Vesicular Trafficking, a Mechanism Controlled by Cascade Activation of Rab Proteins: Focus on Rab27
Abstract
:Simple Summary
Abstract
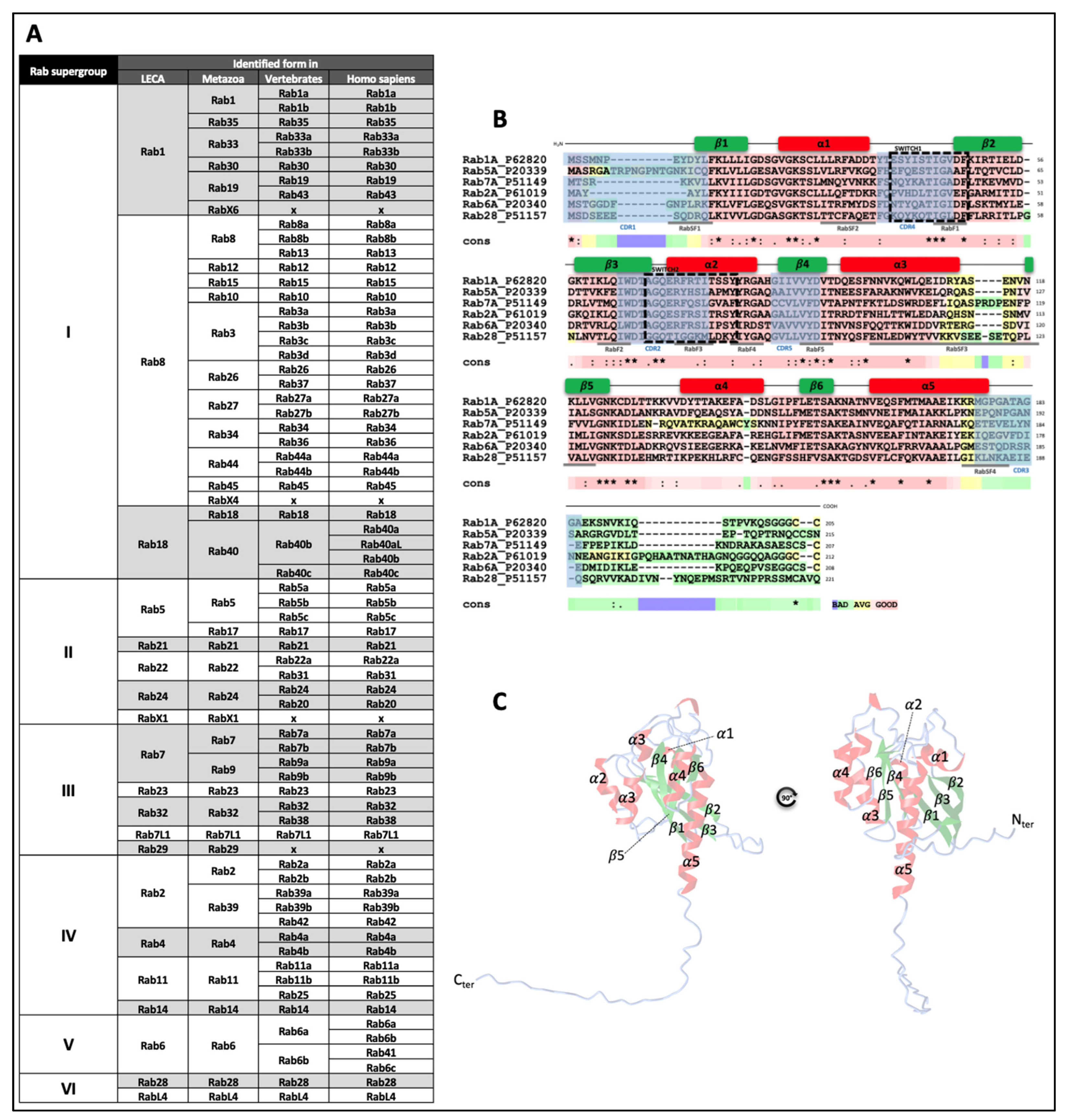
1. Introduction
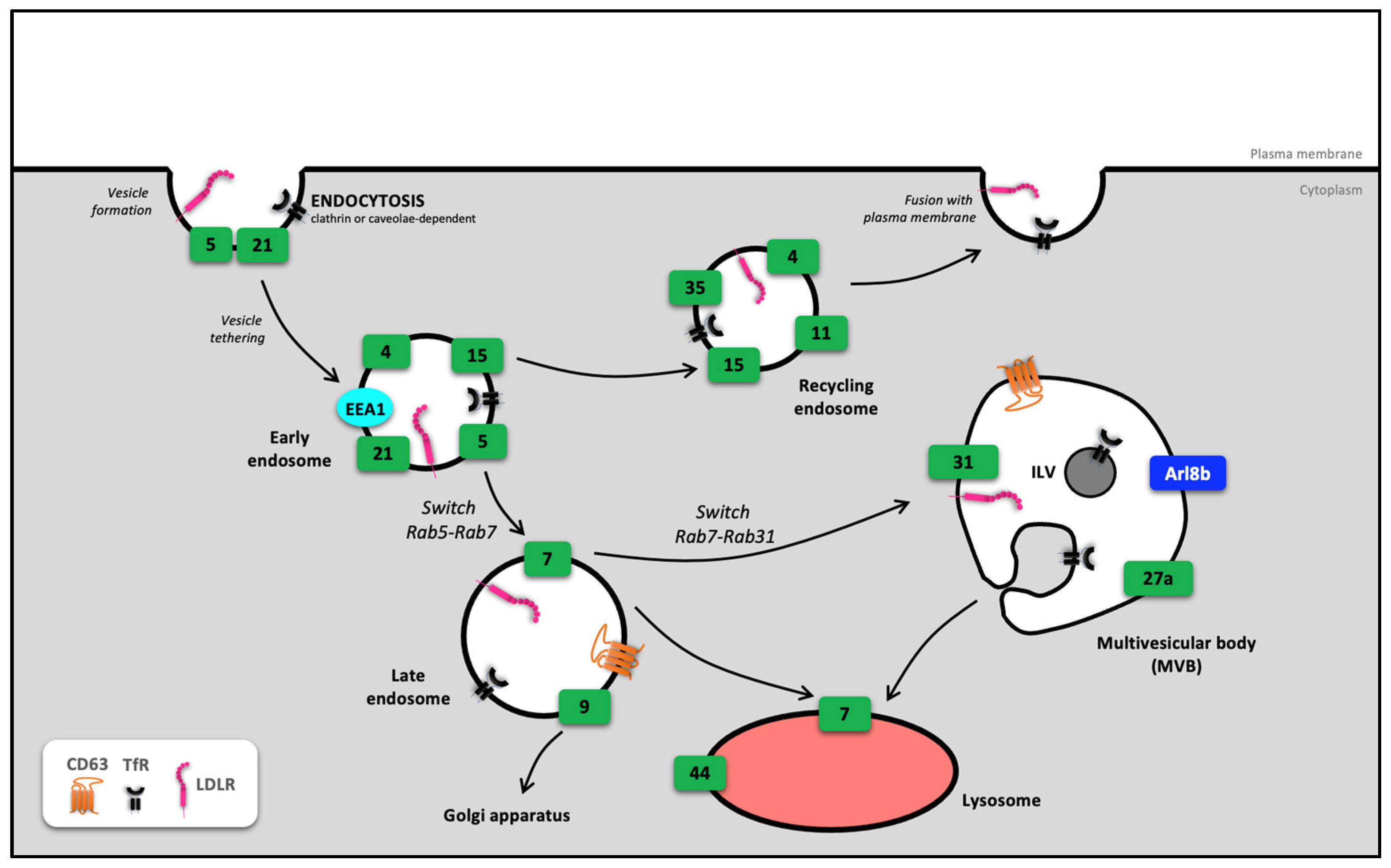
2. Involvement of Rab in Endosome Routing
2.1. From Early Endosome to Recycling Endosome
2.2. From the Early Endosome to the Late Endosome
2.3. Fate of the Late Endosome
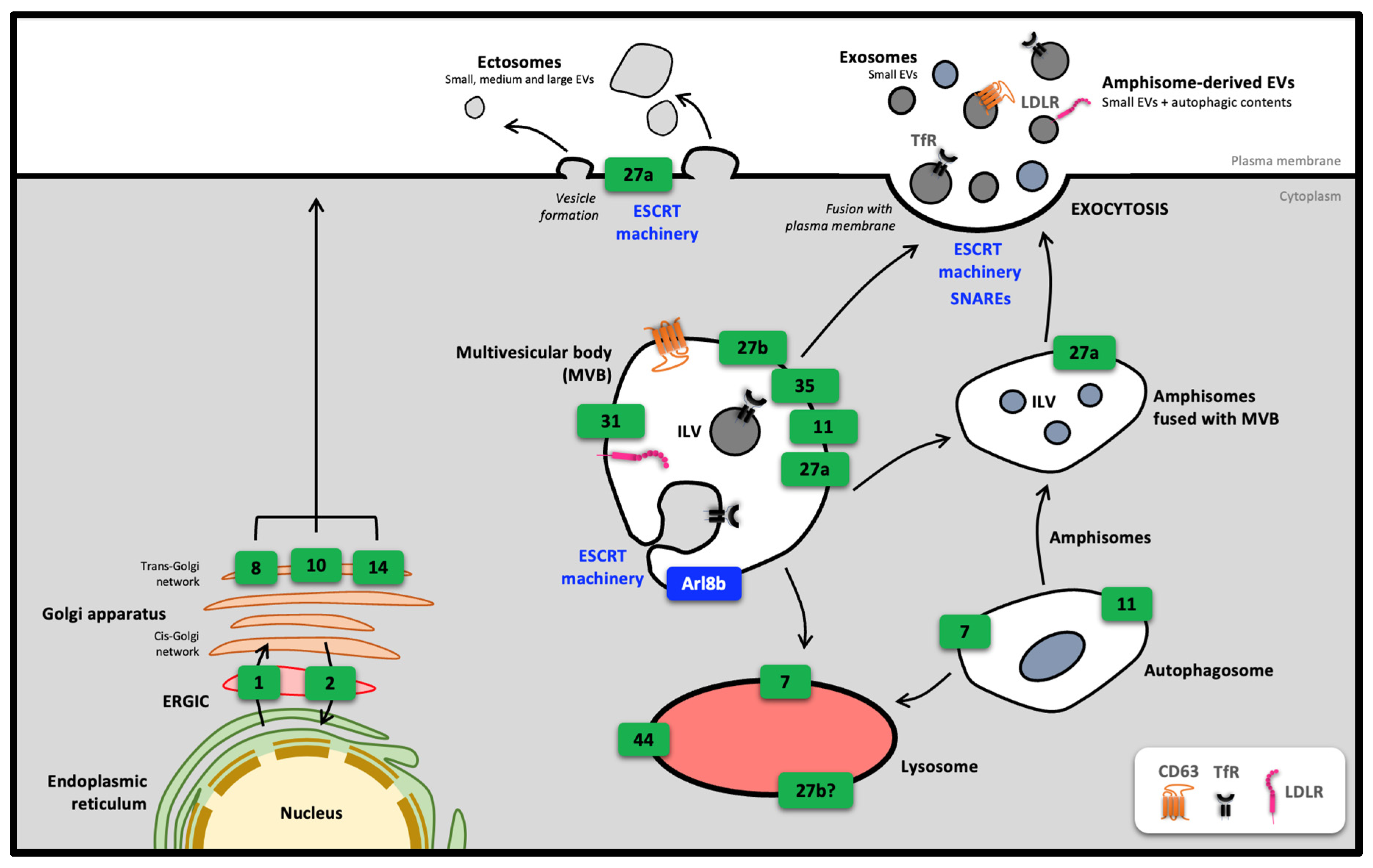
3. Role of Rab Proteins in the Biogenesis and Secretion of Extracellular Vesicles
3.1. Biogenesis and Secretion of Extracellular Vesicles
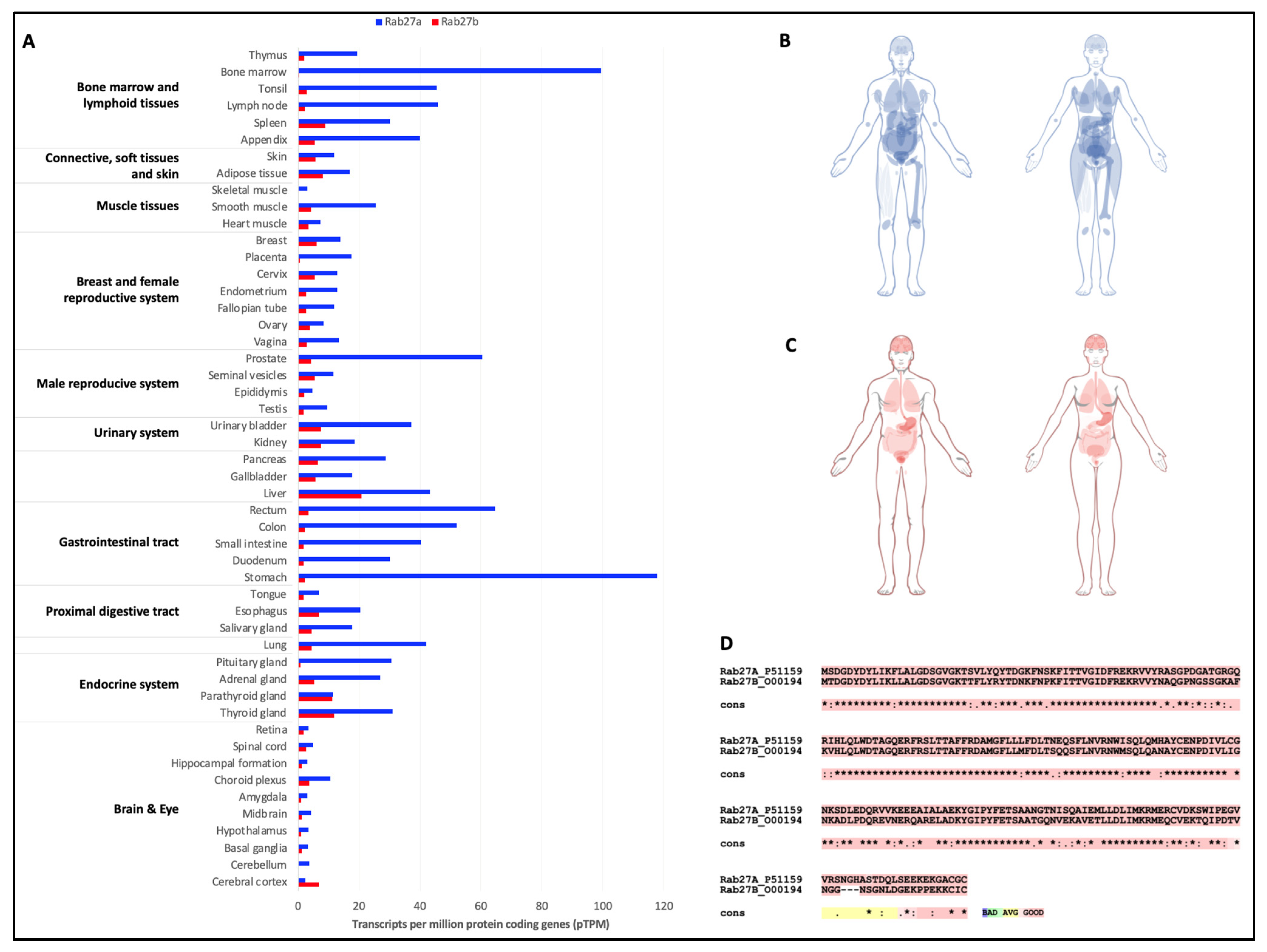
3.2. Importance of Rab27 in Pathophysiological Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Touchot, N.; Chardin, P.; Tavitian, A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: Molecular cloning of YPT-related cDNAs from a rat brain library. Proc. Natl. Acad. Sci. USA 1987, 84, 8210–8214. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Rab GTPases: Master regulators of membrane trafficking. Curr. Opin. Cell Biol. 1994, 6, 522–526. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Unsolved mysteries in membrane traffic. Annu. Rev. Biochem. 2007, 76, 629–645. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Pereira-Leal, J.B.; Seabra, M.C. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000, 301, 1077–1087. [Google Scholar] [CrossRef]
- Waschbusch, D.; Khan, A.R. Phosphorylation of Rab GTPases in the regulation of membrane trafficking. Traffic 2020, 21, 712–719. [Google Scholar] [CrossRef]
