Metal Interactions in the Ni Hyperaccumulating Population of Noccaea caerulescens Monte Prinzera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
- (1)
- Control (Ctrl): half-strength nutrient solution amended with 10 μM NiSO4;
- (2)
- 100 μM Ni: half-strength nutrient solution amended with 100 μM NiSO4;
- (3)
- −Zn: half-strength nutrient solution lacking Zn salts and amended with 10 μM NiSO4;
- (4)
- −Fe: half-strength nutrient solution lacking Fe salts and amended with 10 μM NiSO4;
- (5)
- 100 μM Zn: half-strength nutrient solution amended with 10 μM NiSO4 and 100 μM ZnSO4;
- (6)
- 50 μM Co: half-strength nutrient solution amended with 10 μM NiSO4 and 50 μM CoSO4.
2.2. RNA Extraction and Gene Expression Analysis
2.3. Metal Content Quantification and Calculation of the Translocation Factors
2.4. Chlorophyll Quantification
2.5. Quantification of Metal Chelating Agents
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Analysis of Plants
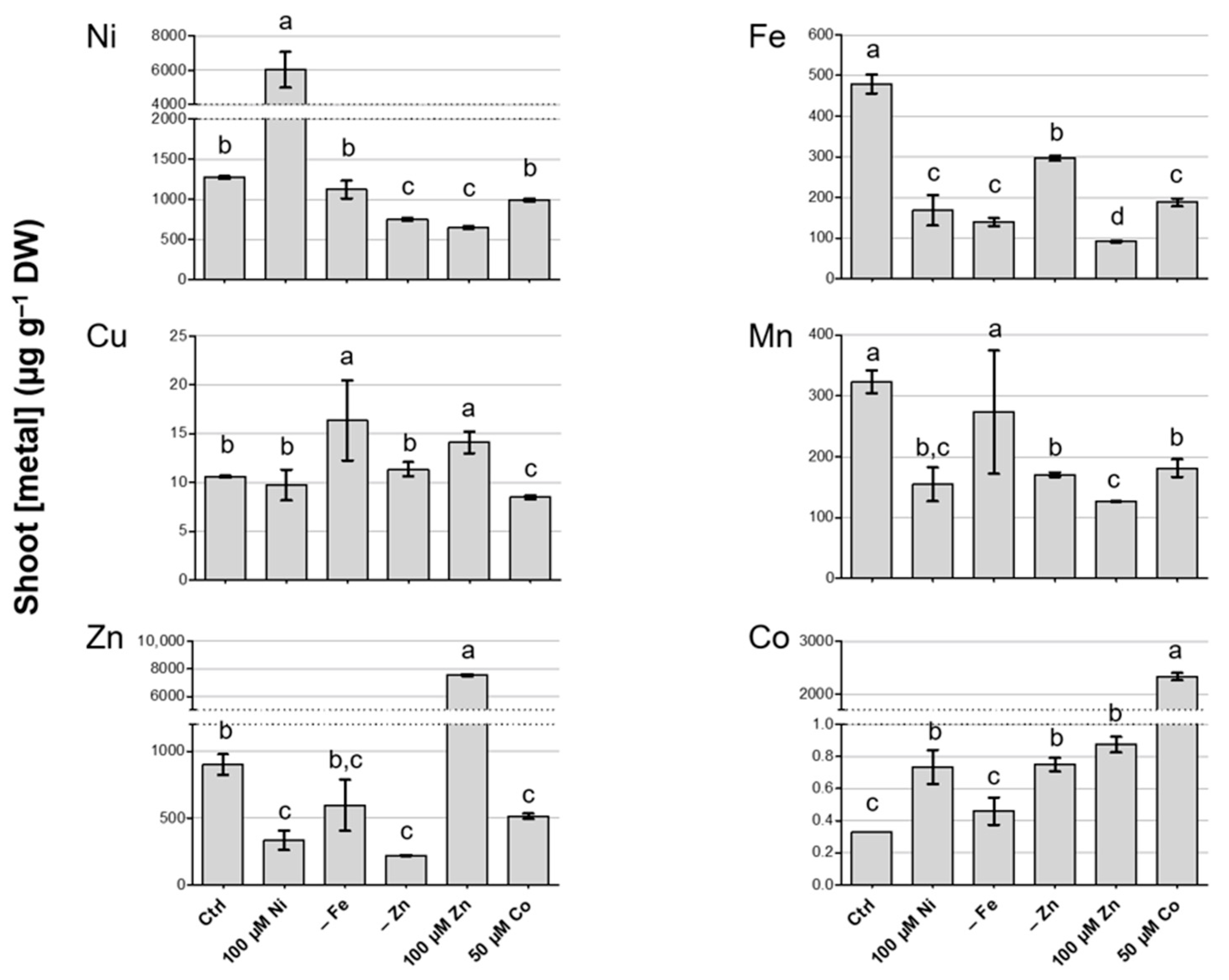
3.2. Metal Concentration in Shoots of N. caerulescens MP
3.3. Metal Concentration in Roots of N. caerulescens MP and Effect on Root-to-Shoot Translocation
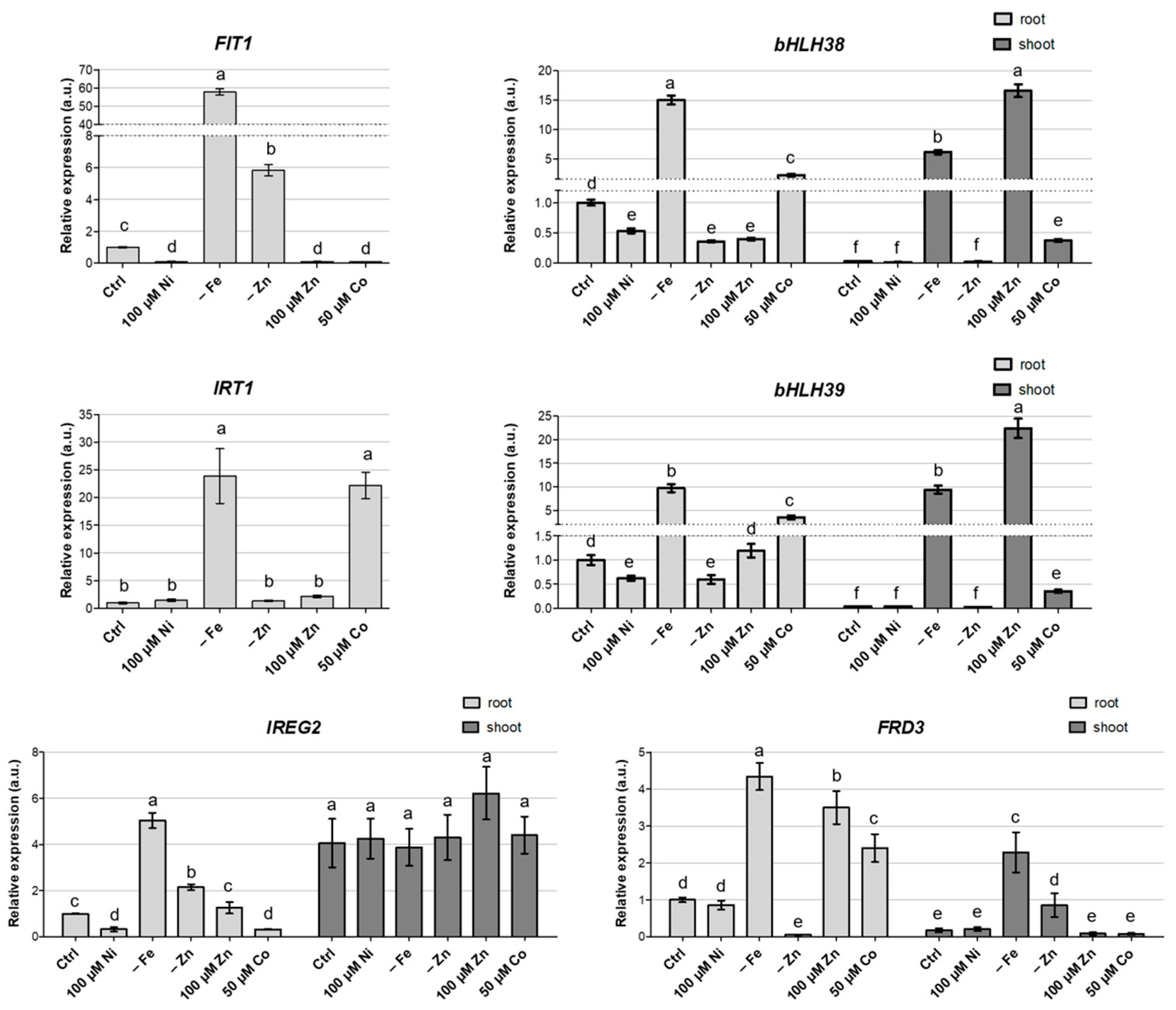
3.4. Gene Expression Analysis
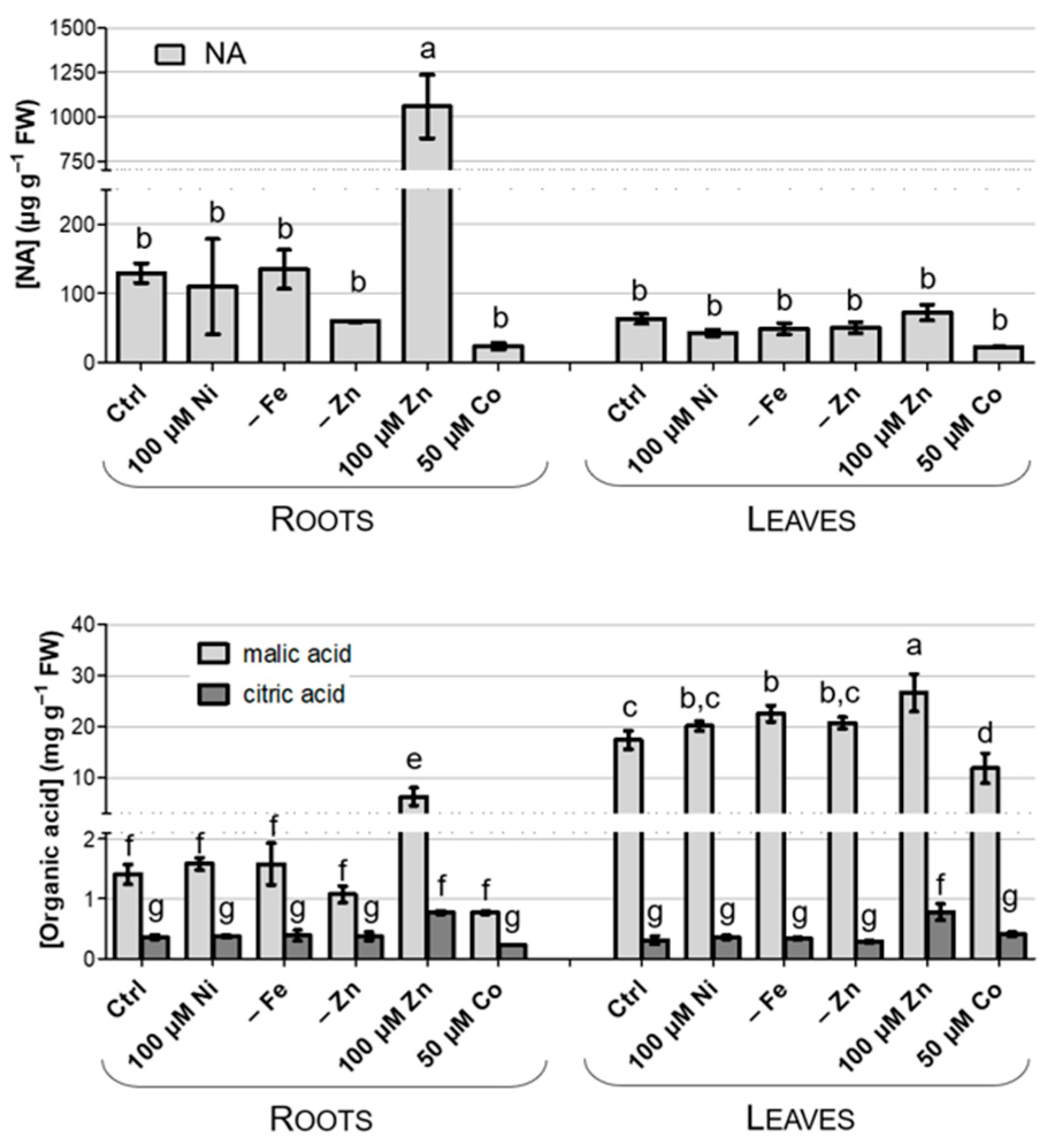
3.5. Accumulation of Chelating Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaspari, M. The invisible hand of the periodic table: How micronutrients shape ecology. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 199–219. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G. Heavy metal toxicity in plants. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lam, E.J.; Keith, B.F.; Bech, J.; Alvarez, F.A.; Zetola, V.; Pereira, L.H.; Montofré, Í.L. Characteristic curve modeling of plant species behavior in soils with heavy metals. Environ. Geochem. Health 2022, 1–14. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Furini, A.; DalCorso, G. Evolution of the metal hyperaccumulation and hypertolerance traits. Plant Cell Environ. 2020, 43, 2969–2986. [Google Scholar] [CrossRef]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef]
- Al-Hiyaly, S.A.; McNeilly, T.; Bradshaw, A.D. The effect of zinc contamination from electricity pylons—Evolution in a replicated situation. New Phytol. 1988, 110, 571–580. [Google Scholar] [CrossRef]
