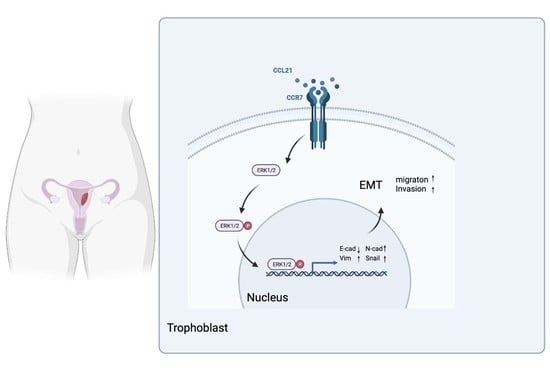
CCL21/CCR7 Axis Contributes to Trophoblastic Cell Migration and Invasion in Preeclampsia by Affecting the Epithelial Mesenchymal Transition via the ERK1/2 Signaling Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Clinical Samples
2.2. Animal Model
2.3. Cell Culture
2.4. RNA Extraction and qRT-PCR
2.5. Western Blotting
2.6. Immunohistochemistry Staining
2.7. Immunofluorescence Staining
2.8. Scratch Wound-Healing Assay
2.9. Matrigel Invasion and Transwell Migration Assay
2.10. DNA Synthesis Assay
2.11. TUNEL Assay
2.12. Cell Immunofluorescence
2.13. Transfection
2.14. Bioinformatic Analysis
2.14.1. Data Acquisition
2.14.2. Gene Set Enrichment Analysis
2.14.3. Blood Pressure Correlation Analysis
2.15. Statistical Analysis
3. Results
3.1. CCR7 Has Been Linked to the Pathogenesis of PE According to Bioinformatics Analysis
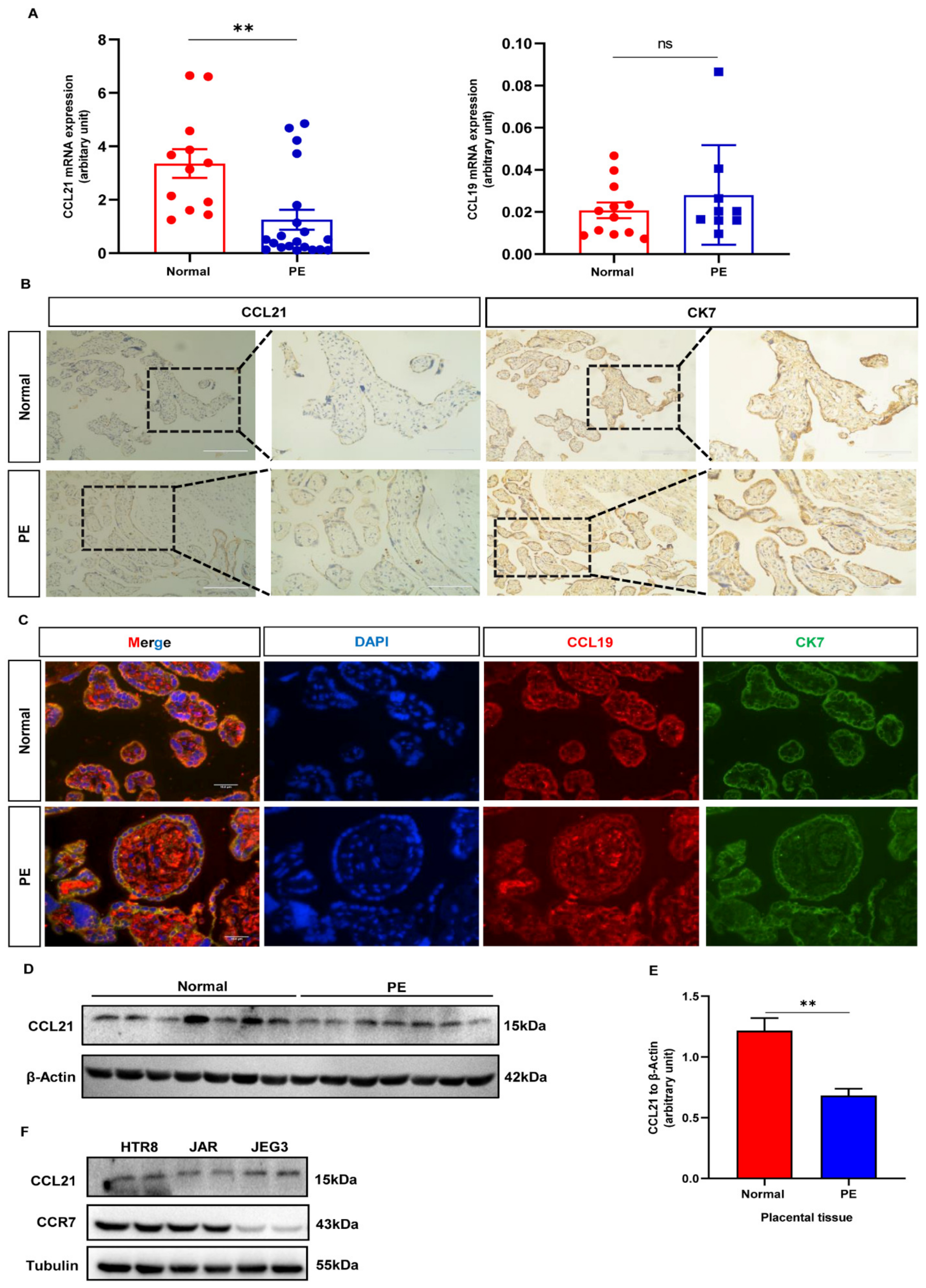
3.2. CCR7 Is Upregulated in the Preeclamptic Placenta
3.3. Expression of CCL21 Is Downregulated in the Placenta of PE
3.4. CCL21 Promotes Trophoblasts Mobility
3.5. CCL21-Induced Trophoblasts Migration and Invasion Are Dependent on the EMT Process
3.6. CCL21 Affects the EMT Process in a Receptor-Dependent Manner and Further Affects the Migration and Invasion of Trophoblasts
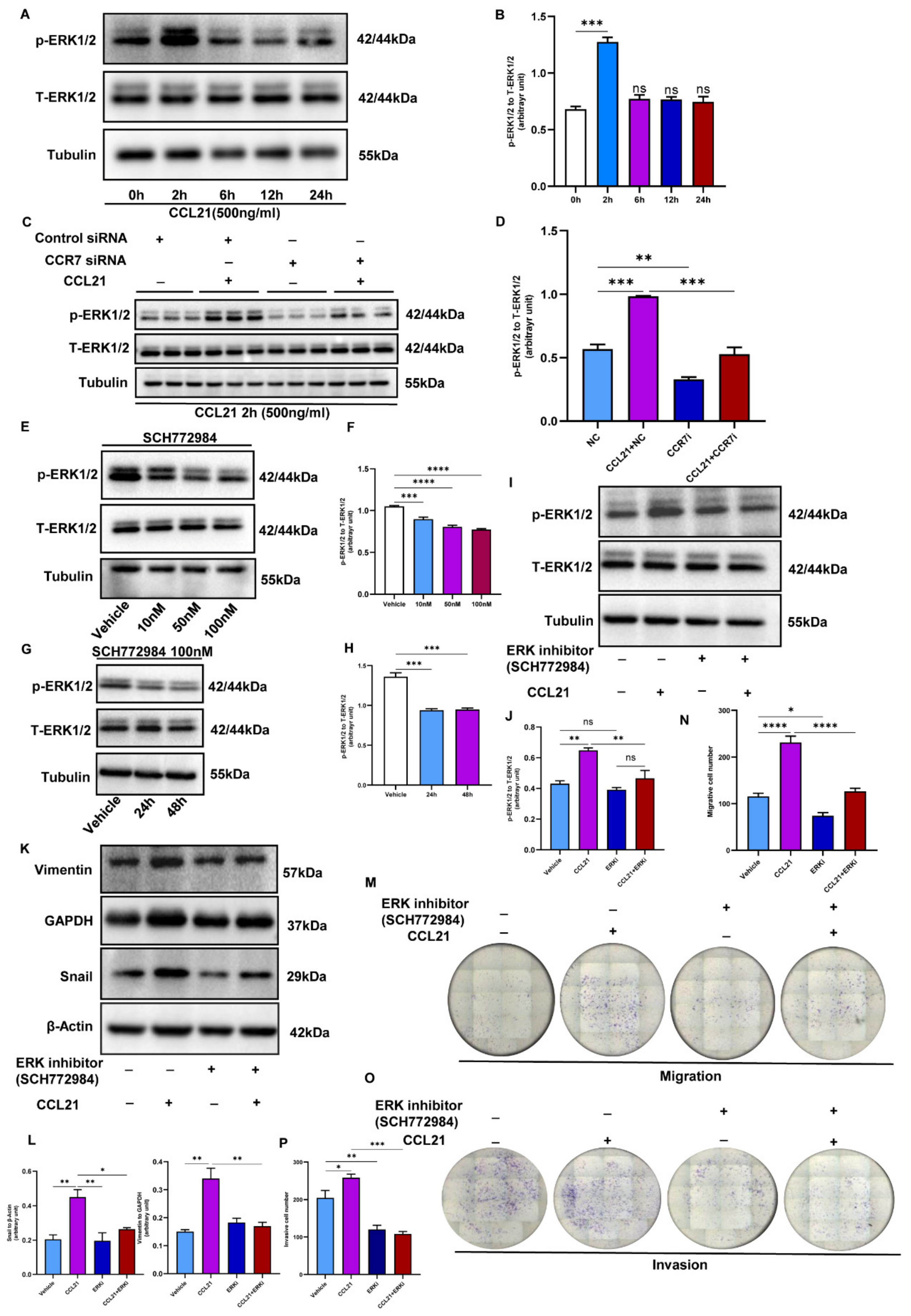
3.7. CCL21/CCR7 Axis Activates the ERK1/2 Signaling Pathway to Induce EMT in Trophoblasts
3.8. Role of CCL21/CCR7 Axis to Induce EMT in the PE Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, L.; van Dijk, M.; Chye, S.T.J.; Messerschmidt, D.M.; Chng, S.C.; Ong, S.; Yi, L.K.; Boussata, S.; Goh, G.H.-Y.; Afink, G.B.; et al. ELABELA Deficiency Promotes Preeclampsia and Cardiovascular Malformations in Mice. Science 2017, 357, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J.M. Preeclampsia: Short and Long-Term Consequences for Mother and Neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Schmieder, R.E. New Approaches in the Treatment of Hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous Trophoblast and Decidual Natural Killer Cells: A Remodelling Partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Preeclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Chen, X.; Tong, C.; Li, H.; Peng, W.; Li, R.; Luo, X.; Ge, H.; Ran, Y.; Li, Q.; Liu, Y.; et al. Dysregulated Expression of RPS4Y1 (Ribosomal Protein S4, Y-Linked 1) Impairs STAT3 (Signal Transducer and Activator of Transcription 3) Signaling to Suppress Trophoblast Cell Migration and Invasion in Preeclampsia. Hypertension 2018, 71, 481–490. [Google Scholar] [CrossRef]
- Kim, R.H.; Ryu, B.J.; Lee, K.M.; Han, J.W.; Lee, S.K. Vitamin D Facilitates Trophoblast Invasion through Induction of Epithelial-Mesenchymal Transition. Am. J. Reprod. Immunol. 2018, 79, e12796. [Google Scholar] [CrossRef]
- Peng, P.; Song, H.; Xie, C.; Zheng, W.; Ma, H.; Xin, D.; Zhan, J.; Yuan, X.; Chen, A.; Tao, J.; et al. MiR-146a-5p-Mediated Suppression on Trophoblast Cell Progression and Epithelial-Mesenchymal Transition in Preeclampsia. Biol. Res. 2021, 54, 30. [Google Scholar] [CrossRef]
- Perez-Garcia, V.; Lea, G.; Lopez-Jimenez, P.; Okkenhaug, H.; Burton, G.J.; Moffett, A.; Turco, M.Y.; Hemberger, M. BAP1/ASXL Complex Modulation Regulates Epithelial-Mesenchymal Transition during Trophoblast Differentiation and Invasion. eLife 2021, 10, e63254. [Google Scholar] [CrossRef]
- Jessica Davies; Jürgen Pollheimer; Hannah E J Yong Epithelial-Mesenchymal Transition during Extravillous Trophoblast Differentiation. Cell Adhes. Migr. 2016, 10, 310–321. [CrossRef]
- Zhang, H.; Hou, L.; Li, C.M.; Zhang, W.Y. The Chemokine CXCL6 Restricts Human Trophoblast Cell Migration and Invasion by Suppressing MMP-2 Activity in the First Trimester. Hum. Reprod. 2013, 28, 2350–2362. [Google Scholar] [CrossRef]
- Salem, A.; Alotaibi, M.; Mroueh, R.; Basheer, H.A.; Afarinkia, K. CCR7 as a Therapeutic Target in Cancer. Biochim. Et Biophys. Acta 2021, 1875, 188499. [Google Scholar] [CrossRef]