- Ostermeier, C.; Brunger, A.T. Structural basis of Rab effector specificity: Crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell 1999, 96, 363–374. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Wang, J.; Youkharibache, P.; Zhang, D.; Lanczycki, C.J.; Geer, R.C.; Madej, T.; Phan, L.; Ward, M.; Lu, S.; Marchler, G.H.; et al. iCn3D, a web-based 3D viewer for sharing 1D/2D/3D representations of biomolecular structures. Bioinformatics 2020, 36, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kadowaki, T.; Tokuhisa, M.; Yamaguchi, Y.; Umeda, M.; Tsukuba, T. Role of the EF-hand and coiled-coil domains of human Rab44 in localisation and organelle formation. Sci. Rep. 2020, 10, 19149. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.R.; Maddika, S. Post translational modifications of Rab GTPases. Small GTPases 2018, 9, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Kondapalli, C.; Lehneck, R.; Procter, J.B.; Dill, B.D.; Woodroof, H.I.; Gourlay, R.; Peggie, M.; Macartney, T.J.; Corti, O.; et al. Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J. 2015, 34, 2840–2861. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016, 5, e12813. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, P.; Hull, M.; Webster, P.; Male, P.; Goud, B.; Mellman, I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992, 70, 729–740. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Reed, G.L. Rab6 is phosphorylated in thrombin-activated platelets by a protein kinase C-dependent mechanism: Effects on GTP/GDP binding and cellular distribution. Biochem. J. 1999, 342 Pt 2, 353–360. [Google Scholar] [CrossRef]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef]
- Jin, H.; Tang, Y.; Yang, L.; Peng, X.; Li, B.; Fan, Q.; Wei, S.; Yang, S.; Li, X.; Wu, B.; et al. Rab GTPases: Central Coordinators of Membrane Trafficking in Cancer. Front. Cell Dev. Biol. 2021, 9, 648384. [Google Scholar] [CrossRef]
