The Impact of Long-Term Clinoptilolite Administration on the Concentration Profile of Metals in Rodent Organisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zeolites
2.2. Animals
2.3. Drugs and Treatment Schedule
- Group 1 (n = 10): I (received drinking water);
- Group 2 (n = 10): TMAZ;
- Group 3 (n = 10): PMA zeolite;
- Group 4 (n = 10): colloidal silica (Ludox AS-40).
2.4. Tissue Sampling
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.6. Statistical Analysis
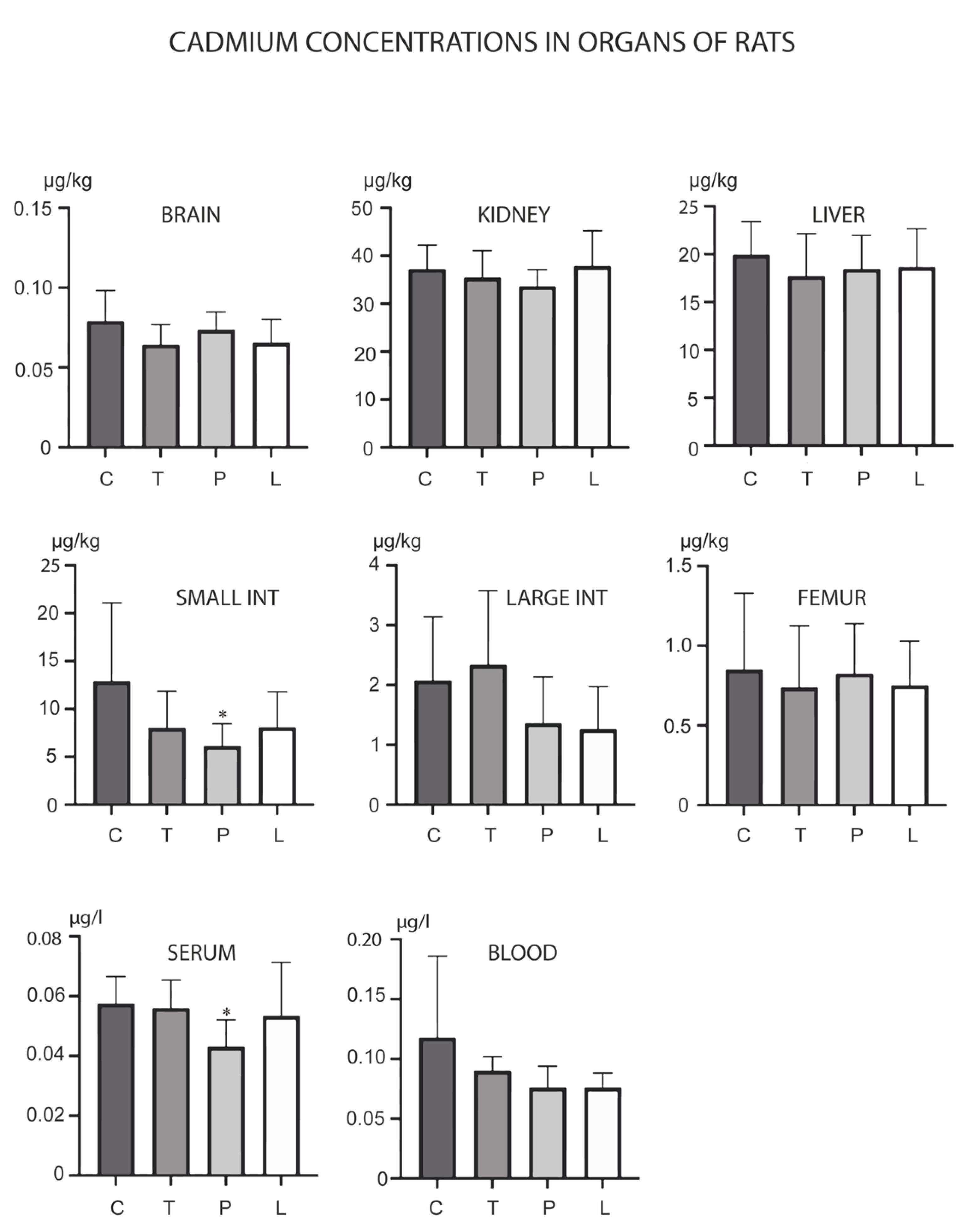
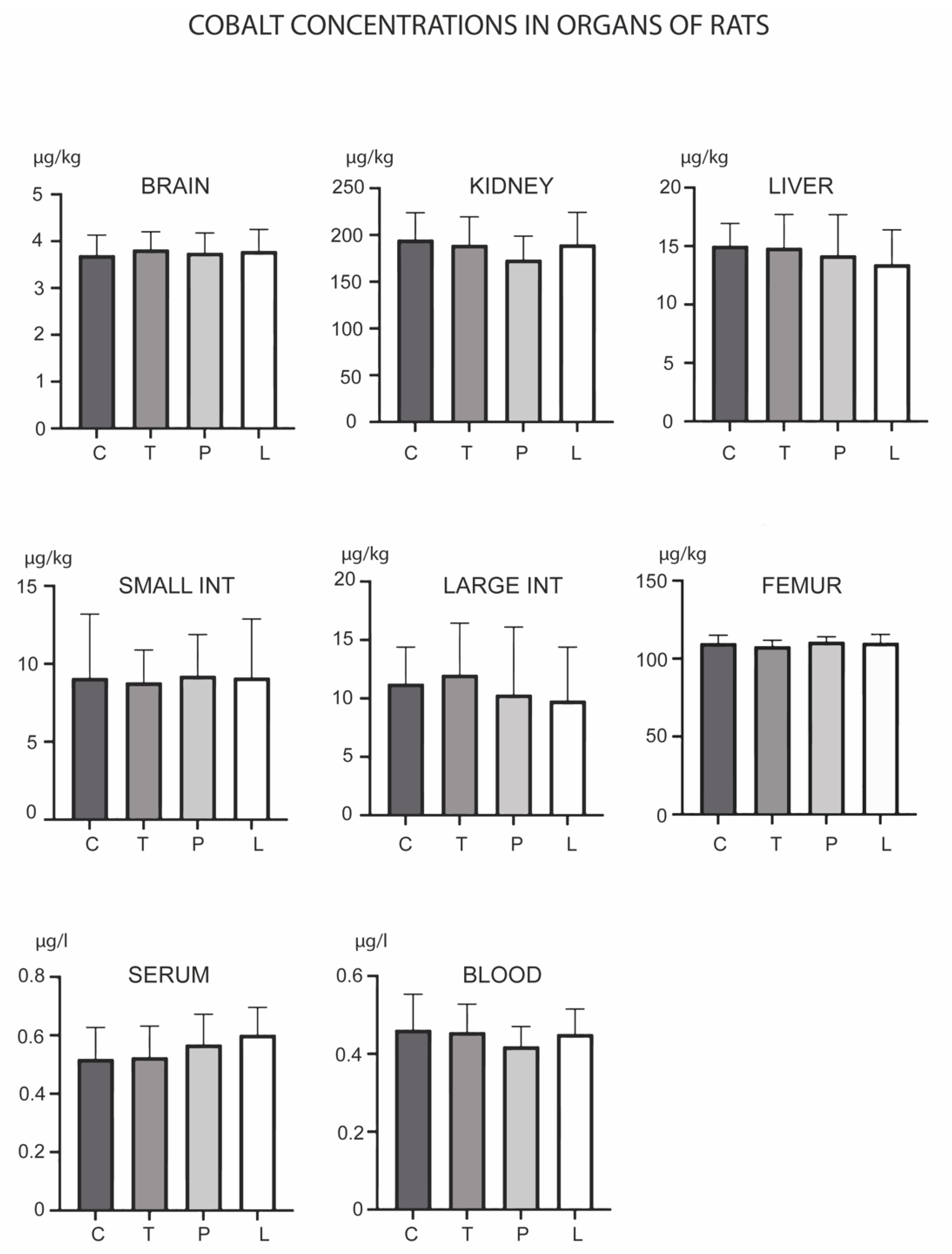
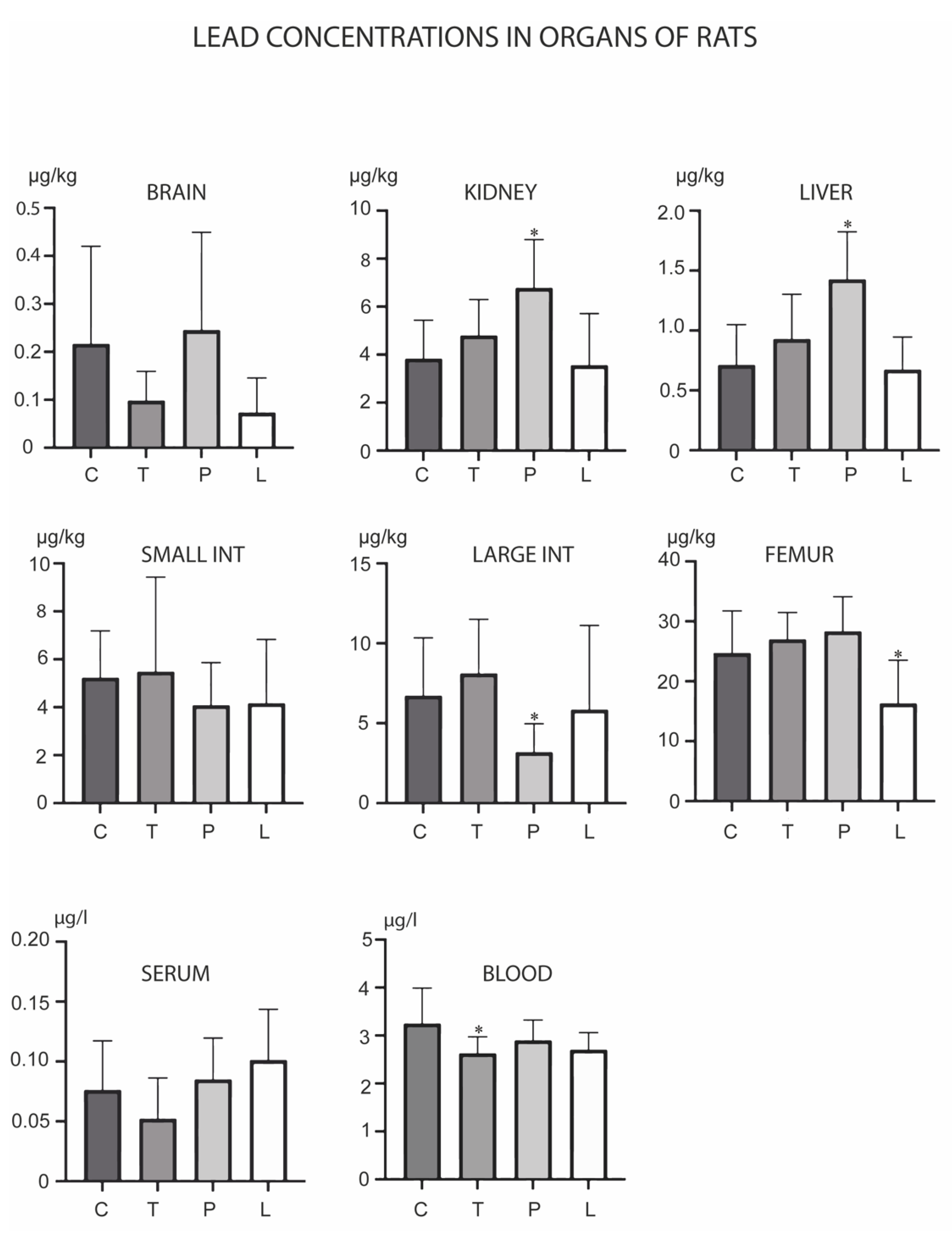
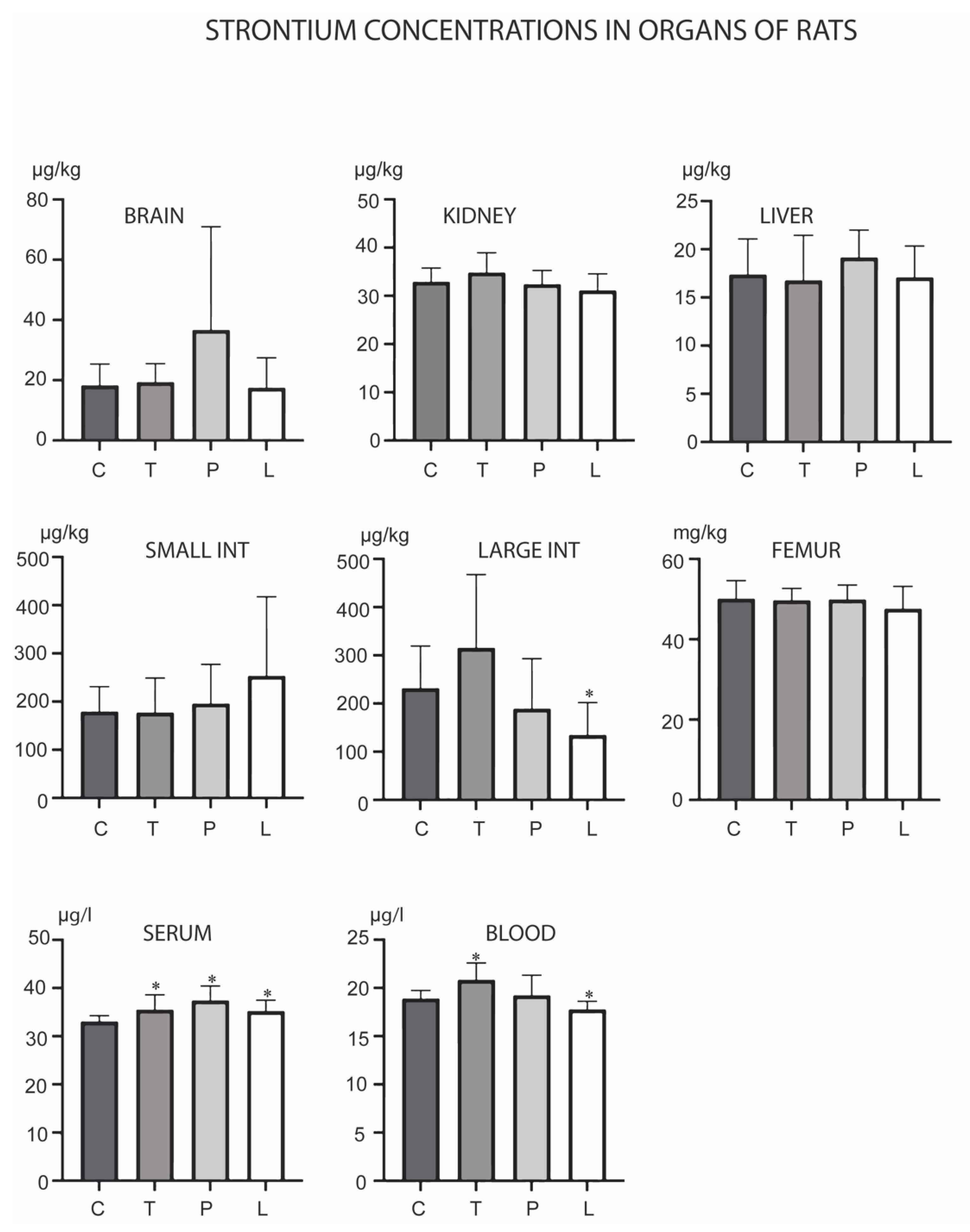
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell′Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals-A review. Sci. Total Environ. 2019, 670, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Kunhikrishnan, A.; Seshadri, B.; Choppala, G.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Zhang, M.; Li, C.-G.; Li, F.; et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ. Int. 2017, 108, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Lash, L.H. Environmental and Genetic Factors Influencing Kidney Toxicity. Semin. Nephrol. 2019, 39, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Al Osman, M.; Yang, F. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Zhou, F.; Yin, G.; Gao, Y.; Liu, D.; Xie, J.; Ouyang, L.; Fan, Y.; Yu, H.; Zha, Z.; Wang, K.; et al. Toxicity assessment due to prenatal and lactational exposure to lead, cadmium and mercury mixtures. Environ. Int. 2019, 133 Pt B, 105192. [Google Scholar] [CrossRef]
