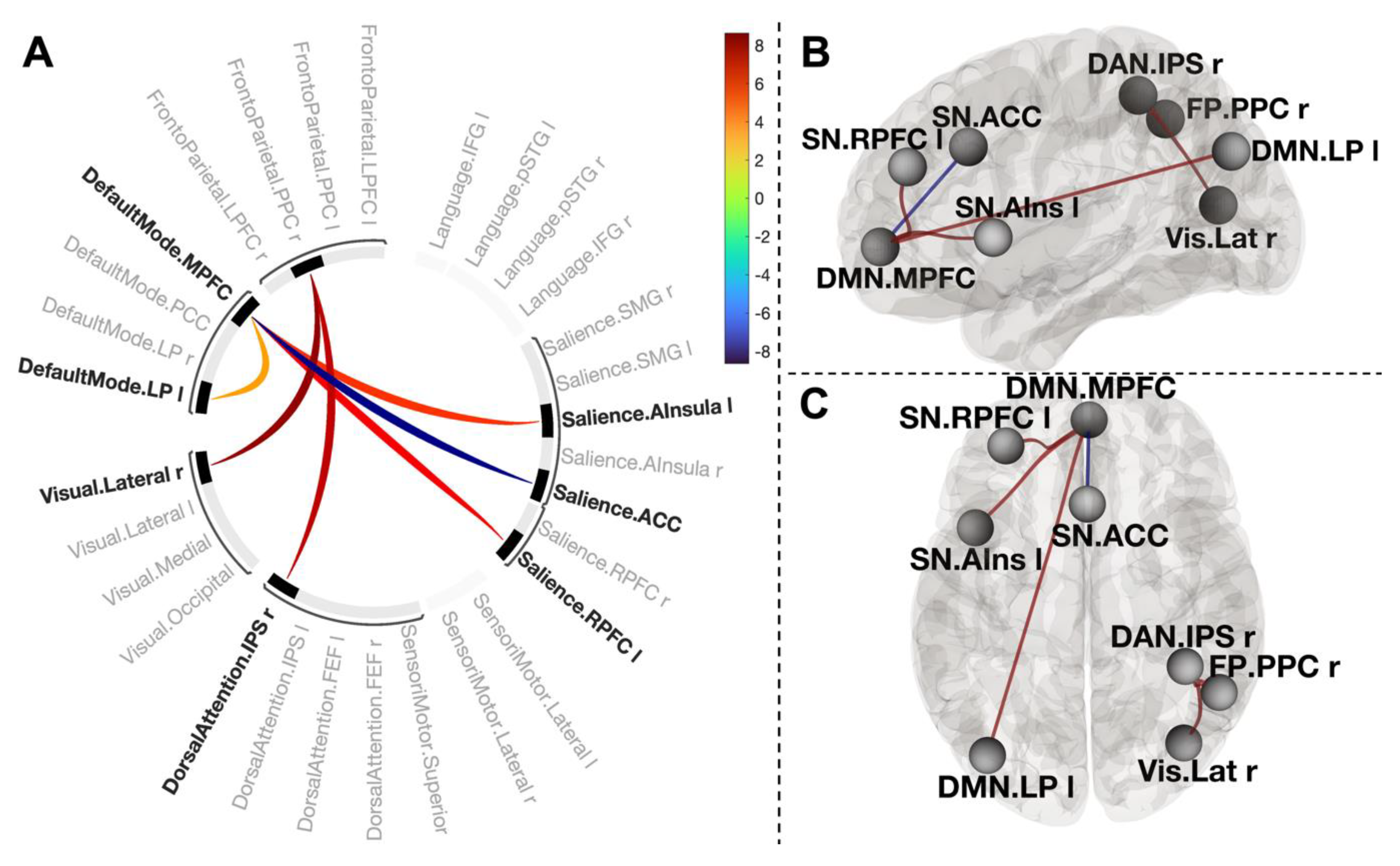
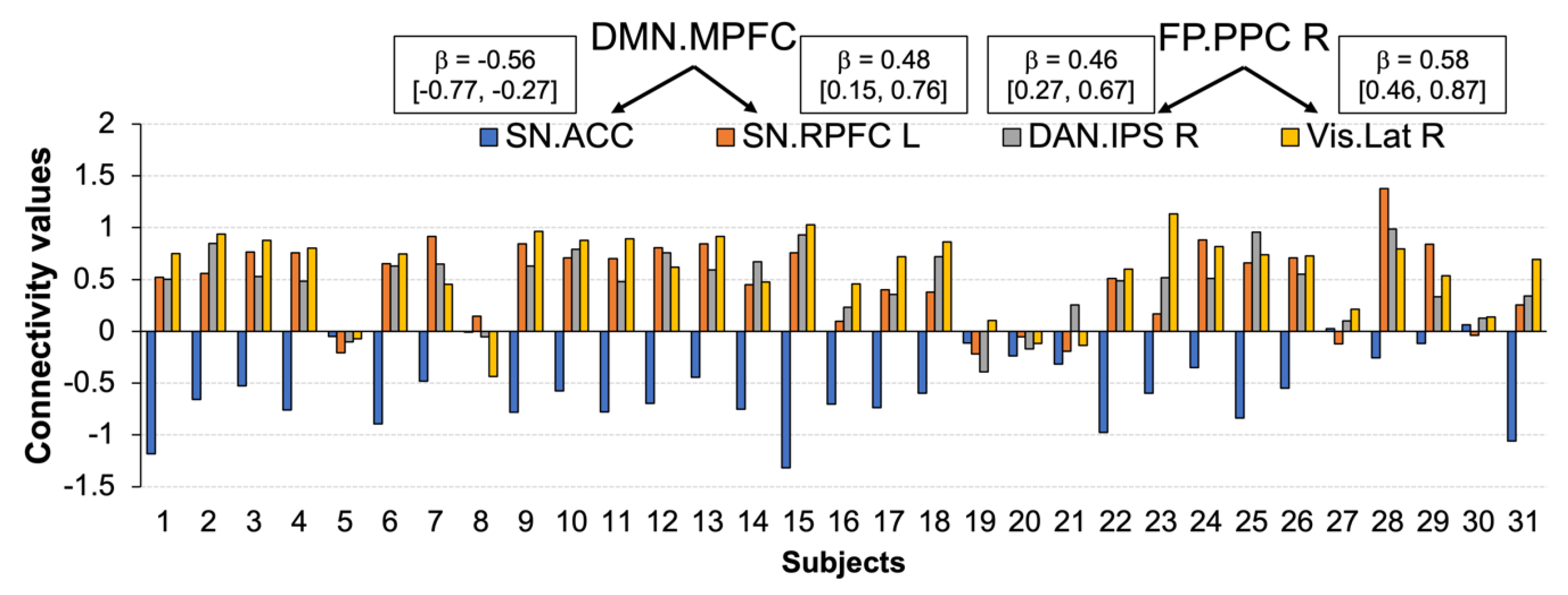
3.4. Functional Connections Explaining Enhanced Positive Affect after CWI
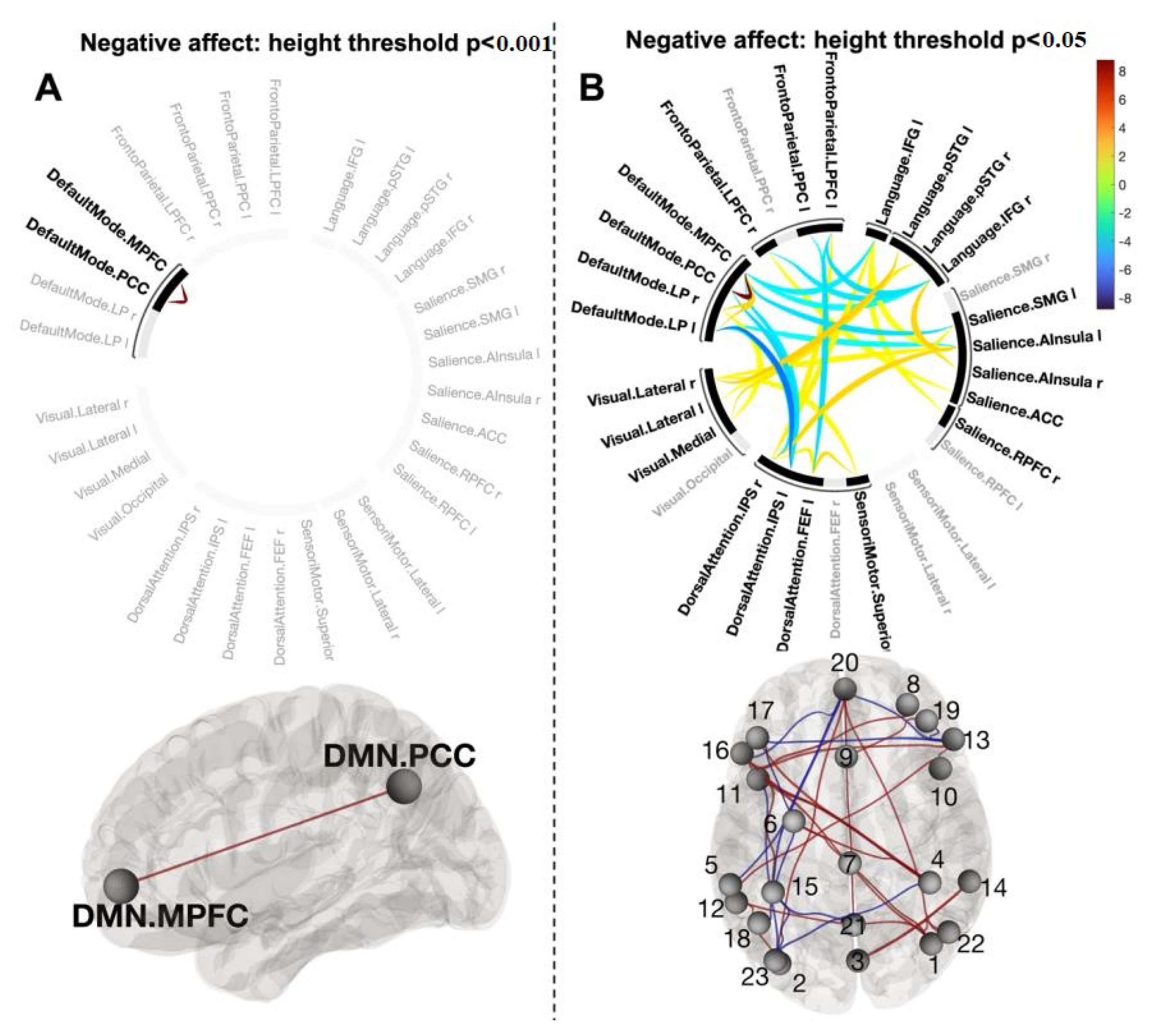
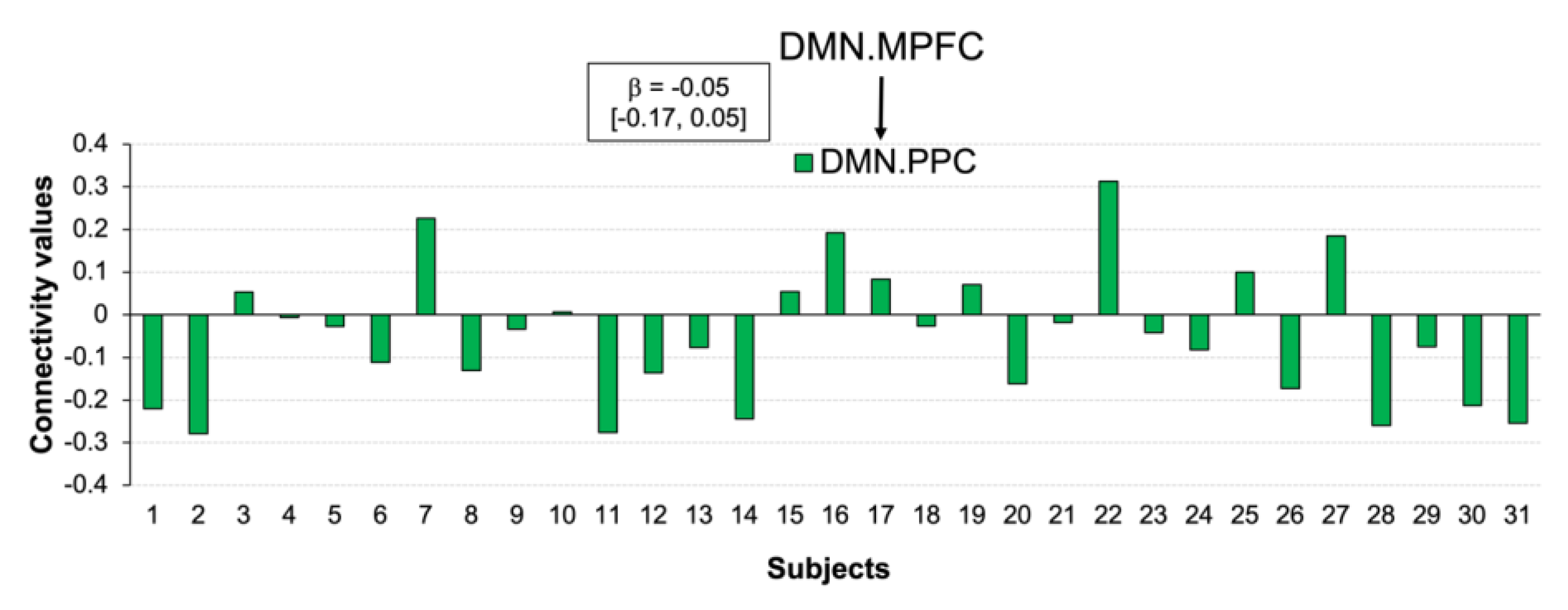
3.5. Functional Connections Explaining Reducing Negative Affect after CWI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, S.; Turner, Y. Trends in Outdoor Swimming. Second Edition. 2022. Available online: https://www.outdoorswimmershop.com/products/outdoor-swimmer-trends-report-22 (accessed on 18 December 2022).
- Tipton, M.J.; Collier, N.; Massey, H.; Corbett, J.; Harper, M. Cold water immersion: Kill or cure? Exp. Physiol. 2017, 102, 1335–1355. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Waśkiewicz, Z.; Sousa, C.V.; Hill, L.; Nikolaidis, P.T. Cold Water Swimming—Benefits and Risks: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 8984. [Google Scholar] [CrossRef]
- Demori, I.; Piccinno, T.; Saverino, D.; Luzzo, E.; Ottoboni, S.; Serpico, D.; Chiera, M.; Giuria, R. Effects of winter sea bathing on psychoneuroendocrinoimmunological parameters. Explore 2021, 17, 122–126. [Google Scholar] [CrossRef]
- Bleakley, C.M.; Davison, G.W. What is the biochemical and physiological rationale for using cold-water immersion in sports recovery? A systematic review. Br. J. Sports Med. 2010, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Han, H.S.; Cheng, D.; Sun, G.H.; Yenari, M.A. Mild Hypothermia Inhibits Inflammation After Experimental Stroke and Brain Inflammation. Stroke 2003, 34, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.J.; Nadler, E.; Busch, C. Effects of hot and cold temperature exposure on performance: A meta-analytic review. Ergonomics 2002, 45, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Delahunty, E.T.; Bisset, L.M.; Kavanagh, J. Intracortical motor networks are affected in both the contralateral and ipsilateral hemisphere during single limb cold water immersion. Exp. Physiol. 2019, 104, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Van Tulleken, C.; Tipton, M.; Massey, H.; Harper, C.M. Open water swimming as a treatment for major depressive disorder. BMJ Case Rep. 2018, 2018, bcr2018225007. [Google Scholar] [CrossRef] [PubMed]