- van der Sluijs, P.; Hull, M.; Huber, L.A.; Male, P.; Goud, B.; Mellman, I. Reversible phosphorylation–dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992, 11, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Candela, P.; Gosselet, F.; Miller, F.; Buee-Scherrer, V.; Torpier, G.; Cecchelli, R.; Fenart, L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: Transcytosis through brain capillary endothelial cells in vitro. Endothelium 2008, 15, 254–264. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Gibieza, P.; Petrikaite, V. The dual functions of Rab11 and Rab35 GTPases-regulation of cell division and promotion of tumorigenicity. Am. J. Cancer Res. 2021, 11, 1861–1872. [Google Scholar] [PubMed]
- Singh, R.D.; Puri, V.; Valiyaveettil, J.T.; Marks, D.L.; Bittman, R.; Pagano, R.E. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 2003, 14, 3254–3265. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, M.; Ito, S.; Nishimura, Y.V.; Akagawa, R.; Fukuda, M.; Yuzaki, M.; Nabeshima, Y.I.; Kawauchi, T. Rab21 regulates caveolin-1-mediated endocytic trafficking to promote immature neurite pruning. EMBO Rep. 2023, 24, e54701. [Google Scholar] [CrossRef]
- Bielli, A.; Thornqvist, P.O.; Hendrick, A.G.; Finn, R.; Fitzgerald, K.; McCaffrey, M.W. The small GTPase Rab4A interacts with the central region of cytoplasmic dynein light intermediate chain-1. Biochem. Biophys. Res. Commun. 2001, 281, 1141–1153. [Google Scholar] [CrossRef]
- Cormont, M.; Meton, I.; Mari, M.; Monzo, P.; Keslair, F.; Gaskin, C.; McGraw, T.E.; Le Marchand-Brustel, Y. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic 2003, 4, 97–112. [Google Scholar] [CrossRef]
- de Renzis, S.; Sonnichsen, B.; Zerial, M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 2002, 4, 124–133. [Google Scholar] [CrossRef]
- Strick, D.J.; Elferink, L.A. Rab15 effector protein: A novel protein for receptor recycling from the endocytic recycling compartment. Mol. Biol. Cell 2005, 16, 5699–5709. [Google Scholar] [CrossRef]