- Boyd, R.S.; Martens, S.N. The raison d’être for metal for metal hyperaccumulation by plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 279–289. [Google Scholar]
- Boyd, R.S. Plant defense using toxic inorganic ions: Conceptual models of the defensive enhancement and joint effects hypotheses. Plant Sci. 2012, 195, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, B.D.; Ketterer, M.E.; Shuster, S.M. Elemental allelopathy by an arsenic hyperaccumulating fern, Pteris vittata L. J. Plant Ecol. 2018, 11, 553–559. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sterckeman, T.; Cazes, Y.; Gonneau, C.; Sirguey, C. Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction. Plant Soil 2017, 418, 523–540. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Seregin, I.V.; Aarts, M.G.; Schat, H. Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens. Plant Soil 2020, 452, 479–498. [Google Scholar] [CrossRef]
- Deng, T.H.B.; Tang, Y.T.; Sterckeman, T.; Echevarria, G.; Morel, J.L.; Qiu, R.L. Effects of the interactions between nickel and other trace metals on their accumulation in the hyperaccumulator Noccaea caerulescens. Environ. Exp. Bot. 2019, 158, 73–79. [Google Scholar] [CrossRef]
- Visioli, G.; Vincenzi, S.; Marmiroli, M.; Marmiroli, N. Correlation between phenotype and proteome in the Ni hyperaccumulator Noccaea caerulescens subsp. caerulescens. Environ. Exp. Bot. 2012, 77, 156–164. [Google Scholar] [CrossRef]
- Fasani, E.; Li, M.; Varotto, C.; Furini, A.; DalCorso, G. Metal Detoxification in land plants: From Bryophytes to Vascular Plants. State of the art andopportunities. Plants 2022, 11, 237. [Google Scholar] [CrossRef]
- Milner, M.J.; Mitani-Ueno, N.; Yamaji, N.; Yokosho, K.; Craft, E.; Fei, Z.; Ebbs, S.; Zambrano, M.C.; Ma, J.F.; Kochian, L.V. Root and shoot transcriptome analysis of two ecotypes of Noccaea caerulescens uncovers the role of NcNramp1 in cd hyperaccumulation. Plant J. 2014, 78, 398–410. [Google Scholar] [CrossRef]