- Mohammed, S.; Gill, A.R.; Alsafadi, K.; Hijazi, O.; Yadav, K.K.; Hasan, M.A.; Khan, A.H.; Islam, S.; Pinto, M.M.S.; Harsany, E. An overview of greenhouse gases emissions in Hungary. J. Clean. Prod. 2021, 314, 127865. [Google Scholar] [CrossRef]
- Fisher, R.M.; Gupta, V. Heavy Metals; StatPearls Publishing©: Treasure Island, FL, USA, 2021. [Google Scholar]
- Tavker, N.; Yadav, V.K. Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers 2021, 13, 234. [Google Scholar] [CrossRef]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Zheng, F.; Gonçalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020, 34, 101475. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Roskams, A.J.; Connor, J.R. Aluminum access to the brain: A role for transferrin and its receptor. Proc. Natl. Acad. Sci. USA 1990, 87, 9024–9027. [Google Scholar] [CrossRef]
- Gupta, V.B.; Anitha, S.; Hegde, M.L.; Zecca, L.; Garruto, R.M.; Ravid, R.; Shankar, S.K.; Stein, R.; Shanmugavelu, P.; Rao, K.S.J. Aluminium in Alzheimer’s disease: Are we still at a crossroad? Cell Mol. Life Sci. 2005, 62, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, C.S.; Jureschi, M.; Drochioiu, G. Aluminium Binding to Modified Amyloid-β Peptides: Implications for Alzheimer′s Disease. Molecules 2020, 25, 4536. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Davenward, S.; Bentham, P.; Wright, J.; Crome, P.; Job, D.; Polwart, A.; Exley, C. Silicon-rich mineral water as a non-invasive test of the ′aluminum hypothesis′in Alzheimer′s disease. J. Alzheimers Dis. 2013, 33, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Hua, H.Y.; Naranmandura, H.; Zhu, H.H. Balance between the toxicity and anticancer activity of arsenic trioxide in treatment of acute promyelocytic leukemia. Toxicol. Appl. Pharmacol. 