- Foley, R. Swimming in Ireland: Immersions in therapeutic blue space. Health Place 2015, 35, 218–225. [Google Scholar] [CrossRef]
- Huttunen, P.; Kokko, L.; Ylijukuri, V. Winter swimming improves general well-being. Int. J. Circumpolar Health 2004, 63, 140–144. [Google Scholar] [CrossRef]
- Massey, H.; Kandala, N.; Davis, C.; Harper, M.; Gorczynski, P.; Denton, H. Mood and well-being of novice open water swimmers and controls during an introductory outdoor swimming programme: A feasibility study. Lifestyle Med. 2020, 1, e12. [Google Scholar] [CrossRef]
- Kelly, J.S.; Bird, E. Improved mood following a single immersion in cold water. Lifestyle Med. 2021, 3, e53. [Google Scholar] [CrossRef]
- Hirvonen, J.; Lindeman, S.; Joukamaa, M.; Huttunen, P. Plasma catecholamines, serotonin and their metabolites and beta-endorphin of winter swimmers during one winter. Possible correlations to psychological traits. Int. J. Circumpolar Health 2002, 61, 363–372. [Google Scholar] [CrossRef]
- Gu, S.; Wang, W.; Wang, F.; Huang, J.H. Neuromodulator and Emotion Biomarker for Stress Induced Mental Disorders. Neural Plast. 2016, 2016, 2609128. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Lesch, K.-P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.-H. Stress and the dopaminergic reward system. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Lin, S.-H.; Lee, L.-T.; Yang, Y.K. Serotonin and Mental Disorders: A Concise Review on Molecular Neuroimaging Evidence. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2014, 12, 196–202. [Google Scholar] [CrossRef]
- Jarrahi, B.; Martucci, K.T.; Nilakantan, A.S.; Mackey, S. Investigating the BOLD spectral power of the intrinsic connectivity networks in fibromyalgia patients: A resting-state fMRI study. Annu. Int. Conf. IEEE Eng. Med. Biol. 2017, 2017, 497–500. [Google Scholar] [CrossRef]