- Ullrich, O.; Reinsch, S.; Urbe, S.; Zerial, M.; Parton, R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996, 135, 913–924. [Google Scholar] [CrossRef]
- Hales, C.M.; Vaerman, J.P.; Goldenring, J.R. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 2002, 277, 50415–50421. [Google Scholar] [CrossRef]
- Kouranti, I.; Sachse, M.; Arouche, N.; Goud, B.; Echard, A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 2006, 16, 1719–1725. [Google Scholar] [CrossRef]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef]
- Gilleron, J.; Zeigerer, A.; Marsico, G.; Galvez, T.; Zerial, M. Key role of Rab5: From endosome biogenesis to liver metabolism. Med. Sci. 2012, 28, 1041–1044. [Google Scholar] [CrossRef]
- Dong, B.; Kakihara, K.; Otani, T.; Wada, H.; Hayashi, S. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat. Commun. 2013, 4, 1358. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Carroll, K.; Bittova, L.; Pfeffer, S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol. Biol. Cell 2004, 15, 5420–5430. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Kuchitsu, Y.; Homma, Y.; Fujita, N.; Fukuda, M. Rab7 knockout unveils regulated autolysosome maturation induced by glutamine starvation. J. Cell Sci. 2018, 131, jcs215442. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamaguchi, Y.; Ogawa, K.; Tokuhisa, M.; Okamoto, K.; Tsukuba, T. Rab44 isoforms similarly promote lysosomal exocytosis, but exhibit differential localization in mast cells. FEBS Open Bio. 2021, 11, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F. Small but sturdy: Neuronal-derived exosomes control brain vasculature integrity. Med. Sci. 2018, 34, 303–306. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Jongsma, M.L.; Bakker, J.; Cabukusta, B.; Liv, N.; van Elsland, D.; Fermie, J.; Akkermans, J.L.; Kuijl, C.; van der Zanden, S.Y.; Janssen, L.; et al. SKIP-HOPS recruits TBC1D15 for a Rab7-to-Arl8b identity switch to control late endosome transport. EMBO J. 2020, 39, e102301. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Gronborg, M.; Mobius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peng, X.; Li, Y.; Zhang, X.; Ma, Y.; Wu, C.; Fan, Q.; Wei, S.; Li, H.; Liu, J. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol. Cancer 2019, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Bebelman, M.P.; George, A.E.; Couty, M.; Becot, A.; Palmulli, R.; Heiligenstein, X.; Sires-Campos, J.; Raposo, G.; Pegtel, D.M.; et al. ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J. Cell Biol. 2022, 221, e202112032. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab27 and its effectors in secretory granule exocytosis: A novel docking machinery composed of a Rab27.effector complex. Biochem. Soc. Trans. 2006, 34, 691–695. [Google Scholar] [CrossRef]
- Tsuboi, T.; Fukuda, M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J. Cell Sci. 2006, 119, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Shen, Y.T.; Gu, Y.; Su, W.F.; Zhong, J.F.; Jin, Z.H.; Gu, X.S.; Chen, G. Rab27b is Involved in Lysosomal Exocytosis and Proteolipid Protein Trafficking in Oligodendrocytes. Neurosci. Bull. 2016, 32, 331–340. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Fukuda, M. Rab GTPases: Key players in melanosome biogenesis, transport, and transfer. Pigment Cell Melanoma Res. 2021, 34, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Barral, D.C.; Ramalho, J.S.; Anders, R.; Hume, A.N.; Knapton, H.J.; Tolmachova, T.; Collinson, L.M.; Goulding, D.; Authi, K.S.; Seabra, M.C. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Investig. 2002, 110, 247–257. [Google Scholar] [CrossRef]