- Ueno, D.; Milner, M.J.; Yamaji, N.; Yokosho, K.; Koyama, E.; Clemencia Zambrano, M.; Ma, J.F. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J. 2011, 66, 852–862. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van Der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Macnair, M.R. Within and between population variation for zinc and nickel accumulation in two species of Thlaspi (Brassicaceae). New Phytol 2006, 169, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Eroglu, S.; Grillet, L.; Nozoye, T. Editorial: Metal Transport in Plants. Front. Plant Sci. 2021, 12, 644960. [Google Scholar] [CrossRef] [PubMed]
- Halimaa, P.; Lin, Y.F.; Ahonen, V.H.; Blande, D.; Clemens, S.; Gyenesei, A.; Häikiö, E.; Kärenlampi, S.O.; Laiho, A.; Aarts, M.G.; et al. Gene expression differences between Noccaea caerulescens ecotypes help to identify candidate genes for metal phytoremediation. Environ. Sci. Technol. 2014, 48, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Richau, K.H.; Schat, H. Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens. Plant Soil 2009, 314, 253–262. [Google Scholar] [CrossRef]
- Nishida, S.; Tanikawa, R.; Ishida, S.; Yoshida, J.; Mizuno, T.; Nakanishi, H.; Furuta, N. Elevated expression of vacuolar Nickel transporter gene IREG2 is associated with reduced root-to-shoot Nickel translocation in Noccaea japonica. Front. Plant Sci. 2020, 11, 610. [Google Scholar] [CrossRef]
- Schmittgen, T.; Livak, K. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D.P.; Kumar, N.; Bhargava, S.K.; Barman, S.C. Accumulation and translocation of heavy metals in soil and plants; from fly ash contaminated area. J. Environ. Biol. 2010, 31, 421–430. [Google Scholar]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Van de Mortel, J.E.; Almar Villanueva, L.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; Ver Loren van Themaat, E.; Koornneef, M.; Aarts, M.G. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Talke, I.N.; Hanikenne, M.; Krämer, U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006, 142, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Aisu, A.; Mizuno, T. Induction of IRT1 by the nickel-induced iron deficient response in Arabidopsis. Plant Signal Behav. 2012, 7, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Honsbein, A.; Meda, A.R.; Kirchner, S.; Wipf, D.; von Wirén, N. AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J. Biol. Chem. 2006, 281, 25532–25540. [Google Scholar] [CrossRef] [PubMed]