- Bitar, N.; Dugré, J.R.; Marchand, S.; Potvin, S. Medial Orbitofrontal De-Activation During Tonic Cold Pain Stimulation: A fMRI Study Examining the Opponent-Process Theory. J. Pain Res. 2020, 13, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Grouper, H.; Löffler, M.; Flor, H.; Eisenberg, E.; Pud, D. Increased functional connectivity between limbic brain areas in healthy individuals with high versus low sensitivity to cold pain: A resting state fMRI study. PLoS ONE 2022, 17, e0267170. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, K.A.; Satpute, A.B.; Wager, T.D.; Weber, J.; Barrett, L.F. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb. Cortex 2016, 26, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.J.; Gollan, J.; Berntson, G.G.; Cacioppo, J.T. The current status of research on the structure of evaluative space. Biol. Psychol. 2010, 84, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.; Russell, J. Independence and bipolarity in the structure of current affect. J. Personal. Soc. Psychol. 1998, 74, 967–984. [Google Scholar] [CrossRef]
- Lewis, P.; Critchley, H.; Rotshtein, P.; Dolan, R. Neural Correlates of Processing Valence and Arousal in Affective Words. Cereb. Cortex 2007, 17, 742–748. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Amunts, K. A short review on emotion processing: A lateralized network of neuronal networks. Brain Struct. Funct. 2021, 227, 673–684. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Barrett, L.F. A functional architecture of the human brain: Emerging insights from the science of emotion. Trends Cogn. Sci. 2012, 16, 533–540. [Google Scholar] [CrossRef]
- Rueschkamp, J.M.G.; Brose, A.; Villringer, A.; Gaebler, M. Neural correlates of up-regulating positive emotions in fMRI and their link to affect in daily life. Soc. Cogn. Affect. Neurosci. 2019, 14, 1049–1059. [Google Scholar] [CrossRef]
- Cole, M.W.; Bassett, D.S.; Power, J.D.; Braver, T.S.; Petersen, S.E. Intrinsic and Task-Evoked Network Architectures of the Human Brain. Neuron 2014, 83, 238–251. [Google Scholar] [CrossRef]