- Izumi, T. In vivo Roles of Rab27 and Its Effectors in Exocytosis. Cell Struct. Funct. 2021, 46, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.M.; Jang, B.G.; Kim, D.C. Prognostic significance of Rab27 expression in solid cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 14136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, Q.; Huang, F.; Tang, Z.; Huang, J. Effects of Rab27A and Rab27B on Invasion, Proliferation, Apoptosis, and Chemoresistance in Human Pancreatic Cancer Cells. Pancreas 2017, 46, 1173–1179. [Google Scholar] [CrossRef]
- Ren, J.G.; Xing, B.; Lv, K.; O’Keefe, R.A.; Wu, M.; Wang, R.; Bauer, K.M.; Ghazaryan, A.; Burslem, G.M.; Zhang, J.; et al. RAB27B controls palmitoylation-dependent NRAS trafficking and signaling in myeloid leukemia. J. Clin. Investig. 2023, 133, e165510. [Google Scholar] [CrossRef]

| Rab Proteins | Effector(s) and Partners | Role(s) in Vesicular Trafficking |
|---|---|---|
| Rab1 | p115-GM130 | ER-Golgi trafficking |
| Giantin-Golgin84 | Tethering | |
| Rab2 | RUND-1 | ER-Golgi trafficking |
| CCCP-1 | Tethering | |
| Rab4 | Rabaptin-4,5,5β | Protein sorting and recycling |
| Rabex-5 | Endocytic recycling to plasma membrane | |
| Rab5 | Rabaptin-5,5β-p150-Vac1-EEA1 | Endocytic internalization and early endosome formation |
| Rabenosyn-5-Vps34,45-CORVET | ||
| Rabkinesin-6-Rabex-5-Rabphillin-3 | Tethering and fusion | |
| Syntaxin13,16 | ||
| Rab7 | Rabring | Late endocytic trafficking |
| HOPS complex | Vesicle fusion | |
| Rab8 | Rab8IP | Transport between Golgi and TGN |
| Rab9 | p40 | Cargo adaptor, sorting and fusion |
| TIP47 | Exchanges between late endosomes and trans-Golgi | |
| Rab10 | MICAL1 | Transport between Golgi and TGN |
| MYO5A-B-C | Transport between TGN to plasma membrane | |
| RIMS1 | ||