- Hindt, M.N.; Guerinot, M.L. Getting a sense for signals: Regulation of the plant iron deficiency response. Biochim. Biophys. Acta 2012, 1823, 1521–1530. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.; Ling, H.Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef]
- Schwarz, B.; Bauer, P. FIT, a regulatory hub for iron deficiency and stress signaling in roots, and FIT-dependent and -independent gene signatures. J. Exp. Bot. 2020, 71, 1694–1705. [Google Scholar] [CrossRef]
- Barberon, M.; Berthomieu, P.; Clairotte, M. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol. 2008, 180, 608–619. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.; Du, X.; Shah, A.A.; Ahmad, B.; Yang, L.; Mu, Z. Response and Tolerance of Macleaya cordata to Excess Zinc Based on Transcriptome and Proteome Patterns. Plants 2023, 12, 2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, F.H.; Li, J.X.; Chen, J.; Wang, G.H.; Wang, W.H.; Hu, W.J.; Gao, L.J.; Wang, Z.L.; Chen, J.H.; et al. Glutathione homeostasis and Cd tolerance in the Arabidopsis sultr1; 1-sultr1; 2 double mutant with limiting sulfate supply. Plant Cell Rep. 2016, 35, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, G.; Gai, Z.; Zhang, W.; Han, Y.; Li, W. Changes in the gene expression profile of Arabidopsis thaliana under chromium stress. Ecotoxicol. Environ. Saf. 2020, 193, 110302. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kutrowska, A.; Szelag, M. Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol. Plant 2014, 36, 1957–1968. [Google Scholar] [CrossRef]
- Bouain, N.; Krouk, G.; Lacombe, B.; Rouached, H. Getting to the root of plant mineral nutrition: Combinatorial nutrient stresses reveal emergent properties. Trends Plant Sci. 2019, 24, 542–552. [Google Scholar] [CrossRef]
- Gonneau, C.; Noret, N.; Gode, C.; Frerot, H.; Sirguey, C.; Sterckeman, T.; Pauwels, M. Demographic history of the trace metal hyperaccumulator Noccaea caerulescens (J. Presl and C. Presl) FK Mey. in Western Europe. Mol. Ecol 2017, 26, 904–922. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Martins, P.D.C.; De Folter, S.; Vooijs, R.; Schat, H.; Aarts, M.G.M. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2001, 24, 217–226. [Google Scholar] [CrossRef]