- Kieliba, P.; Madugula, S.; Filippini, N.; Duff, E.P.; Makin, T.R. Large-scale intrinsic connectivity is consistent across varying task demands. PLoS ONE 2019, 14, e0213861. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.W.; Ito, T.; Bassett, D.S.; Schultz, D.H. Activity flow over resting-state networks shapes cognitive task activations. Nat. Neurosci. 2016, 19, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, S.; Poline, J.-B.; Kleinschmidt, A.; D’Esposito, M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc. Natl. Acad. Sci. USA 2015, 112, 8463–8468. [Google Scholar] [CrossRef] [PubMed]
- Rohr, C.S.; Okon-Singer, H.; Craddock, C.; Villringer, A.; Margulies, D.S. Affect and the Brain’s Functional Organization: A Resting-State Connectivity Approach. PLOS ONE 2013, 8, e68015. [Google Scholar] [CrossRef]
- Balaev, V.; Orlov, I.; Petrushevsky, A.; Martynova, O. Functional connectivity between salience, default mode and frontoparietal networks in post-stroke depression. J. Affect. Disord. 2018, 227, 554–562. [Google Scholar] [CrossRef]
- Rai, S.; Griffiths, K.R.; Breukelaar, I.A.; Barreiros, A.R.; Chen, W.; Boyce, P.; Hazell, P.; Foster, S.L.; Malhi, G.S.; Harris, A.W.F.; et al. Default-mode and fronto-parietal network connectivity during rest distinguishes asymptomatic patients with bipolar disorder and major depressive disorder. Transl. Psychiatry 2021, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Jao, T.; Vértes, P.E.; Alexander-Bloch, A.F.; Tang, I.-N.; Yu, Y.-C.; Chen, J.-H.; Bullmore, E.T. Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage 2013, 69, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Bagiella, E.; Sloan, R.P.; Heitjan, D.F. Mixed-effects models in psychophysiology. Psychophysiology 2000, 37, 13–20. [Google Scholar] [CrossRef]
- Šrámek, P.; Šimečková, M.; Janský, L.; Šavlíková, J.; Vybíral, S. Human physiological responses to immersion into water of different temperatures. Eur. J. Appl. Physiol. 2000, 81, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Eimonte, M.; Paulauskas, H.; Daniuseviciute, L.; Eimantas, N.; Vitkauskiene, A.; Dauksaite, G.; Solianik, R.; Brazaitis, M. Residual effects of short-term whole-body cold-water immersion on the cytokine profile, white blood cell count, and blood markers of stress. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2021, 38, 696–707. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Siegel, J.S.; Power, J.D.; Dubis, J.W.; Vogel, A.C.; Church, J.A.; Schlaggar, B.L.; Petersen, S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014, 35, 1981–1996. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020. [Google Scholar]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. Neuroimage 2010, 53, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Zalesky, A.; Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Tipton, M. Respiratory responses to cold water immersion: Neural pathways, interactions, and clinical consequences awake and asleep. J. Appl. Physiol. 2006, 100, 2057–2064. [Google Scholar] [CrossRef]
- Button, C.; Croft, J.L.; Cotter, J.D.; Graham, M.J.; Lucas, S.J. Integrative physiological and behavioural responses to sudden cold-water immersion are similar in skilled and less-skilled swimmers. Physiol. Behav. 2015, 138, 254–259. [Google Scholar] [CrossRef]
- Targum, S.D.; Fava, M. Fatigue as a residual symptom of depression. Innov. Clin. Neurosci. 2011, 8, 40–43. [Google Scholar]
- Massey, H.; Gorczynski, P.; Harper, C.M.; Sansom, L.; McEwan, K.; Yankouskaya, A.; Denton, H. Perceived Impact of Outdoor Swimming on Health: Web-Based Survey. Interact. J. Med Res. 2022, 11, e25589. [Google Scholar] [CrossRef]
- Fredrickson, B.L. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. Am. Psychol. 2001, 56, 218–226. [Google Scholar] [CrossRef]
- Schmukle, S.C.; Egloff, B.; Burns, L.R. The relationship between positive and negative affect in the Positive and Negative Affect Schedule. J. Res. Pers. 2002, 36, 463–475. [Google Scholar] [CrossRef]
- Yankouskaya, A.; Sui, J. Self-prioritization is supported by interactions between large-scale brain networks. Eur. J. Neurosci. 2022, 55, 1244–1261. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Fink, G.R.; Chau, P.M.-L.; Dolan, R.J. Neural activation during selective attention to subjective emotional responses. Neuroreport 1997, 8, 3969–3972. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020–11025. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef]