- Luan, X.; Li, S.; Zhao, J.; Zhai, J.; Liu, X.; Chen, Z.-J.; Li, W.; Du, Y. Down-Regulation of CCR7 via AKT Pathway and GATA2 Inactivation Suppressed Trophoblast Migration and Invasion in Recurrent Spontaneous Abortion. Biol. Reprod. 2020, 102, 424–433. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Li, Y.; Liu, Y.; Xie, X.; Wu, Y.; Zhou, Y.; Ren, J.; Zhang, J.; Zhu, H.; et al. CCL21/CCR7 Axis Contributed to CD133+ Pancreatic Cancer Stem-Like Cell Metastasis via EMT and Erk/NF-ΚB Pathway. PLoS ONE 2016, 11, e0158529. [Google Scholar] [CrossRef]
- Chen, Y. CCL21/CCR7 Interaction Promotes EMT and Enhances the Stemness of OSCC via a JAK2/STAT3 Signaling Pathway. J. Cell Physiol. 2020, 235, 5995–6009. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of Chemokine Receptors in Breast Cancer Metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Ishida, K.; Iwahashi, M.; Nakamori, M.; Nakamura, M.; Yokoyama, S.; Iida, T.; Naka, T.; Nakamura, Y.; Yamaue, H. High CCR7 MRNA Expression of Cancer Cells Is Associated with Lymph Node Involvement in Patients with Esophageal Squamous Cell Carcinoma. Int. J. Oncol. 2009, 34, 915–922. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal 2021, 34, 118–136. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, K.; Umar, S.; Shahrara, S. The Pathogenic Importance of CCL21 and CCR7 in Rheumatoid Arthritis. Cytokine Growth Factor Rev. 2020, 55, 86–93. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, F.; Li, X.; Chen, Z.; Feng, D.; Jiang, H.; Chen, W.; Zhang, X. CCL21/CCR7 Interaction Promotes Cellular Migration and Invasion via Modulation of the MEK/ERK1/2 Signaling Pathway and Correlates with Lymphatic Metastatic Spread and Poor Prognosis in Urinary Bladder Cancer. Int. J. Oncol. 2017, 51, 75–90. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of Preeclampsia with Aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Velicky, P.; Knöfler, M.; Pollheimer, J. Function and Control of Human Invasive Trophoblast Subtypes: Intrinsic vs. Maternal Control. Cell Adh. Migr. 2016, 10, 154–162. [Google Scholar] [CrossRef]