2020, 409, 115299. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Cabral Pinto, M.M.S.; Silva, M.M.V.; Ferreira da Silva, E.A.; Marinho-Reis, A.P. The Cancer and Non-Cancer Risk of Santiago Island (Cape Verde) Population due to Potential Toxic Elements Exposure from Soils. Geosciences 2017, 7, 78. [Google Scholar] [CrossRef]
- Ohba, K.I. Transport and Toxicity of Cadmium. Nihon Eiseigaku Zasshi 2018, 73, 269–274. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Cadmium as a testicular toxicant: A Review. J. Appl. Toxicol. 2021, 41, 105–117. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Rinaldi, M.; Micali, A.; Marini, H.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–206. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef]
- Krzywy, I.; Krzywy, E.; Pastuszak-Gabinowska, M.; Brodkiewicz, A. (Eds.) Lead—Is there Something to Be Afraid of? Annales Academiae Medicae Stetinensis: London, UK, 2010. [Google Scholar]
- Shinkai, Y.; Kaji, T. Cellular Defense Mechanisms against Lead Toxicity in the Vascular System. Biol. Pharm. Bull. 2012, 35, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Halmo, L.; Nappe, T.M. Lead Toxicity; StatPearls Publishing©: Treasure Island, FL, USA, 2021. [Google Scholar]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Miranda, M.L.; Kim, D.; Galeano, M.A.O.; Paul, C.J.; Hull, A.P.; Morgan, S.P. The Relationship between Early Childhood Blood Lead Levels and Performance on End-of-Grade Tests. Environ. Health Perspect. 