- Lombini, A.; Dinelli, E.; Ferrari, C.; Simoni, A. Plant–soil relationships in the serpentinite screes of Mt. Prinzera (Northern Apennines, Italy). J. Geochem. Expl. 1998, 64, 19–33. [Google Scholar] [CrossRef]
- Keeling, S.M.; Stewart, R.B.; Anderson, C.W.N.; Robinson, B.H. Nickel and cobalt phytoextraction by the hyperaccumulator Berkheya coddii: Implications for polymetallic phytomining and phytoremediation. Int. J. Phytoremediation 2003, 5, 235–244. [Google Scholar] [CrossRef]
- Van Der Ent, A.; Mak, R.; De Jonge, M.D.; Harris, H.H. Simultaneous hyperaccumulation of nickel and cobalt in the tree Glochidion cf. sericeum (Phyllanthaceae): Elemental distribution and chemical speciation. Sci. Rep. 2018, 8, 9683. [Google Scholar] [PubMed]
- Malik, M.; Chaney, R.L.; Brewer, E.P.; Li, Y.M.; Angle, J.S. Phytoextraction of soil cobalt using hyperaccumulator plants. Int. J. Phytoremediation 2000, 2, 319–329. [Google Scholar] [CrossRef]
- Tappero, R.; Peltier, E.; Gräfe, M.; Heidel, K.; Ginder-Vogel, M.; Livi, K.J.T.; Sparks, D.L. Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol. 2007, 175, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Rue, M.; Paul, A.L.; Echevarria, G.; van Der Ent, A.; Simonnot, M.O.; Morel, J.L. Uptake, translocation and accumulation of nickel and cobalt in Berkheya coddii, a ‘metal crop’ from South Africa. Metallomics 2020, 12, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Zulfiqar, U.; Mumtaz, M.Z.; Radziemska, M.; Haider, F.U.; Holatko, J.; Hammershmiedt, T.; Naveed, M.; Ali, H.; Kintl, A.; et al. Nickel (Ni) phytotoxicity and detoxification mechanisms: A review. Chemosphere 2023, 328, 138574. [Google Scholar] [CrossRef]
- Yang, X.; Baligar, V.C.; Martens, D.C.; Clark, R.B. Plant tolerance to nickel toxicity: II Nickel effects on influx and transport of mineral nutrients in four plant species. J. Plant Nutr. 1996, 19, 265–279. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Tappero, R.V.; Maugel, T.K.; Erbe, E.F.; Sparks, D.L.; Chaney, R.L. Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum. Plant Soil 2009, 314, 35–48. [Google Scholar] [CrossRef]
- Ghasemi, R.; Ghaderian, S.M.; Krämer, U. Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol. 2009, 184, 566–580. [Google Scholar] [CrossRef]