- Du, M.-R.; Wang, S.-C.; Li, D.-J. The Integrative Roles of Chemokines at the Maternal-Fetal Interface in Early Pregnancy. Cell Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the Immune Response to Cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Li, J.; Sun, R.; Tao, K.; Wang, G. The CCL21/CCR7 Pathway Plays a Key Role in Human Colon Cancer Metastasis through Regulation of Matrix Metalloproteinase-9. Dig. Liver Dis. 2011, 43, 40–47. [Google Scholar] [CrossRef]
- Shi, M.; Chen, D.; Yang, D.; Liu, X.-Y. CCL21-CCR7 Promotes the Lymph Node Metastasis of Esophageal Squamous Cell Carcinoma by up-Regulating MUC1. J. Exp. Clin. Cancer Res. 2015, 34, 149. [Google Scholar] [CrossRef]
- Ferretti, C.; Bruni, L.; Dangles-Marie, V.; Pecking, A.P.; Bellet, D. Molecular Circuits Shared by Placental and Cancer Cells, and Their Implications in the Proliferative, Invasive and Migratory Capacities of Trophoblasts. Hum. Reprod. Update 2007, 13, 121–141. [Google Scholar] [CrossRef]
- Red-Horse, K.; Drake, P.M.; Fisher, S.J. Human Pregnancy: The Role of Chemokine Networks at the Fetal-Maternal Interface. Expert Rev. Mol. Med. 2004, 6, 1–14. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.-H.; Shang, W.-Q.; Liu, L.-B.; Chen, X.; Yuan, M.-M.; Jin, L.-P.; Li, M.-Q.; Li, D.-J. Chemokine CCL24 Promotes the Growth and Invasiveness of Trophoblasts through ERK1/2 and PI3K Signaling Pathways in Human Early Pregnancy. Reproduction 2015, 150, 417–427. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Du, M.-R.; Dong, L.; Yu, J.; Li, D.-J. Chemokine CXCL12 Promotes the Cross-Talk between Trophoblasts and Decidual Stromal Cells in Human First-Trimester Pregnancy. Hum. Reprod. 2008, 23, 2669–2679. [Google Scholar] [CrossRef]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef]