2007, 115, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.I.; Hendricks, M.; Jones, K.S. Lead Toxicity in Children: An Unremitting Public Health Problem. Pediatr. Neurol. 2020, 113, 51–55. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; Khan, S.A.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity-an overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, M. Strontium overload and toxicity: Impact on renal osteodystrophy. Nephrol. Dial. Transpl. 2002, 17 (Suppl. S2), 30–34. [Google Scholar] [CrossRef]
- Li, Y.; Yue, J.; Liu, Y.; Wu, J.; Guan, M.; Chen, D.; Pan, H.; Zhao, X.; Lu, W.W. Strontium regulates stem cell fate during osteogenic differentiation through asymmetric cell division. Acta Biomater. 2021, 119, 432–443. [Google Scholar] [CrossRef]
- Fernandes, G.; Vanyo, S.T.; Alsharif, S.B.A.; Andreana, S.; Visser, M.B.; Dziak, R. Strontium Effects on Human Gingival Fibroblasts. J. Oral Implantol. 2019, 45, 274–280. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Fanta, F.T.; Dubale, A.A.; Bebizuh, D.F.; Atlabachew, M. Copper doped zeolite composite for antimicrobial activity and heavy metal removal from waste water. BMC Chem. 2019, 13, 44. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.V. Definition of a zeolite. Zeolites 1984, 4, 309–310. [Google Scholar] [CrossRef]
- Pavelic, K.; Hadzija, M. Medical applications of zeolites. In Handbook of Zeolite Science and Technology; Dekker: New York, NY, USA, 2003; pp. 1143–1174. [Google Scholar]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Laurino, C.; Palmieri, B. Zeolite: The Magic Stone; Main Nutritional, Environmental, Experimental and Clinical Fields of Application. Nutr. Hosp. 2015, 32, 573–581. [Google Scholar] [PubMed]