| Rab11 | Rabphylin11-Rab11BP | Exocytosis, transport and recycling of endosomes |
| FIP2-4-Sec15 | Transport from the Golgi | |
| RIP11-Sec13 | Endocytic recycling | |
| Rab14 | KIF16B-RUFY1-ZFYVE20 | Transport between Golgi and early endosomes |
| Rab15 | REP15 | Exit from recycling endosomes |
| Inhibitor of endocytin internalization | ||
| Rab21 | APPL | Endocytic internalization |
| ITGA2-ITGA11 | Cytokinesis | |
| Rab27a | MLPH-SLP2A-Rabphilin-3 | Exocytosis |
| Noc2-Granuphilin-CORO1C | ||
| MYO5A-MYRIP-RPH3A | ||
| RPH3AL-SYTL1-5-UNC13D | ||
| Rab27b | SYP4-EXPH5 | Exocytosis |
| Rab31 | OCRL-TBC1D2B | Bidirectional transport between TGN and early endosomes |
| Rab35 | ACAP2-FSCN1-MICALL1-OCRL | Endocytic recycling |
| Rab44 | Coronin1C | (Putative) Lysosomal function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menaceur, C.; Dusailly, O.; Gosselet, F.; Fenart, L.; Saint-Pol, J. Vesicular Trafficking, a Mechanism Controlled by Cascade Activation of Rab Proteins: Focus on Rab27. Biology 2023, 12, 1530. https://doi.org/10.3390/biology12121530
Menaceur C, Dusailly O, Gosselet F, Fenart L, Saint-Pol J. Vesicular Trafficking, a Mechanism Controlled by Cascade Activation of Rab Proteins: Focus on Rab27. Biology. 2023; 12(12):1530. https://doi.org/10.3390/biology12121530
Chicago/Turabian StyleMenaceur, Camille, Océane Dusailly, Fabien Gosselet, Laurence Fenart, and Julien Saint-Pol. 2023. "Vesicular Trafficking, a Mechanism Controlled by Cascade Activation of Rab Proteins: Focus on Rab27" Biology 12, no. 12: 1530. https://doi.org/10.3390/biology12121530
APA StyleMenaceur, C., Dusailly, O., Gosselet, F., Fenart, L., & Saint-Pol, J. (2023). Vesicular Trafficking, a Mechanism Controlled by Cascade Activation of Rab Proteins: Focus on Rab27. Biology, 12(12), 1530. https://doi.org/10.3390/biology12121530