- Wrege, J.S.; Ruocco, A.C.; Euler, S.; Preller, K.H.; Busmann, M.; Meya, L.; Schmidt, A.; Lang, U.E.; Borgwardt, S.; Walter, M. Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cogn. Affect. Behav. Neurosci. 2019, 19, 1273–1285. [Google Scholar] [CrossRef]
- Yoshimura, S.; Okamoto, Y.; Onoda, K.; Matsunaga, M.; Ueda, K.; Suzuki, S.-I.; Yamawaki, S. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J. Affect. Disord. 2010, 122, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Yue, Z. Positive Emotion Facilitates Cognitive Flexibility: An fMRI Study. Front. Psychol. 2017, 8, 1832. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, L.; Xu, J.; Evans, A.C.; Fan, L.; Ge, H.; Tang, Y.; Khundrakpam, B.; Wang, J.; Liu, S. Anatomical Substrates of the Alerting, Orienting and Executive Control Components of Attention: Focus on the Posterior Parietal Lobe. PLOS ONE 2012, 7, e50590. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, E.; Laufs, H. Decoding Wakefulness Levels from Typical fMRI Resting-State Data Reveals Reliable Drifts between Wakefulness and Sleep. Neuron 2014, 82, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. The mathematical theory of communication. 1963. M.D. Comput. Comput. Med. Pract. 1997, 14, 306–317. [Google Scholar]
- Ursino, M.; Ricci, G.; Magosso, E. Transfer Entropy as a Measure of Brain Connectivity: A Critical Analysis with the Help of Neural Mass Models. Front. Comput. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, L.; Tuzhilina, E.; Glasser, M.F.; Hastie, T.J.; Williams, L.M. Relating whole-brain functional connectivity to self-reported negative emotion in a large sample of young adults using group regularized canonical correlation analysis. Neuroimage 2021, 237, 118137. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Yeo, B.T.T.; Spreng, R.N. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 2019, 32, 926–942. [Google Scholar] [CrossRef]
- Li, J.; Curley, W.H.; Guerin, B.; Dougherty, D.D.; Dalca, A.V.; Fischl, B.; Horn, A.; Edlow, B.L. Mapping the subcortical connectivity of the human default mode network. Neuroimage 2021, 245, 118758. [Google Scholar] [CrossRef]