- Ghaderian, S.M.; Ghasemi, R.; Hajihashemi, F. Interaction of nickel and manganese in uptake, translocation and accumulation by the nickel-hyperaccumulator plant, Alyssum bracteatum (Brassicaceae). Aust. J. Bot. 2015, 63, 47–55. [Google Scholar] [CrossRef]
- Montargès-Pelletier, E.; Chardot, V.; Echevarria, G.; Michot, L.J.; Bauer, A.; Morel, J.L. Identification of nickel chelators in three hyperaccumulating plants: An X-ray spectroscopic study. Phytochemistry 2008, 69, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Mari, S.; Gendre, D.; Pianelli, K.; Ouerdane, L.; Lobinski, R.; Briat, J.F.; Lebrun, M.; Czernic, P. Root-to-shoot long-distance circulation of nicotianamine and nicotianamine–nickel chelates in the metal hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 2006, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pineau, C.; Loubet, S.; Lefoulon, C.; Chalies, C.; Fizames, C.; Lacombe, B.; Ferrand, M.; Loudet, O.; Berthomieu, P.; Richard, O. Natural variation at the FRD3 MATE transporter locus reveals cross-talk between Fe homeostasis and Zn tolerance in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1003120. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233, Erratum in: Plant Cell 2021, 33, 439–440. [Google Scholar] [CrossRef]
- Fasani, E.; DalCorso, G.; Zorzi, G.; Agrimonti, C.; Fragni, R.; Visioli, G.; Furini, A. Overexpression of ZNT1 and NRAMP4 from the Ni hyperaccumulator Noccaea caerulescens population Monte Prinzera in Arabidopsis thaliana perturbs Fe, Mn and Ni accumulation. Int. J. Mol. Sci. 2021, 22, 11896. [Google Scholar] [CrossRef]
- Mai, H.J.; Pateyron, S.; Bauer, P. Iron homeostasis in Arabidopsis thaliana: Transcriptomic analyses reveal novel FIT-regulated genes, iron deficiency marker genes and functional gene networks. BMC Plant Biol. 2016, 16, 211. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Phytochelatins: Sulfur-Containing Metal(loid)-Chelating Ligands in Plants. Int. J. Mol. Sci. 2023, 24, 2430. [Google Scholar] [CrossRef]


| Target Gene | Primer in Forward | Primer in Reverse |
|---|---|---|
| FIT1 | CTCTAACCTAAGCTCTCCTTC | AAGTGATCCAGTGATCCACAG |
| IREG2 | CATGCTCATGGCTGGAGTTG | CCAAAAGATTAGCCATCAAGT |