- Halvorsen, B.; Dahl, T.B.; Smedbakken, L.M.; Singh, A.; Michelsen, A.E.; Skjelland, M.; Krohg-Sørensen, K.; Russell, D.; Höpken, U.E.; Lipp, M.; et al. Increased Levels of CCR7 Ligands in Carotid Atherosclerosis: Different Effects in Macrophages and Smooth Muscle Cells. Cardiovasc. Res. 2014, 102, 148–156. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, D.; Hui, B.; Shu, L.; Tang, X.; Wang, C.; Xie, J.; Yin, Y.; Sagnelli, M.; Yang, N.; et al. A Novel Regulatory Mechanism Network Mediated by LncRNA TUG1 That Induces the Impairment of Spiral Artery Remodeling in Preeclampsia. Mol. Ther. 2022, 30, 1692–1705. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, H.; Tachibana, R.; Yoshikawa, K.; Kawamura, T.; Takakura, S.; Takeuchi, H.; Ikeda, T. Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental MTOR Signaling. Int. J. Mol. Sci. 2022, 23, 1474. [Google Scholar] [CrossRef]
- Schanz, A.; Red-Horse, K.; Hess, A.P.; Baston-Büst, D.M.; Heiss, C.; Krüssel, J.S. Oxygen Regulates Human Cytotrophoblast Migration by Controlling Chemokine and Receptor Expression. Placenta 2014, 35, 1089–1094. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef]
- Xiong, L.; Ye, X.; Chen, Z.; Fu, H.; Li, S.; Xu, P.; Yu, J.; Wen, L.; Gao, R.; Fu, Y.; et al. Advanced Maternal Age-Associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation. Aging Cell 2021, 20, e13491. [Google Scholar] [CrossRef]
- Pang, M.-F.; Georgoudaki, A.-M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-Β1-Induced EMT Promotes Targeted Migration of Breast Cancer Cells through the Lymphatic System by the Activation of CCR7/CCL21-Mediated Chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef]
- Li, G.; Yang, Y.; Xu, S.; Ma, L.; He, M.; Zhang, Z. Slug Signaling Is Up-Regulated by CCL21/CCR7 [Corrected] to Induce EMT in Human Chondrosarcoma. Med. Oncol. 2015, 32, 478. [Google Scholar] [CrossRef]
- Nakamura, A.; Goto, Y.; Kondo, Y.; Aoki, K. Shedding Light on Developmental ERK Signaling with Genetically Encoded Biosensors. Development 2021, 148, dev199767. [Google Scholar] [CrossRef]