- Kraljević Pavelić, S.; Micek, V.; Filošević, A.; Gumbarević, D.; Žurga, P.; Bulog, A.; Orct, T.; Yamamoto, Y.; Preocanin, J.; Peter, R.; et al. Novel, oxygenated clinoptilolite material efficiently removes aluminium from aluminium chloride-intoxicated rats in vivo. Microporous Mesoporous Mater. 2017, 249, 146–156. [Google Scholar] [CrossRef]
- Vitale, M.G.; Barbato, C.; Crispo, A.; Habetswallner, F.; Martino, B.M.; Riccardi, F.; Maione, A.; Eisenwagen, S.; Vitale, G.; Carteni, G. ZeOxaNMulti Trial: A Randomized, Double-Blinded, Placebo-Controlled Trial of Oral PMA-zeolite to prevent Chemotherapy-Induced Side Effects, in particular, Peripheral Neuropathy. Molecules 2020, 25, 2297. [Google Scholar] [CrossRef]
- Loizidou, M.; Townsend, R.P. Exchange of cadmium into the sodium and ammonium forms of the natural zeolites clinoptilolite, mordenite, and ferrierite. J. Chem. Soc. Dalt. Trans 1911, 8, 1911–1916. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Burgess, R.M.; Perron, M.M.; Cantwell, M.G.; Ho, K.T.; Serbst, J.R.; Pelletier, M.C. Use of zeolite for removing ammonia and ammonia-caused toxicity in marine toxicity identification evaluations. Arch. Environ. Contam. Toxicol. 2004, 47, 440–447. [Google Scholar] [CrossRef]
- Eisenwagen, S.; Pavelic, K. Potential Role of Zeolites in Rehabilitation of Cancer Patients. Arch. Physiother. Rehabil. 2020, 3, 29–40. [Google Scholar]
- Kraljević Pavelić, S.; Saftić Martinović, L.; Simović Medica, J.; Žuvić, M.; Perdija, Ž.; Krpan, D.; Eisenwagen, S.; Orct, T.; Pavelic, K. Clinical Evaluation of a Defined Zeolite-Clinoptilolite Supplementation Effect on the Selected Blood Parameters of Patients. Front. Med. 2022, 9, 851782. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.; Schütz, B.; Eisenwagen, S.; Muss, C.; Mosgoeller, W. PMA-zeolite can modulate inflammation associated markers in irritable bowel disease-An explorative randomized, double blinded, controlled pilot trial. Neuro Endocrinol. Lett. 2021, 42, 1–12. [Google Scholar] [PubMed]
- Kraljević Pavelić, S.; Micek, V.; Bobinac, D.; Bazdulj, E.; Gianoncelli, A. Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial. Exp. Biol. Med. 2021, 246, 529–537. [Google Scholar] [CrossRef]
- Dumková, J.; Smutná, T.; Vrlíková, L.; Dočekal, B.; Kristeková, D.; Večeřa, Z.; Husáková, Z.; Jakešová, V.; Jedličková, A.; Mikuška, P.; et al. A Clearance Period after Soluble Lead Nanoparticle Inhalation Did Not Ameliorate the Negative Effects on Target Tissues Due to Decreased Immune Response. Int. J. Mol. Sci. 2020, 21, 8738. [Google Scholar] [CrossRef] [PubMed]
- Dumková, J.; Smutná, T.; Vrlíková, L.; Dočekal, B.; Kristeková, D.; Večeřa, Z.; Husáková, Z.; Jakešová, V.; Jedličková, A.; Mikuška, P.; et al. Variability in the Clearance of Lead Oxide Nanoparticles Is Associated with Alteration of Specific Membrane Transporters. ACS Nano 2020, 14, 3096–3120. [Google Scholar] [CrossRef]
- Koch, M.A. Experimental modeling and research methodology. In The Laboratory Rat; Elsevier: Amsterdam, The Netherlands, 2006; pp. 587–625. [Google Scholar]
- Ambrose, A.; Larson, P.S.; Borzelleca, J.F.; Hennigar, G.R., Jr. Long term toxicologic assessment of nickel in rats and dogs. J. Food Sci. Technol. 1976, 13, 181–187. [Google Scholar]