| HMA4 | GTGGCAGAAGAGTTACTTCGA | TTTGGAACGGGGAGATGAGG |
| bHLH38 | GTCTCTTCAGAGGGAAATGAG | TCTGAGGCTGGAAGGCACG |
| bHLH39 | AGGGAAATGGAATAGACAACC | CCGTCTGAGGAATACTTAGCT |
| ZIP10 | TCCTCCAGGCAGAATACACG | TGTTATGAGAGAGGAAGGGCT |
| IRT1 | CCGACGGGAACATTTTCACC | GAAATTTGTGCCACGGGTTCT |
| SULTR1,1 | CAGTTGACAGTCCCGCTGAA | GCTGTTGGAGAGCGATTGTG |
| FRD3 | TGTGTTAGGACTTGGACTGTC | AGATGCTCCAAAATTGACTC |
| TUB | CCTACGCACCAGTCATCTCT | CGAGATCACCTCCTGGAACA |
| EF1α | GGATACAAATGAAGAAGAGAGG | CCAGCACACCAATGTCCGC |
| Ni | Cu | Zn | Fe | Mn | Co | |
|---|---|---|---|---|---|---|
| 10 µM Ni | 6.02 ± 1.51 a | 0.34 ± 0.03 a | 0.10 ± 0.05 a | 0.19 ± 0.01 a | 1.09 ± 0.05 a | 2.53 ± 0.14 a |
| 100 µM Ni | 1.14 ± 0.06 b | 0.28 ± 0.03 a | 0.45 ± 0.12 b | 0.04 ± 0.01 b | 0.59 ± 0.11 a | 1.32 ± 0.18 b |
| −Zn | 4.01 ± 0.10 c | 0.24 ± 0.01 b | 1.34 ± 0.05 c | 0.09 ± 0.01 c | 1.73 ± 0.07 b | 0.74 ± 0.26 b |
| −Fe | 9.07 ± 2.23 a | 0.54 ± 0.10 c | 2.23 ± 0.43 d | 0.23 ± 0.01 d | 3.58 ± 0.75 c | 0.43 ± 0.30 c |
| 100 µM Zn | 3.14 ± 1.40 c | 0.44 ± 0.18 a,c | 0.21 ± 0.01 e | 0.02 ± 0.00 b | 1.32 ± 0.06 b | 3.34 ± 0.42 a |
| 50 µM Co | 4.44 ± 0.94 a,c | 0.22 ± 0.00 b | 1.55 ± 0.05 c | 0.04 ± 0.00 b | 0.73 ± 0.05 a | 7.51 ± 1.63 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasani, E.; Zamboni, A.; Sorio, D.; Furini, A.; DalCorso, G. Metal Interactions in the Ni Hyperaccumulating Population of Noccaea caerulescens Monte Prinzera. Biology 2023, 12, 1537. https://doi.org/10.3390/biology12121537
Fasani E, Zamboni A, Sorio D, Furini A, DalCorso G. Metal Interactions in the Ni Hyperaccumulating Population of Noccaea caerulescens Monte Prinzera. Biology. 2023; 12(12):1537. https://doi.org/10.3390/biology12121537
Chicago/Turabian StyleFasani, Elisa, Anita Zamboni, Daniela Sorio, Antonella Furini, and Giovanni DalCorso. 2023. "Metal Interactions in the Ni Hyperaccumulating Population of Noccaea caerulescens Monte Prinzera" Biology 12, no. 12: 1537. https://doi.org/10.3390/biology12121537
APA StyleFasani, E., Zamboni, A., Sorio, D., Furini, A., & DalCorso, G. (2023). Metal Interactions in the Ni Hyperaccumulating Population of Noccaea caerulescens Monte Prinzera. Biology, 12(12), 1537. https://doi.org/10.3390/biology12121537