- Zhang, J.; Mo, H.-Q.; Tian, F.-J.; Zeng, W.-H.; Liu, X.-R.; Ma, X.-L.; Li, X.; Qin, S.; Fan, C.-F.; Lin, Y. EIF5A1 Promotes Trophoblast Migration and Invasion via ARAF-Mediated Activation of the Integrin/ERK Signaling Pathway. Cell Death Dis. 2018, 9, 926. [Google Scholar] [CrossRef]
- Du, M.-R.; Zhou, W.-H.; Yan, F.-T.; Zhu, X.-Y.; He, Y.-Y.; Yang, J.-Y.; Li, D.-J. Cyclosporine A Induces Titin Expression via MAPK/ERK Signalling and Improves Proliferative and Invasive Potential of Human Trophoblast Cells. Hum. Reprod. 2007, 22, 2528–2537. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Meng, T. Overexpression of Let-7b Exerts Beneficial Effects on the Functions of Human Placental Trophoblasts by Activating the ERK1/2 Signaling Pathway. Mol. Reprod. Dev. 2022, 89, 39–53. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; He, J.; Jin, P.; Ran, Y.; Yin, N.; Qi, H. CCL21/CCR7 Axis Contributes to Trophoblastic Cell Migration and Invasion in Preeclampsia by Affecting the Epithelial Mesenchymal Transition via the ERK1/2 Signaling Pathway. Biology 2023, 12, 150. https://doi.org/10.3390/biology12020150
Liu Z, He J, Jin P, Ran Y, Yin N, Qi H. CCL21/CCR7 Axis Contributes to Trophoblastic Cell Migration and Invasion in Preeclampsia by Affecting the Epithelial Mesenchymal Transition via the ERK1/2 Signaling Pathway. Biology. 2023; 12(2):150. https://doi.org/10.3390/biology12020150
Chicago/Turabian StyleLiu, Zheng, Jie He, Pingsong Jin, Yuxin Ran, Nanlin Yin, and Hongbo Qi. 2023. "CCL21/CCR7 Axis Contributes to Trophoblastic Cell Migration and Invasion in Preeclampsia by Affecting the Epithelial Mesenchymal Transition via the ERK1/2 Signaling Pathway" Biology 12, no. 2: 150. https://doi.org/10.3390/biology12020150
APA StyleLiu, Z., He, J., Jin, P., Ran, Y., Yin, N., & Qi, H. (2023). CCL21/CCR7 Axis Contributes to Trophoblastic Cell Migration and Invasion in Preeclampsia by Affecting the Epithelial Mesenchymal Transition via the ERK1/2 Signaling Pathway. Biology, 12(2), 150. https://doi.org/10.3390/biology12020150