- Rodríguez, J.; Mandalunis, P.M. A review of metal exposure and its effects on bone health. J. Toxicol. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Xing, M.; Wang, X.; Wang, E.; Gao, L.; Chang, J. Bone tissue engineering strategy based on the synergistic effects of silicon and strontium ions. Acta Biomater. 2018, 72, 381–395. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolanc, I.; Ferhatović Hamzić, L.; Orct, T.; Micek, V.; Šunić, I.; Jonjić, A.; Jurasović, J.; Missoni, S.; Čoklo, M.; Pavelić, S.K. The Impact of Long-Term Clinoptilolite Administration on the Concentration Profile of Metals in Rodent Organisms. Biology 2023, 12, 193. https://doi.org/10.3390/biology12020193
Dolanc I, Ferhatović Hamzić L, Orct T, Micek V, Šunić I, Jonjić A, Jurasović J, Missoni S, Čoklo M, Pavelić SK. The Impact of Long-Term Clinoptilolite Administration on the Concentration Profile of Metals in Rodent Organisms. Biology. 2023; 12(2):193. https://doi.org/10.3390/biology12020193
Chicago/Turabian StyleDolanc, Ivan, Lejla Ferhatović Hamzić, Tatjana Orct, Vedran Micek, Iva Šunić, Antonija Jonjić, Jasna Jurasović, Saša Missoni, Miran Čoklo, and Sandra Kraljević Pavelić. 2023. "The Impact of Long-Term Clinoptilolite Administration on the Concentration Profile of Metals in Rodent Organisms" Biology 12, no. 2: 193. https://doi.org/10.3390/biology12020193
APA StyleDolanc, I., Ferhatović Hamzić, L., Orct, T., Micek, V., Šunić, I., Jonjić, A., Jurasović, J., Missoni, S., Čoklo, M., & Pavelić, S. K. (2023). The Impact of Long-Term Clinoptilolite Administration on the Concentration Profile of Metals in Rodent Organisms. Biology, 12(2), 193. https://doi.org/10.3390/biology12020193