- Kim, J.H.; Taylor, A.J.; Himmelbach, M.; Hagberg, G.E.; Scheffler, K.; Ress, D. Characterization of the blood oxygen level dependent hemodynamic response function in human subcortical regions with high spatiotemporal resolution. Front. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Noble, S.; Spann, M.N.; Tokoglu, F.; Shen, X.; Constable, R.T.; Scheinost, D. Influences on the Test–Retest Reliability of Functional Connectivity MRI and its Relationship with Behavioral Utility. Cereb. Cortex 2017, 27, 5415–5429. [Google Scholar] [CrossRef]
- Shah, L.M.; Cramer, J.A.; Ferguson, M.A.; Birn, R.M.; Anderson, J.S. Reliability and reproducibility of individual differences in functional connectivity acquired during task and resting state. Brain Behav. 2016, 6, e00456. [Google Scholar] [CrossRef]
- Birn, R.M.; Molloy, E.K.; Patriat, R.; Parker, T.; Meier, T.B.; Kirk, G.R.; Nair, V.A.; Meyerand, M.E.; Prabhakaran, V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 2013, 83, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Horien, C.; Shen, X.; Scheinost, D.; Constable, R.T. The individual functional connectome is unique and stable over months to years. Neuroimage 2019, 189, 676–687. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankouskaya, A.; Williamson, R.; Stacey, C.; Totman, J.J.; Massey, H. Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks. Biology 2023, 12, 211. https://doi.org/10.3390/biology12020211
Yankouskaya A, Williamson R, Stacey C, Totman JJ, Massey H. Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks. Biology. 2023; 12(2):211. https://doi.org/10.3390/biology12020211
Chicago/Turabian StyleYankouskaya, Ala, Ruth Williamson, Cameron Stacey, John James Totman, and Heather Massey. 2023. "Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks" Biology 12, no. 2: 211. https://doi.org/10.3390/biology12020211
APA StyleYankouskaya, A., Williamson, R., Stacey, C., Totman, J. J., & Massey, H. (2023). Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks. Biology, 12(2), 211. https://doi.org/10.3390/biology12020211